Abstract
Polybrominated diphenyl ether (PBDE) flame retardants have become widespread environmental contaminants. Body burden in the U.S. population has been shown to be higher than in other countries, and infants and toddlers have highest exposure through maternal breast milk and household dust. The primary concern for adverse health effects of PBDEs relates to their potential developmental neurotoxicity, which has been found in a number of animal studies. Information on the possible mechanisms of PBDE neurotoxicity is limited, though some studies have suggested that PBDEs may elicit oxidative stress. The present study examined the in vitro neurotoxicity of DE-71, a penta-BDE mixture, in primary neurons and astrocytes obtained from wild-type and Gclm knockout mice, which lack the modifier subunit of glutamate-cysteine ligase and, as a consequence, have very low levels of glutathione (GSH). These experiments show that neurotoxicity of DE-71 in these cells is modulated by cellular GSH levels. Cerebellar granule neurons (CGNs) from Gclm (−/−) mice displayed a higher sensitivity to DE-71 toxicity compared to CGNs from wild-type animals. DE-71 neurotoxicity in CGNs from Gclm (+/+) mice was exacerbated by GSH depletion, and in CGNs from both genotypes it was antagonized by increasing GSH levels and by anti-oxidants. DE-71 caused an increase in reactive oxygen species and in lipid peroxidation in CGNs, that was more pronounced in Gclm (−/−) mice. Toxicity of DE-71 was mostly due to the induction of apoptotic cell death. An analysis of DE-71-induced cytotoxicity and apoptosis in neurons and astrocytes from different brain areas (cerebellum, hippocampus, cerebral cortex) in both mouse genotypes showed a significant correlation with intracellular GSH levels. As an example, DE-71 caused cytotoxicity in hippocampal neurons with IC50s of 2.2 and 0.3 µM, depending on genotype, and apoptosis with IC50s of 2.3 and 0.4 µM, respectively. These findings suggest that the developmental neurotoxicity of PBDE may involve oxidative stress, and that individual with genetic polymorphisms leading to lower GSH levels may be more susceptible to their adverse effects.
Introduction
The use of flame retardants has contributed to a drastic drop in the incidence of fires in the past thirty years. Among chemicals used as fire retardants, several are brominated compounds, such as tetrabisphenol A, hexabromocyclododecane, and polybrominated diphenyl ethers (PBDEs). The latter are extensively used in a variety of consumer products such as textile, carpets, polyurethane foams, television sets, small appliances and computers. There are 209 possible PBDE congeners; commercially used PBDEs are penta-, octa-, and deca-brominated mixtures. The first two were banned in the EU in 2004, and in several states in the U.S. in from 2006, while decaBDE continues to be widely used. PBDEs are not fixed to the polymer product through chemical binding, and can thus leak into the environment (Hale et al. 2003; Law et al. 2006; Wang et al. 2007). PBDEs are chemically and toxicologically similar to non-coplanar polychlorinated biphenyls (PCBs) (Kodavanti et al. 2005; Sanders et al. 2005; Coburn et al. 2007). Although PCBs were banned decades ago, they are still widespread environmental contaminants. Similarly, PBDEs have become persistent environmental pollutants, and like PCBs, they bioaccumulate in the environment, and biomagnify in the food chain (Darnerud et al. 2001; de Wit 2002; Birbaum and Staskal, 2004).
PBDEs have been detected in outdoor and indoor air, in sediments, soil, house dust, foods, birds, fish, and marine and terrestrial animals (Darnerud et al. 2001; Law et al. 2006), as well as in human tissues, including adipose tissue, blood and breast milk (Sjodin et al. 2004; Furst, 2006). Exposure of humans to PBDEs can occur in the occupational setting (Sjodin et al. 1999), through the diet (Schecter et al. 2006; 2008), and in the indoor environment, particularly through house dust (Jones-Otazo et al. 2005; Wilford et al. 2005). High concentrations of PBDEs are present in human breast milk, with levels usually one to two orders of magnitude higher in North America than in Europe (Schecter et al. 2005; Lind et al. 2003; Furst, 2006). In contrast to PCBs, dioxins and furans, whose concentrations in human tissues and breast milk have been declining in the past 20 years (Schecter et al. 2005), levels of PBDEs have been drastically increasing worldwide (Thompsen et al. 2002; Schecter et al. 2005). The highest body burden is found in infants and toddlers, because of exposure through maternal milk and house dust (Schecter et al. 2006; Fischer et al. 2006; Zuurbier et al. 2006). In addition, PBDEs can easily cross the placenta, thus exposing the fetus (Antignac et al. 2006). The PBDE congeners found at highest levels in human tissues are BDE-47 (a tetra BDE), BDE-99 (a pentaBDE) and BDE-153 (an hexaBDE).
The high exposure to PBDEs during development has raised concerns for their potential developmental toxicity. Emerging evidence indicates that a major aspect of toxicity relates to their effects on the developing nervous system (Branchi et al. 2003; Birnbaum and Staskal, 2004; McDonald, 2005; Costa and Giordano, 2007). A number of studies in rodents have shown that pre-and post-natal exposures to PBDEs cause significant behavioral alterations, particularly in the domains of locomotor activity and cognition (e.g. Eriksson et al. 2001; Branchi et al. 2002; 2003; Viberg et al. 2003; 2006; Dufault et al. 2005; Rice et al. 2007; Gee and Moser, 2008; additional references in Costa and Giordano, 2007). These effects are seen at levels of PBDE exposure that are in the range of exposure of infants and toddlers in North America, and only slightly higher than exposure levels in Europe or Japan ( McDonald, 2005; Costa and Giordano, 2007).
The mechanism(s) underlying such developmental neurotoxic effects of PBDEs are not known. PBDEs have been shown to decrease serum levels of thyroid hormones (T4) (Zhou et al. 2002; Skarman et al. 2005; Rice et al. 2007), and, given the relevance of thyroid hormones for brain development (Haddow et al. 1999), such effect may contribute to their developmental neurotoxicity. Direct effects of PBDEs on the developing nervous system have also been reported. Some studies have shown that PBDEs can affect signal transduction pathways, particularly the homeostasis of calcium and protein kinase C (e.g. Madia et al. 2004; Kodavanti and Ward, 2005; Dingemans et al. 2007). Aditionally, a few in vitro studies in neuronal and astroglial cells have shown that PBDEs may cause apoptotic cell death of neurons by a mechanism that involves oxidative stress (Reistad et al. 2006; He et al. 2008a; 2008b).
Oxidative stress refers to the cytotoxic consequences of reactive oxygen species (ROS), which are generated as by-products of normal and aberrant metabolic processes that use molecular oxygen. The tripeptide glutathione (GSH; γ-glutamyl-cysteinylglycine) is one of the most abundant cellular thiols. GSH is a major player in cellular defense against ROS, because it nonenzymatically scavenges both singlet oxygen and hydroxyl radicals, and is used by glutathione peroxidases and glutathione transferases to limit the levels of certain reactive aldehydes and peroxides within the cell. When ROS production exceeds the antioxidant defense capacity of the cell, oxidative stress ensues, leading to damage to DNA, proteins and membrane lipids. The first and rate-limiting step in the synthesis of GSH is carried out by glutamate-cysteine ligase (GCL; Griffin and Mulcahy, 1999; Dringen, 2000). The enzyme consists of two subunit, a larger (73 kD) catalytic subunit (GCLC), and a smaller (31 kD) modifier subunit (GCLM), which are encoded by separate genes (Tu and Anders, 1998). GCLC alone provides catalytic activity, and is the site of GSH feedback inhibition. By lowering the Km of GCL for glutamate and raising the Ki for GSH, GCLM, although enzymatically inactive, plays an important regulatory function. Indeed, the holoenzyme (GCLholo) has been shown to have much higher catalytic efficiency than GCLC (Chen et al. 2005). Disruption of the Gclm gene in mice does not produce any overt phenotype (Chen et al. 2005); however, in the absence of GCLM, the ability of GCLC to synthesize GSH is drastically reduced, resulting in GSH levels that are only 9–20% of those found in Gclm (+/+) animals (Yang et al. 2002; Giordano et al. 2006; McConnachie et al. 2007). Cerebellar granule neurons derived from Gclm knockout mice have been shown to be particularly sensitive to the toxicity of agents that elicit oxidative stress (Giordano et al. 2006; 2007a).
In the present study, we utilized neurons and astrocytes from different brain areas obtained from Gclm (+/+) and Gclm (−/−) mice to investigate whether intracellular GSH levels may modulate the in vitro neurotoxicity of a PBDE. The penta-BDE mixture DE-71, which contains all major PBDEs most commonly found in human tissues, was used in these experiments.
Materials and Methods
Materials
DE-71 (Lot # 05500F16P) was purchased from Wellington Laboratories (Guelph, ON, Canada). The composition of DE-71 is reported as follows: BDE-99, 44%; BDE-47, 32%; BDE-100, 9%; BDE-153, 4%; other PBDEs, 11%. Other DE-71 mixtures have been reported to contain detectable amounts of polybrominated dibenzofurans and polybrominated dibenzodioxins (Hanari et al. 2006; Sanders et al. 2005). None were reported by the vendor, and no chemical analysis of the DE-71 used in this study was carried out. Glutathione ethyl ester (GSHEE), dimethylsulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), N-t-butyl-α-phenylnitrone (PBN), N-acetylcysteine and buthionine sulfoximine (BSO) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). The apoptotic DNA ladder kit and DNase I were from Roche Diagnostics (Indianapolis, IN, USA). Neurobasal-A medium, fetal bovine serum (FBS), gentamycin, naphthalene dicarboxaldehyde, and 2,7’-dichlorofluorescin diacetate were from Invitrogen (Carlsbad, CA, USA). Hoechst DNA binding dye was from Molecular Probes (Eugene, OR, USA). Tris (2-carboxyethyl)-phosphine hydrochloride (TCEP) and the protein bicinchoninic acid assay were from Pierce Chemical (Rockford, IL). Melatonin was from Tocris Cookson Inc. (Ellisville, MO).
Generation of Gclm-null mice and genotyping
All procedures for animal use were in accordance with the National Institute of Health Guide for the Use and Care of Laboratory Animals, and were approved by the University of Washington Animal Care and Use Committee. Gclm-null [Gclm (−/−)] mice were derived by homologous recombination techniques in mouse embryonic stem cells, as previously described in detail (Giordano et al. 2006; McConnachie et al. 2007). Pups were genotyped as described by Giordano et al. (2006).
Cultures of cerebellar granule neurons, hippocampal neurons and cortical neurons
Cultures of cerebellar granule neurons (CGN) were prepared from 7 day-old mice, as described by Giordano et al. (2006). Neurons were grown for 10–12 days before treatments. Hippocampal and cerebral cortical neurons were prepared from PND 0.5 mice, as described by Ahlemeyer and Baumgart-Vogt (2005). Briefly, hippocampi and cerebral cortices were collected in HBSS medium containing 0.02% BSA and 10 mM HEPES. Tissues were digested for 25 min in HBSS containing papain (1 mg/ml) and DNAse 40 ug/ml) and centrifuged at 300 × g for 5 min at room temperature. The supernatant (containing papain) was removed and the pellet was gently triturated in Neurobasal A Medium supplemented with B27 with a Pasteur pipette to dissociate larger aggregates. Cells were centrifuged at 200 × g for 10 min and the cell pellet was gently resuspended. Neurons were then counted, seeded on poly-D-lysine-coated 48-well plates at the density of 5 × 104/cm2, and cultured in neurobasal medium supplemented with B27 (minus AO). Neurons were cultured 8 days before experiments.
Primary cultures of cerebellar, hippocampal and cerebral cortex astrocytes
Primary mouse astrocytes were obtained from PND 0.5 hippocampus and cerebral cortex and from PND 7 cerebellum, as previously described by Giordano et al. (2006) and Guizzetti et al. (1996). Briefly, brain regions were dissected, mechanically dissociated and incubated with trypsin, followed by trituration, repeated washing, and filtering. After counting, cells were plated at a density of 107 cells in 75 cm2 tissue culture flasks pre-coated with poly-D-lysine and grown in DMEM containing 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin at 37 °C in 5% CO2 /95% air. After 10 days in culture, cells were plated in 24-well plates for the experiments at the density of 5×104 astrocytes/well.
Cell treatments
DE-71 was dissolved in DMSO to obtain a stock solution of 25 mM, which was diluted appropriately at the time of use. Three to five different concentrations of DE-71, in triplicate, were used to allow determination of IC50 values. In some experiments only one selected concentrations of DE-71 was used. Cells were incubated with DE-71 for 24 h for cytotoxicity assays and for assessment of apoptosis, or for shorter periods, as appropriate, for other assays. When used, antagonists (e.g. antioxidants) were added 30 min before DE-71. N-acetylcysteine and GSHEE were dissolved in PBS, while PBN and melatonin were dissolved in DMSO. Buthionine sulfoximine (BSO; 25 µM, dissolved in DMSO) was added 24 h before DE-71 to deplete intracellular GSH. All assays were run in parallel, in cells from Gclm (+/+) and Gclm (−/−) mice, in triplicate, and each experiments was repeated at least three times.
Cytotoxicity Assays
Cell viability was quantified by a colorimetric method utilizing the metabolic dye 3-(4,5- dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT). After 24 h of incubation, medium was removed and replaced with 500 µl /well of Locke’s solution containing 2 mg/ml MTT. After 30 min at 37 °C, the MTT solution was removed and the reaction product dissolved in 0.25 ml of DMSO. MTT is reduced to a blue formazan by mitochondrial dehydrogenases in living cells, but not by dying cells or their lytic debris. Absorbance was read at 562 nm, and the results are expressed as percent viability relative to the control. For the Trypan blue exclusion assay, attached cells were collected by trypsinization, followed by the addition of appropriate medium/serum to inactivate the trypsin. Cells were counted in a hemocytometer using equal volumes of cell suspension and trypan blue solution.
Assessment of apoptosis
Apoptosis was assessed by morphologic analysis and by measurement of DNA fragmentation. Following DE-71 treatment, cells were fixed with methanol and stained in 10 µg/mL Hoechst DNA binding dye for 15 min. All cells exhibiting either classical apoptotic bodies or shrunken, rounded nuclei, were scored as being apoptotic. Five fields across a diameter were counted per well, at 20X, using an inverted fluorescence microscope. DNA fragmentation was detected with a commercial kit. Briefly, cells were washed twice with Locke’s buffer, and incubated for 10 minutes with an equal volume of lysis buffer containing 10 mM Tris-HCl (pH 7.4), 6 M guanidine-HCl, 10 mM urea and EDTA, and 0.2% Triton X-100. The samples were passed through glass fiber fleece by centrifugation, and the nucleic acid bound to the glass fibers was eluted. The DNA was applied to a 1.5% agarose gel, and the bands were then visualized by ethidium bromide staining and photographed.
Measurements of GSH levels
Total intracellular GSH levels were measured as described by Giordano et al. (2006). Briefly, cells were homogenized in Locke’s buffer and an aliquot was taken to measure protein concentration by the bicinchoninic acid method. A second aliquot was diluted (1:1) in 10% 5-sulfosalicylic acid (SSA), centrifuged at 12,000 rpm for 5 min at 4°C, and the supernatant was used for GSH determination. Aliquots from the SSA fraction were added to a black flat-bottomed 96-well plate, and pH was adjusted to 7 with 0.2 M N-ethylmorpholine/0.02 M KOH. Oxidized glutathione was reduced by adding 10 µl of 10 mM TCEP for 15 min at room temperature. The pH was then adjusted to 12.5 by using 0.5 NaOH before derivatizing samples with 10 mM naphthalene dicarboxaldehyde for 30 min. Finally, samples were analyzed on a spectrofluorometric plate reader (λEX = 472 nm and λEM = 528 nm). After incubation, the total amount of GSH in the sample was expressed as nanomoles per mg of protein determined from a standard curve obtained by plotting known amount of GSH incubated in the same experimental conditions versus fluorescence.
Measurement of Reactive Oxygen Species formation
ROS formation was determined by fluorescence using 2,7’-dichlorofluorescin diacetate (DCF-DA), as previously described (Giordano et al. 2006). Upon entering cells DCF-DA is de-esterified to DCFH, which is then oxidized by ROS to form the fluorescent 2,7’-dichlorofluorescein (DCF). Cells were first washed with Locke’s solution, and then pre-incubated for 30 min (37°C) with DCF-DA (50 nmol/mg cell protein) in Locke’s solution. DCF-DA was added from a stock solution in DMSO; the quantity of DMSO never exceeded 0.1%, and was also added to the blank. Cells were then washed with Locke’ solution to remove extracellular DCF-DA and fluorescence was immediately read using a fluorescence microplate reader (excitation 488 nm, emission 538 nm).
Measurement of lipid peroxidation
Cells were scraped in 20 mM phosphate buffer (pH 7.4) and aliquots were removed to determine protein content. After addition of an antioxidant (butylhydroxytoluene, 10 µM) to prevent sample oxidation, the homogenate was centrifuged at 3000 × g for 10 min to remove large particles. N-methyl-2-phenylindole and methanesulfonic acid were then added and the samples were incubated at 45°C for 60 min and then centrifuged at 5000 × g for 10 min to obtain a clear supernatant. Absorbance of malonyldialdehyde in the supernatant was read at 586 nm.
Statistical Analysis
Data are expressed as the mean ± SD of at least three independent experiments. IC50 values were calculated from a concentration-response curve with 3–5 concentrations of DE-71 (GraphPad Prism Software), using a non-linear regression with a sigmoidal fit model. Statistical analysis was carried out by Student’s t-test. Correlations between IC50 values from MTT or apoptosis assays were calculated by linear regression analysis and Pearson’s coefficient.
Results
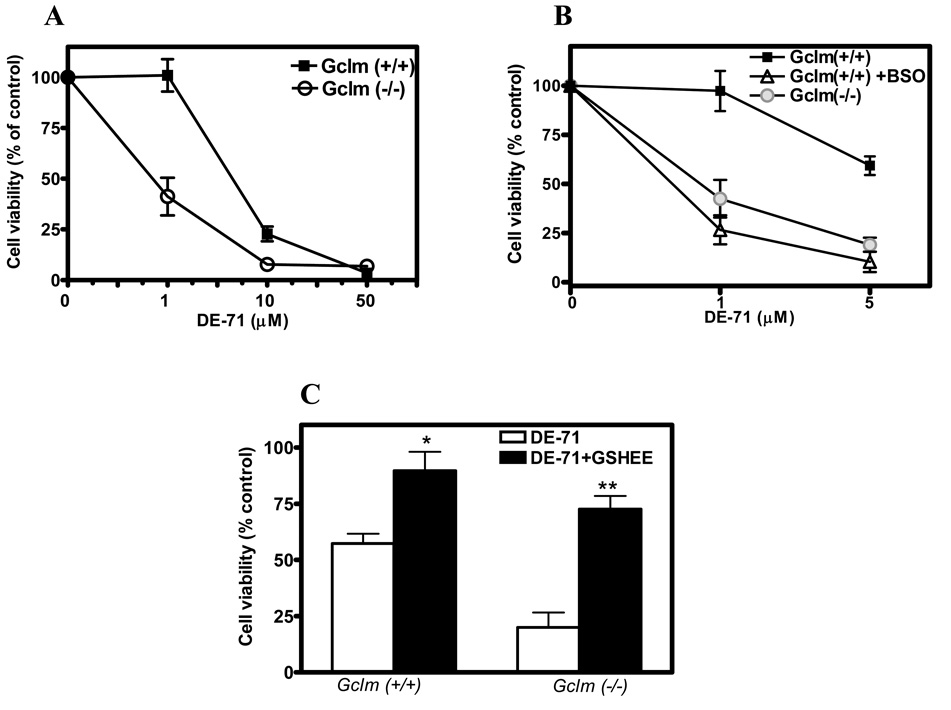
The neurotoxicity of DE-71 was first evaluated in CGNs from Gclm (+/+) and Gclm (−/−) mice by the MTT assay. Levels of GSH in these cells were (nmol/mg protein) 12.4 ± 1.4 and 2.4 ± 1.1, respectively (n =4; p<0.01). As shown in Fig. 1A, the toxicity of DE-71 was higher in CGNs from Gclm knockout mice (IC50 = 0.9 ± 0.2 µM) than in those from wild-type mice (IC50 = 7.7 ± 2.3 µM) (n = 3; p<0.01). When CGNs from Gclm (+/+) mice were exposed to the GSH synthase inhibitor buthionine sulfoximine (BSO; 25 µM for 24 h), levels of GSH decreased to 3.7 ± 0.9 nmol/mg protein, similar to those present in CGNs from Gclm (−/−) mice. Under this condition, CGNs from Gclm (+/+) mice were as sensitive to DE-71 toxicity as those from Gclm (−/−) mice (IC50 = 0.7 ± 0.1 µM; n =3) (Fig. 1B). When CGNs of both genotypes were incubated with the membrane permeable GSH delivery agent GSH ethylester (2.5 mM for 30 min), intracellular levels of GSH increased to 22 and 18 nmol/mg protein in CGNs from Gclm (+/+) and (−/−) mice, respectively (Giordano et al. 2006). Under this condition, toxicity of DE-71 was significantly decreased (Fig. 1C).
Fig. 1. GSH levels modulate DE-71 neurotoxicity in mouse CGNs.
A. Effect of DE-71 on cell viability of CGNs from Gclm (+/+) and (−/−) mice, as assessed by the MTT assay. B. Effect of BSO (25 µM for 24 h) on DE-71 neurotoxicity in CGNs from Gclm (+/+) mice. C. Effect of GSHEE (2.5 mM for 30 min) on DE-71 (5 µM) neurotoxicity in CGNs of both genotypes. Results represent the means (± SD) of at least three separate determinations. *Significantly different from DE-71 alone, p<0.05; **p<0.01.
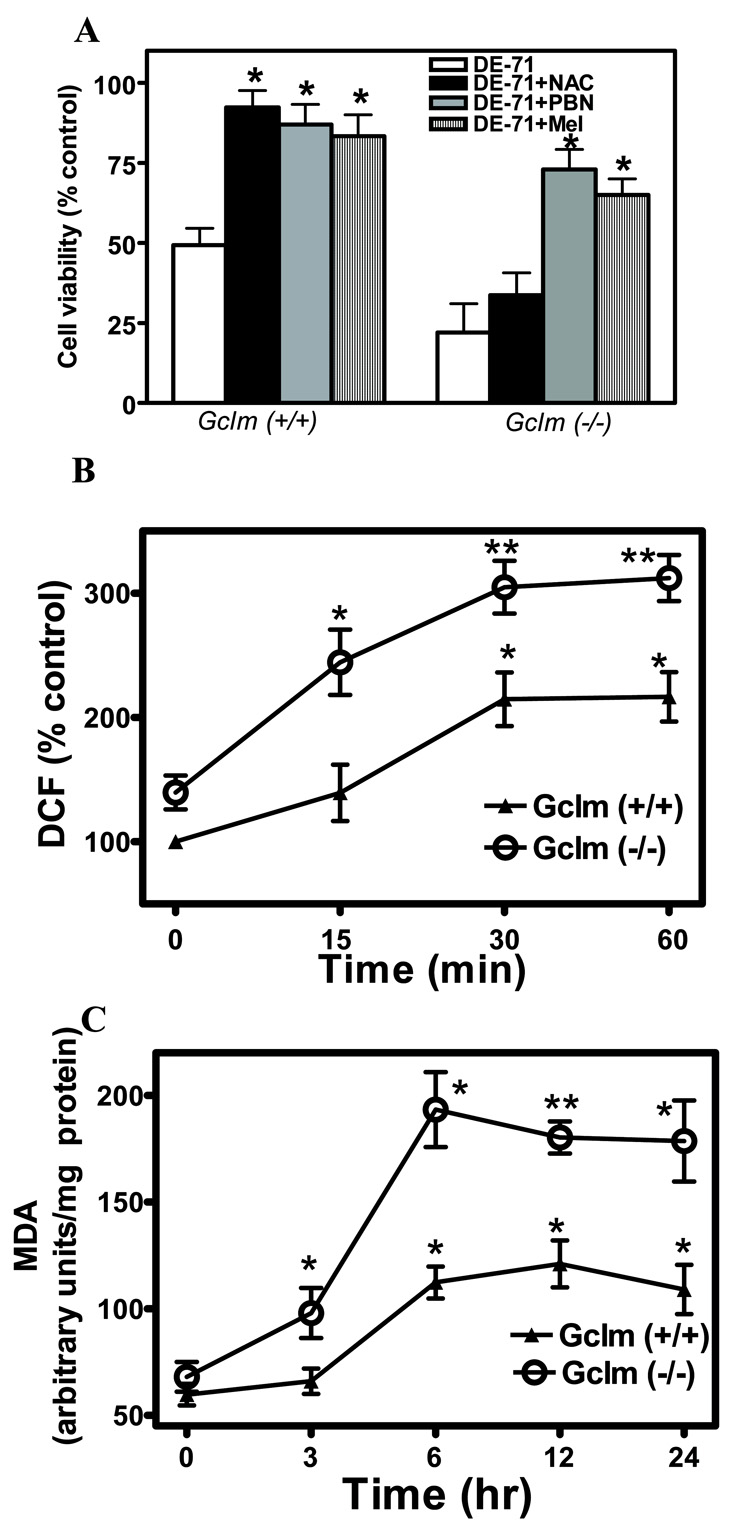
Two antioxidants, PBN and melatonin, protected CGNs of both genotypes from DE-71 toxicity (Fig. 2A). In contrast, N-acetylcysteine, which needs to be converted to GSH, was only effective in CGNs from Gclm (+/+) mice (Fig. 2A). DE-71 also caused a time-dependent increase in the levels of ROS (measured by DCF fluorescence), which was more pronounced in CGNs from Gclm (−/−) mice (Fig. 2B). In addition, DE-71 caused an increase in lipid peroxidation, that was also more evident in Gclm (−/−) CGNs (Fig. 2C). These findings provide initial evidence for a central role of GSH in modulating the neurotoxicity of DE-71, and suggest that its toxicity may involve oxidative stress.
Fig. 2. DE-71 causes oxidative stress in mouse CGNs.
A. Effects of antioxidants N-acetylcysteine (NAC; 2.5 mM), N-t-phenyl-α-butylnitrone (PBN; 100 µM), and melatonin (Mel; 200 µM) on DE-71 (5 µM) neurotoxicity in CGNs from Gclm (+/+) and (−/−) mice. B. Effect of DE-71 (5 µM) on ROS production in CGNs from both mouse genotypes. C. Effect of DE-71 (5 µM) on lipid peroxidation in CGNs from both mouse genotypes. Results represent the means (± SD) of at least three separate determinations. * Significantly different from DE-71 (A) or control (B, C), p<0.05; **p<0.01.
As oxidative stress may be amplified under higher oxygen concentrations, thus leading to an overestimation of actual effects caused by DE-71, we also investigated the neurotoxicity of, and the induction of ROS by DE-71 in CGNs, under conditions of low oxygen (5% vs. 21% in normal cell culturing conditions). Table 1 shows a comparison of the effects of DE-71 under both low and high oxygen levels in CGNs of both genotypes. There were no significant differences in the effects of DE-71 on ROS or cell viability under the two experimental conditions, indicating that the initial findings were not an artifact associated with high oxygen levels.
Table 1.
Toxicity and ROS production induced by DE-71 in mouse cerebellar granule neurons at different oxygen level
| Cytotoxicity | ROS production | |||
|---|---|---|---|---|
| Oxygen | Gclm (+/+) | Gclm (−/−) | Gclm (+/+) | Gclm (−/−) |
| 21 % | 6.8 ± 1.7 | 0.8 ± 0.5 | 160 ± 9 | 266 ± 19 |
| 5% | 10.1 ± 2.1 | 1.4 ± 0.6 | 148 ± 6 | 231 ± 9 |
For cytotoxicity, values represent IC50 (µM) in the MTT assay, derived from concentration-response curves obtained with 3–5 concentrations of DE-71, and are the means (± SD) of three separate determinations. For ROS levels, CGNs were treated with DE-71 (5 µM) for 1 h, and ROS were measured as described in Methods. Results are presented as percent of control and represent the means (± SD) of three separate determinations.
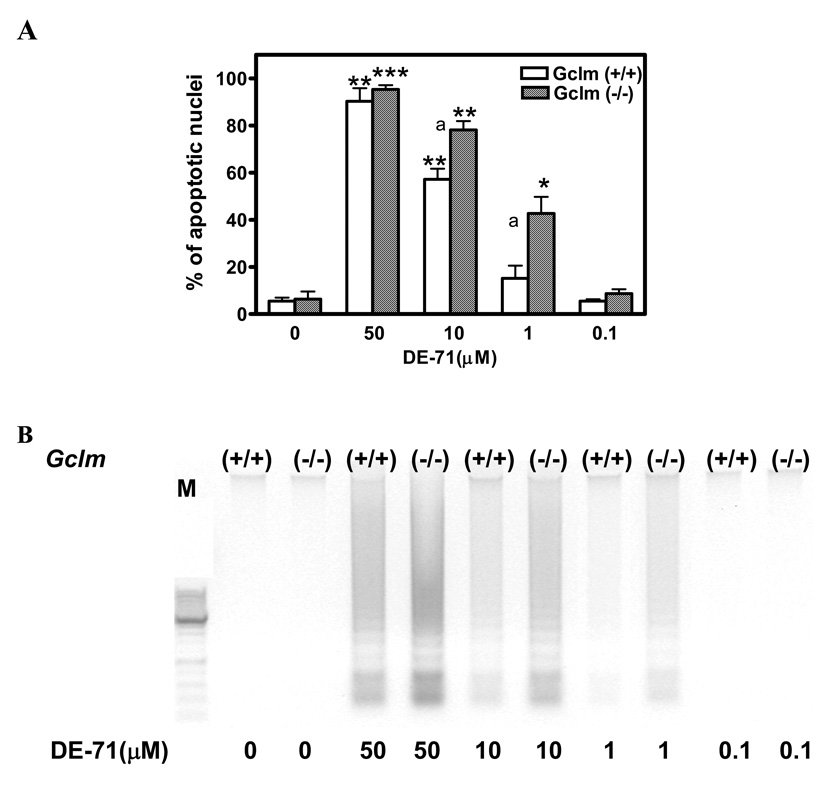
In contrast to the findings obtained with MTT, which provides an indication of mitochondrial integrity, DE-71 had no effect on Trypan blue exclusion at all concentrations tested (1–50 µM; not shown). This is in agreement with previous results obtained in astrocytoma cells with BDE-99, which showed a profound effect on MTT reduction, but no effects on Trypan blue exclusion or lactate dehydrogenase release (Madia et al. 2004). This finding suggests that DE-71 neurotoxicity in CGNs may be ascribed to an apoptotic process. In apoptotic cell death, cell shrink, assume a rounded shape, show plasma membrane blebs, display chromatin condensation, and expose phosphatidylserine on the outer leaf of the cell membrane, and are ultimately engulfed by phagocytes while their membranes are still intact (Hengartner, 2000). As shown in Fig. 3A, DE-71 caused a concentration-dependent increase in apoptotic cell death (measured by Hoechst staining) in mouse CGNs. Apoptotic cell death was more pronounced in cells from Gclm (−/−) mice, particularly at lower DE-71 concentrations. The pattern of cell death closely resembled that observed in the MTT assay; the IC50 values for apoptosis were 11.2 ± 1.5 µM and 2.0 ± 0.5 µM in CGNs from Gclm (+/+) and Gclm (−/−) mice, respectively (n = 3; p<0.05). Apoptotic cell death was confirmed by measuring DNA fragmentation, as shown in Fig. 3B.
Fig. 3. DE-71 causes apoptotic cell death of mouse CGNs.
A. Apoptosis induced by DE-71 in CGNs of Gclm (+/+) and (−/−) mice as determined with Hoechst 33342 (see Methods). Results represent the means (± SD) of at least three separate experiments. *Significantly different from untreated control, p<0.05; **p<0.01; ***p<0.001. aSignificantly different from Gclm (+/+), p<0.05. B. DE-71-induced apoptosis in CGNs of both mouse genotypes as assessed by DNA laddering.
The initial findings of a central role played by intracellular GSH in modulating the neurotoxicity of DE-71 in CGNs prompted us to investigate its toxicity in other cell types. Hippocampal and cerebral cortical neurons, as well as cerebellar, hippocampal and cortical astrocytes were prepared from Gclm (+/+), Gclm (+/−), and Gclm (−/−) mice. These cells displayed significant differences in their GSH content (Table 2). In all cases, cells from Gclm (−/−) mice had lower levels of GSH, as expected. Levels of GSH in cells from Gclm (+/−) mice did not differ from those in Gclm (+/+) animals, confirming previous observations (Yang et al. 2002: Giordano et al. 2006). In all brain areas, GSH levels in astrocytes were higher than those present in neurons, as previously reported for cerebellum (Giordano et al. 2006). While GSH levels in astrocytes from different brain regions were somewhat comparable, those in neurons varied considerably. For example, GSH levels in hippocampal neurons were only 5.5 and 1.4 nmol/mg protein in CGNs from Gclm (+/+) and (−/−) mice, respectively (Table 2).
Table 2.
GSH levels in neurons and astrocytes from different brain areas in Gclm (+/+), Gclm (+/−) and Gclm (−/−) mice
| Cell type | Brain region | Gclm (+/+) | Gclm (+/−) | Gclm (−/−) |
|---|---|---|---|---|
| Neurons | Cerebellum* | 12.4 ± 1.4 | 10.1 ± 1.2 | 2.4 ± 1.1 |
| Hippocampus | 5.5 ± 1.2 | 3.7 ± 0.9 | 1.4 ± 0.6 | |
| Cerebral cortex | 17.8 ± 1.6 | 15.5 ± 2.8 | 5.1 ± 1.0 | |
| Astrocytes | Cerebellum | 19.3 ± 1.6 | 15.6 ± 1.9 | 4.3 ± 1.0 |
| Hippocampus | 21.4 ±2.1 | 20.1 ± 1.7 | 8.3 ± 1.9 | |
| Cerebral cortex | 25.4 ± 3.5 | 23.1 ± 1.3 | 8.0 ± 1.9 | |
GSH was measured as described in Methods and is expressed as nmol/mg protein. Results are the mean (± SD) of three separate determinations. In all cell types, GSH levels in Gclm (−/−) mice are significantly different from Gclm (+/+) mice (p<0.01); GSH levels in Gclm (+/−) mice do not differ from Gclm (+/+) animals (p>0.05).
Cerebellar granule neurons.
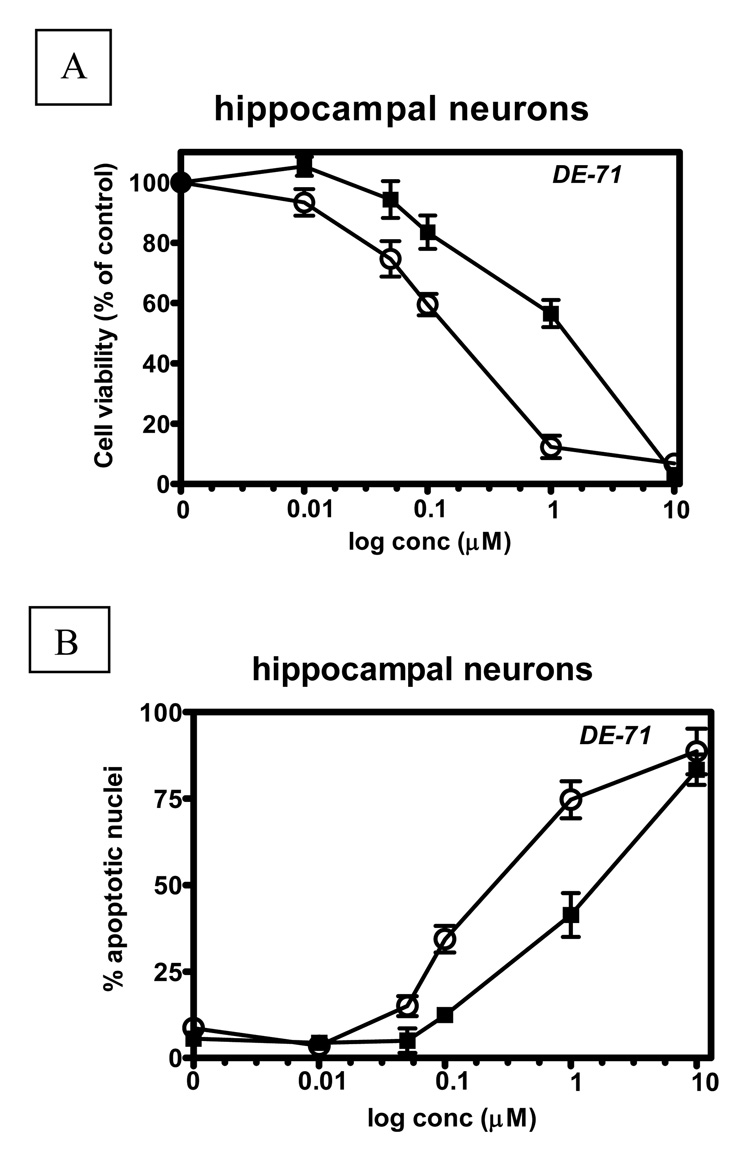
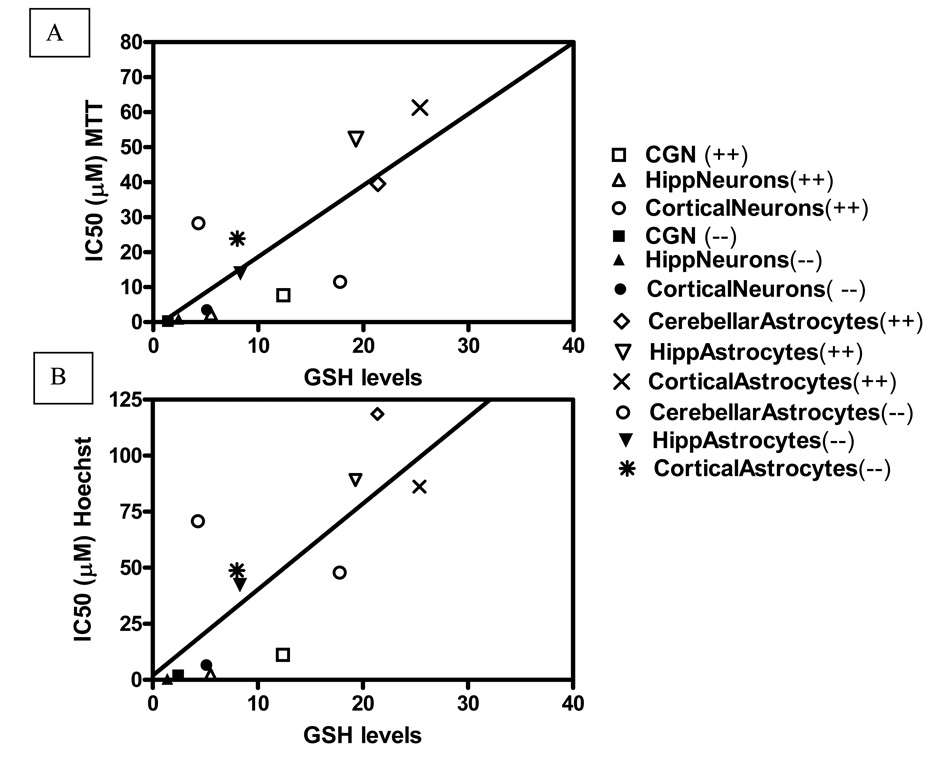
To ascertain whether intracellular GSH levels would influence susceptibility of cells to DE-71 toxicity, we carried out concentration-response curves in each cell type of each genotype, measuring MTT reduction or apoptosis as end-points. Table 3 and 4 show the results of these experiments. The toxicity of DE-71 was consistently higher in neurons than in astrocytes, and was higher in Gclm (−/−) mice than in Gclm (+/+) mice. Neurotoxicity was also significantly higher in hippocampal neurons compared to CGNs or cortical neurons. These observations hold true whether the endpoint was MTT reduction (Table 3) or apoptosis (Table 4). Fig. 4 shows typical concentration response curves for DE-71, in hippocampal neurons. For both end-points, the NOEL (no-observed-effect-level) was 0.05 µM and 0.01 µM for Gclm (+/+) and (−/−) mice, respectively. The LOEL (lowest-observed-effect-level) was 0.1 µM in cells from Gclm (+/+) mice, and 0.05 µM for Gclm (−/−) mice. Fig. 5A, B show the correlation between GSH levels for each cell type from both genotypes, and IC50 values, obtained in the MTT experiments (Fig. 5A) and in the apoptosis experiments (Fig. 5B). In both cases a significant correlation was found between GSH levels and toxicity.
Table 3.
Neurotoxicity of DE-71 in neurons and astrocytes from different brain regions in Gclm (+/+) and Gclm (−/−) mice
| Cell type | Brain region | Gclm (+/+) | Gclm (−/−) | |
|---|---|---|---|---|
| Neurons | Cerebellum* | 7.7 ± 2.3 | 0.9 ± 0.2 | |
| Hippocampus | 2.2 ± 1.7 | 0.3 ± 0.1 | ||
| Cerebral cortex | 11.5 ± 0.9 | 3.5 ± 1.5 | ||
| Astrocytes | Cerebellum | 39.6 ± 1.8 | 13.9 ± 1.2 | |
| Hippocampus | 52.3 ± 3.4 | 28.3 ± 3.4 | ||
| Cerebral cortex | 61.3 ± 4.8 | 23.9 ± 3.2 | ||
Neurotoxicity was assessed by the MTT assay, as describe in Methods. Results represent IC50 values (µM) derived from concentration-response curves utilizing 3–5 concentrations of DE-71, and are the means (± SD) of three separate determinations.
Cerebellar granule neurons.
Table 4.
Induction of apoptosis by DE-71 in neurons and astrocytes from different brain areas in Gclm (+/+) and Gclm (−/−) mice
| Cell type | Brain region | Gclm (+/+) | Gclm (−/−) | |
|---|---|---|---|---|
| Neurons | Cerebellum* | 11.2 ± 1.5 | 2.0 ± 0.5 | |
| Hippocampus | 2.3 ± 0.8 | 0.4 ± 0.2 | ||
| Cerebral cortex | 47.8 ± 1.8 | 6.6 ± 1.4 | ||
| Astrocytes | Cerebellum | 118.6 ± 3.5 | 70.8 ± 5.6 | |
| Hippocampus | 89.1 ± 3.8 | 42.2 ± 2.6 | ||
| Cerebral cortex | 86.2 ± 2.0 | 48.8 ± 2.8 | ||
Apoptosis was assessed by Hoechst staining, as described in Methods. Results represent IC50 values (µM) derived from concentration-response curves utilizing 3–5 concentrations of DE-71, and are the means (± SD) of three separate determinations.
Cerebellar granule neuron
Fig. 4. Concentration-dependent cytotoxicity of DE-71 in hippocampal neurons from Gclm (+/+) (filled squares) and Gclm (−/−) (open circles) mice.
Cytotoxicity was assessed, after 24 h incubation, by the MTT assay (A), and apoptosis was determined by Hoechst staining (B), Results are presented as percent of control for each genotype in A, and as percent of apoptotic nuclei in B. The LOEL (lowest-observed-effect-level) was 0.1 µM in Gclm (+/+) mice and 0.05 µM in Gclm (−/−) mice in both A and B.
Fig. 5. Correlation between intracellular GSH levels and DE-71 neurotoxicity.
A. Correlation between GSH levels in neurons and astrocytes from both mouse genotypes (Table 2) and neurotoxicity (IC50s) in the MTT assay (Table 3). r2 = 0.63; p = 0.0019. B. Correlation between GSH levels in neurons and astrocytes from both mouse genotypes (Table 2) and apoptosis (IC50s) in the Hoechst 33342 assay (Table 4). r2 = 0.59; p = 0.0035.
Discussion
The main finding of this study is that the in vitro neurotoxicity of the penta-BDE mixture DE-71 in neurons and astrocytes is modulated by intracellular GSH levels. As GSH is a major component of the cellular antioxidant defense mechanism, these results suggest that DE-71 toxicity is mediated by oxidative stress. This is supported by the findings that various antioxidants protect against DE-71 neurotoxicity, and that DE-71 causes an increase in ROS levels and in lipid peroxidation. Furthermore, our results also show that DE-71 toxicity in both neurons and astrocytes is primarily apoptotic in nature.
The ability of PBDEs to cause apoptotic cell death has been reported in various cell types. DE-71 caused apoptosis in rat cerebellar granule cells (Reistad et al. 2006), while BDE-99 induced apoptosis in human astrocytoma cells (Madia et al. 2004);BDE-47 was reported to cause apoptotic cell death in hippocampal neurons,human neuroblastoma cells, and human fetal liver hematopoietic cells (He et al. 2008a; 2008b; Shao et al. 2008); and BDE-209 caused apoptosis in human hepatoma cells Hep G2 (Hu et al. 2007). The mechanisms involved in PBDE-induced apoptosis have not been yet investigated, but are most likely related to oxidative stress and may involve the mitochondrial pathway. There is supporting evidence for the ability of PBDEs to induce oxidative stress. Reistad and Mariussen (2005) reported that DE-71 and BDE-47 increased the production of ROS in human neutrophil granulocytes; in contrast, DE-71 did not increase ROS levels in rat cerebellar granule cells, though its neurotoxicity was antagonized by an antioxidant (Reistad et al. 2006). BDE-47 was reported to cause oxidative stress in human neuroblastoma cells (He et al. 2008b), in hippocampal neurons (He et al. 2008a), in human fetal hematopoietic cells (Shao et al. 2008), while BDE-209 was shown to increase oxidative stress in human hepatoma cells HepG2 (Hu et al. 2007). An increase in lipid peroxidation and in the levels of oxidized glutathione were also found in the liver of American kestrels (Falco sparverius) treated in ovo with a mixture of BDE-47, -99, -100, and -153 (Fernie et al. 2005). The exact mechanisms involved in PBDE-induced oxidative stress and mitochondrial dysfunction, leading to apoptotic cell death, have not been elucidated. A number of in vitro studies have examined the effects of PBDEs on end-points other than oxidative stress or apoptosis. For example, PBDEs have been shown to increase the translocation of protein kinase C (Madia et al. 2004; Kodavanti et al. 2005; Kodavanti and Ward, 2005), to inhibit vesicular dopamine uptake (Mariussen and Fonnum, 2003), and to alter cytosolic and mitochondrial calcium homeostasis (Kodavanti and Ward, 2005; Coburn et al. 2008; Dingemans et al. 2008). This latter effect may be of particular relevance, given the importance of calcium in triggering mitochondrial dysfunction and oxidative stress (cfr. e.g. Giordano et al. 2006).
Our results indicate that intracellular GSH content plays an important role in determining susceptibility of neurons and astrocytes to DE-71-induced cytotoxicity and apoptotic cell death. Indeed, cells from Gclm (−/−) mice had significantly lower levels of GSH than their wild-type counterparts (Table 2), and were consistently more susceptible to DE-71 toxicity (Table 3 and Table 4). There was also a high, significant correlation between GSH levels and DE-71 toxicity, when all neurons and astrocytes from both mouse genotypes were compared (Fig. 5). However, it should be noted that other factors may also play a role in the differential susceptibility of each cell type. For example, cerebellar astrocytes from Gclm (−/−) mice had lower GSH levels than cortical neurons from Gclm (+/+) mice (4.3 vs 17.8 nmol/mg protein), yet cytotoxicity of DE-71 was similar in the two cell types (IC50 in the MTT assay = 13.9 and 11.5, respectively).
The toxic concentrations of DE-71 in this study were in the micromolar range, with some notable exception (see below). Similar concentrations had been found to exert toxicological effects in other studies (e.g. Mariussen and Fonnum, 2003; Kodavanti and Ward, 2005; Reistad et al. 2006). The particular sensitivity of hippocampal neurons to DE-71 toxicity is of some interest. These cells displayed the lowest levels of GSH of any other cell type examined, and the highest sensitivity to DE-71 toxicity. In particular, in hippocampal neurons from Gclm (−/−) mice, the IC50 for DE-71 was 300–400 nM, with a LOEL of 50 nM (Fig. 4). Interestingly, Kodavanti and Ward (2005) also reported that mitochondrial calcium uptake was most affected by DE-71 in the hippocampus than in other brain regions.
The high levels of PBDEs found in human milk and house dust, particularly in North America, and the high body burden of infants and neonates, have raised concerns on possible developmental effects of these flame retardants (Eriksson et al. 2001; Birnbaum and Staskal, 2004; McDonald, 2005; Costa and Giordano, 2007). Animal studies have indicated that pre- or post-natal exposures to several PBDEs can cause developmental neurotoxicity, particularly in the domains of locomotor activity and of cognitive behavior (e.g. Eriksson et al. 2001; Branchi et al. 2002; 2003; Viberg et al. 2003; 2006; Dufault et al. 2005; Rice et al. 2007; Gee and Moser, 2008; additional references in Costa and Giordano, 2007). Of note is that behavioral effects were also seen following a single exposure to very low doses of PBDEs. For example, Kuriyama et al. (2005) reported long term behavioral effects in mice following a single prenatal exposure on gestational day 6 to 0.06 mg/kg of BDE-99. While the reported effects of PBDEs on thyroid hormones levels (Zhou et al. 2002; Skarman et al. 2005; Rice et al. 2007) may contribute to their developmental neurotoxicity, it should be noted that exposures that did not cause any alterations in plasma T3 or T4 levels also resulted in neurodevelopmental abnormalities (Gee and Moser, 2008; Gee et al. 2008). These findings would support the notion that direct effect of PBDEs on the developing nervous system may also play a role in their neurotoxicity.
The reported cognitive and motor effects may suggest an involvement of the hippocampus and the cerebellum in PBDEs’ developmental neurotoxicity. Alterations in the cerebellar glutamate-nitric oxide-cGMP pathway have been reported following developmental exposure to BDE-99 (Llansola et al. 2007), and changes in selected proteins, long-term potentiation, and nicotinic receptors in the hippocampus were found upon developmental exposure to various PBDEs (Dingemans et al. 2007; Viberg et al. 2004; Alm et al. 2006). In preliminary experiments, we have also found that in vivo exposure of mice to BDE-47 (10 mg/kg, per os, on PND 10), causes an increase of oxidative stress and activation of caspase-3 in cerebellum and hippocampus (Giordano et al., unpublished results). The developing brain is particularly sensitive to oxidative stress, possibly due to its richness in free iron and a limited antioxidant capacity (Sola et al. 2007). The relevant role of oxidative stress in contributing to pathological apoptosis in the developing brain has also been recently underscored (Blomgren et al. 2007). An involvement of oxidative stress in developmental neurotoxicity has been reported for other compounds, such as methylmercury (Stringari et al. 2008), chlorpyrifos (Crumpton et al. 2000), and ethanol (Maffi et al. 2008). The latter study is of particular interest, as ethanol-induced oxidative stress and apoptosis in the developing brain were found to be dependent upon the GSH content of selected cell populations (Maffi et al. 2008).
It is important to relate the concentrations of DE-71 utilized in the present study with levels of PBDEs found in human populations. As said, infants and toddlers in North America have the highest body burden, with blood levels as high as 400–600 ng/g lipid (Mazdai et al. 2003; Fischer et al. 2006). In a study in rats, behavioral effects were seen with BDE-99 at plasma levels of 475 ng/g lipid (Lichtensteiger et al. 2004; Lilientahl et al. 2006; Ceccatelli et al. 2006). The highest concentrations found in humans are about 6–12 nM, i.e. two to three orders of magnitude lower than the IC50 values in the present study. However, in some cases, the toxic concentration of DE-71 was much lower; for example, the LOEL of DE-71 in hippocampal neurons from Gclm (−/−) mice was 50 nM. This may be of relevance, as individuals with genetic predisposition leading to low GSH levels may display a higher susceptibility to PBDEs developmental neurotoxicity. Several polymorphisms in glutamate-cysteine ligase have been described (Dalton et al. 2004), including some in the Gclm gene (Nakamura et al. 2002), that cause very low levels of GSH. In this respect, the Gclm (−/−) mouse utilized in this study may represent an useful animal model for such genetic alterations, amenable to further in vitro as well as in vivo studies.
Acknowledgments
This study was supported in part by grant P30ES07933 from the National Institute for Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlemeyer B, Baumgart-Vogt E. Optimized protocols for the simultaneous preparation of primary cultures of the neocortex, hippocampus and cerebellum from individual newborn (P0.5) C57B1/6J mice. J. Neurosci. Methods. 2005;149:110–120. doi: 10.1016/j.jneumeth.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Alm H, Scholz B, Fischer C, Kultima K, Viberg H, Eriksson P, Dencker L, Stigson M. Proteomic evaluation of neonatal exposure to 2,2’,4,4’5-pentabromodiphenyl ether. Environ. Health Perspect. 2006;114:254–259. doi: 10.1289/ehp.8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antignac JP, Maume D, Marchand P, Monteau F, Zalko D, Beerebi A, Cravedi JP, Andre F, Le Bizec B, Cariou R. Exposure assessment of fetus and newborn to brominated flame retardants in France: preliminary data. Organohalogen Compounds. 2006;68:790–793. doi: 10.1002/mnfr.200700077. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environ. Health Perspect. 2004;112:9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomgren K, Leist M, Groc L. Pathological apoptosis in the developing brain. Apoptosis. 2007;12:993–1010. doi: 10.1007/s10495-007-0754-4. [DOI] [PubMed] [Google Scholar]
- Branchi I, Alleva E, Costa LG. Effects of perinatal exposure to a polybrominated diphenyl ether (PBDE 99) on mouse neurobehavioral development. Neurotoxicology. 2002;23:375–384. doi: 10.1016/s0161-813x(02)00078-5. [DOI] [PubMed] [Google Scholar]
- Branchi I, Capone F, Alleva E, Costa LG. Polybrominated diphenyl ethers: neurobehavioral effects following developmental exposure. Neurotoxicology. 2003;24:449–462. doi: 10.1016/S0161-813X(03)00020-2. [DOI] [PubMed] [Google Scholar]
- Ceccatelli R, Faass O, Schlumpf M, Lichtensteiger W. Gene expression and estrogen sensitivity in rat uterus after developmental exposure to the polybrominated diphenyl ether PBDE 99 and PCB. Toxicology. 2006;20:104–116. doi: 10.1016/j.tox.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Chen Y, Shertzer HG, Schneider SN, Nebert DW, Dalton TP. Glutamate cysteine ligase catalysis: dependence on ATP and modifier subunit for regulation of tissue glutathione levels. J. Biol. Chem. 2005;280:33766–33774. doi: 10.1074/jbc.M504604200. [DOI] [PubMed] [Google Scholar]
- Coburn CG, Curras-Collazo MC, Kodavanti PR. Polybrominated diphenyl ethers and ortho-substituted polychlorinated biphenyls as neuroendocrine disruptors of vasopressin release:effects during physiological activation in vitro and structure-activity relationships. Toxicol. Sci. 2007;98:178–186. doi: 10.1093/toxsci/kfm086. [DOI] [PubMed] [Google Scholar]
- Coburn CG, Curraz-collazo MC, Kodavanti PRS. In vitro effects of environmentally relevant polybrominated diphenyl ether (PBDE) congeners on calcium buffering mechanisms in rat brain. Neurochem. Res. 2008;3:355–364. doi: 10.1007/s11064-007-9430-x. [DOI] [PubMed] [Google Scholar]
- Costa LG, Giordano G. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology. 2007;28:1047–1067. doi: 10.1016/j.neuro.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpton TL, Seidler FJ, Slotkin TA. Is oxidative stress involved in the developmental neurotoxicity of chlorpyrifos? Dev. Brain Res. 2000;121:189–195. doi: 10.1016/s0165-3806(00)00045-6. [DOI] [PubMed] [Google Scholar]
- Dalton TP, Chen Y, Schneider SN, Nebert DW, Shertzer HG. Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Rad. Biol. Med. 2004;37:1511–1526. doi: 10.1016/j.freeradbiomed.2004.06.040. [DOI] [PubMed] [Google Scholar]
- Darnerud PO, Eriksen GS, Johannesson T, Larsen PB, Viluksela M. Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ. Health Perspect. 2001;109 Suppl. 1:49–68. doi: 10.1289/ehp.01109s149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- Dingemans MML, Ramakers GMJ, Gardoni F, van Kleef GDM, Bergman A, Di Luca M, van der Berg M, Westerink RHS, Vijverberg HPM. Neonatal exposure to brominated flame retardant BDE-47 reduces long-term potentiation and postsynaptic protein levels in mouse hippocampus. Environ. Health. Perspect. 2007;115:865–870. doi: 10.1289/ehp.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans MML, de Groot A, van Kleef RGDM, Bergman A, van der Berg M, Vijverberg HPM, Westerink RHS. Hydroxylation increases the neurotoxic potential of BDE-47 to affect exocytosis and calcium homeostasis in PC12 cells. Environ. Health Perspect. 2008;116:637–643. doi: 10.1289/ehp.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R. Metabolism and function of glutathione in brain. Progr. Neurobiol. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- Dufault C, Poles G, Driscoll LL. Brief postnatal PBDE exposure alters learning and the cholinergic modulation of attention in rats. Toxicol. Sci. 2005;88:172–180. doi: 10.1093/toxsci/kfi285. [DOI] [PubMed] [Google Scholar]
- Eriksson P, Jakobson E, Fredriksson A. Brominated flame retardants: a novel class of developmental neurotoxicants in our environment? Environ. Health Perspect. 2001;109:903–908. doi: 10.1289/ehp.01109903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie KJ, Shutt JL, Mayne G, Hoffman D, Letcher RJ, Drouilard KG, Ritchie IJ. Exposure to polybrominated deiphenyl ethers (PBDEs): changes in thyroid, vitamin A, glutathione homeostasis, and oxidative stress in American kestrels (Falco sparverius) Toxicol. Sci. 2005;88:375–383. doi: 10.1093/toxsci/kfi295. [DOI] [PubMed] [Google Scholar]
- Fischer D, Hooper K, Athanasiadou M, Athanassiadis I, Bergman A. Children show highest levels of polybrominated diphenyl ethers in a California family of four: a case study. Environ. Health Perspect. 2006;114:1581–1584. doi: 10.1289/ehp.8554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst P. Dioxins, polychlorinated biphenyls and other organohalogen compounds in human milk. Mol. Nutr. Food Res. 2006;50:922–933. doi: 10.1002/mnfr.200600008. [DOI] [PubMed] [Google Scholar]
- Gee JR, Moser VC. Acute postnatal exposure to brominated diphenylether 47 delays neuromotor ontogeny and alters motor activity in mice. Neurotoxicol. Teratol. 2008;30:79–87. doi: 10.1016/j.ntt.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Gee JR, Hedge JM, Moser VC. Lack of alterations in thyroid hormones following exposure to polybrominated diphenylether 47 during a period of rapid brain development in mice. Drug Chem. Toxicol. 2008;31:245–254. doi: 10.1080/01480540701873194. [DOI] [PubMed] [Google Scholar]
- Giordano G, White CC, McConnachie LA, Fernandez C, Kavanagh TJ, Costa LG. Neurotoxicity of domoic acid in cerebellar granule neurons in a genetic model of glutathione deficiency. Mol. Pharmacol. 2006;70:2116–2126. doi: 10.1124/mol.106.027748. [DOI] [PubMed] [Google Scholar]
- Giordano G, Afsharinejad Z, Guizzetti M, Vitalone A, Kavanagh TJ, Costa LG. Organophosphorus insecticides chlorpyrifos and diazinon and oxidative stress in neuronal cells in a genetic model of glutathione deficiency. Toxicol. Appl. Pharmacol. 2007a;219:181–189. doi: 10.1016/j.taap.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Giordano G, White CC, Mohar I, Kavanagh TJ, Costa LG. Glutathione levels modulate domoic acid-induced apoptosis in mouse cerebellar granule cells. Toxicol. Sci. 2007b;100:433–444. doi: 10.1093/toxsci/kfm236. [DOI] [PubMed] [Google Scholar]
- Griffith OW, Mulcahy RT. The enzymes of glutathione synthesis: gamma-glutamylcysteine synthetase. Adv. Enzymol. Relat. Areas Mol. Biol. 1999;73:209–267. doi: 10.1002/9780470123195.ch7. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa P, Peters J, Costa LG. Acetylcholine as a mitogen: muscarinic receptor-mediated proliferation of rat astrocytes and human astrocytoma cells. Eur. J. Pharmacol. 1996;297:265–273. doi: 10.1016/0014-2999(95)00746-6. [DOI] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O’Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. New Engl. J. Med. 1999;341:549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- Hale RC, Alaee M, Manchester-Neesvig JB, Stapleton HM, Ikonomou MG. Polybrominated diphenyl ether flame retardants in the North American environment. Environ. Int. 2003;29:771–779. doi: 10.1016/S0160-4120(03)00113-2. [DOI] [PubMed] [Google Scholar]
- Hanari N, Kannan K, Miyake Y, Okazawa T, Kodavanti PRS, Aldous KM, Yamashita N. Occurrence of polybrominated biphenyls, polybrominated dibenzo-p-dioxins, and polybrominated dibenzofurans as impurities in commercial polybrominated diphenl ether mixtures. Environ. Sci. Technol. 2006;40:4400–4405. doi: 10.1021/es060559k. [DOI] [PubMed] [Google Scholar]
- He P, He W, Wang A, Xia T, Xu B, Zhang M, Chen X. PBDE-47-induced oxidative stress, DNA damage and apoptosis in primary cultured hippocampal neurons. Neurotoxicology. 2008a;29:124–129. doi: 10.1016/j.neuro.2007.10.002. [DOI] [PubMed] [Google Scholar]
- He W, He P, Wang A, Xia T, Xu B, Chen X. Effects of BDE-47 on cytotoxicity and genotoxicity in human neuroblastoma cells in vitro. Mutat. Res. 2008b;649:62–70. doi: 10.1016/j.mrgentox.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Hentgartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Hu XZ, Xu Y, Hu DC, Hui Y, Yang FX. Apoptosis induction on human hepatoma cells Hep G2 of decabrominated diphenyl ether (PBDE-209) Toxicol. Lett. 2007;171:19–28. doi: 10.1016/j.toxlet.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Jones-Otazo HA, Clarke JP, Diamond ML, Archbold JA, Ferguson G, Harner T, Richardson GM, Ryan JJ, Wilford B. Is house dust the missing exposure pathway for PBDEs? An analysis of the urban fate and human exposure to PBDEs. Environ. Sci. Technol. 2005;39:5121–5130. doi: 10.1021/es048267b. [DOI] [PubMed] [Google Scholar]
- Kodavanti PRS, Ward TR. Differential effects of commercial polybrominated diphenyl ethers and polychlorinated biphenyl mixtures on intracellular signaling in rat brain in vitro. Toxicol. Sci. 2005;85:952–962. doi: 10.1093/toxsci/kfi147. [DOI] [PubMed] [Google Scholar]
- Kodavanti PRS, Ward TR, Ludewig G, Robertson LW, Birnbaum LS. Polybrominated diphenyl ether (PBDE) effects in rat neuronal cultures: 14C-PBDE accumulation, biological effects, and structure-activity relationships. Toxicol. Sci. 2005;88:181–192. doi: 10.1093/toxsci/kfi289. [DOI] [PubMed] [Google Scholar]
- Kuriyama SN, Talsness CE, Grote K, Chahoud I. Developmental exposure to low-dose PBDE-99: effects on male fertility and neurobehavior in rat offspring. Environ. Health Perspect. 2005;113:149–154. doi: 10.1289/ehp.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law RJ, Allchin CR, deBoer J, Covaci A, Herzke D, Lepom P, Morris S, Tronczynski J, de Wit CA. Levels and trends of brominated flame retardants in the European environment. Chemosphere. 2006;64:187–208. doi: 10.1016/j.chemosphere.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Lichtensteiger W, Faass O, Ceccatelli R, Schlumpf M. Developmental exposure to PBDE 99 and PCB affects estrogen sensitivity of target genes in rat brain regions and female sexual behavior. Organohalog. Compd. 2004;66:3965–3970. [Google Scholar]
- Lilienthal H, Hack A, Roth-Harer A, Wichert Grande S, Talsness CE. Effects of developmental exposure to 2,2’,4,4’,5-pentabromodiphenyl ether (PBDE99) on sex steroids, sexual development, and sexually dimorphic behavior in rats. Environ. Health Perspect. 2006;114:194–201. doi: 10.1289/ehp.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind Y, Darnerud PO, Atuma S, Aune M, Becker W, Bjerselius R, Cnattingius S, Glynn A. Polybrominated diphenyl ethers in breast milk from Uppsala County, Sweden. Environ. Res. 2003;93:186–194. doi: 10.1016/s0013-9351(03)00049-5. [DOI] [PubMed] [Google Scholar]
- Llansola M, Erceg S, Monfort P, Montoliu C, Felipo V. Prenatal exposure to polybrominated diphenyl ether 99 enhances the function of the glutamate-nitric oxide-cGMP pathway in brain in vivo and in cultured neurones. Eur. J. Neurosi. 2007;25:373–379. doi: 10.1111/j.1460-9568.2006.05289.x. [DOI] [PubMed] [Google Scholar]
- Madia F, Giordano G, Fattori V, Vitalone A, Branchi I, Capone F, Costa LG. Differential in vitro neurotoxicity of the flame retardant PBDE-99 and of the PCB Aroclor 1254 in human astrocytoma cells. Toxicol. Lett. 2004;154:11–21. doi: 10.1016/j.toxlet.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Maffi SR, Rathinam ML, Cherian PP, Pate W, Hamby-Mason R, Schenker S, Henderson GI. Glutathione content as a potential mediator of the vulnerability of cultured fetal cortical neurons to ethanol-induced apoptosis. J. Neurosci. Res. 2008;86:1064–1076. doi: 10.1002/jnr.21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariussen E, Fonnum F. The effect of brominated flame retardants on neurotransmitter uptake into rat brain synaptosomes and vesicles. Neurochem. Internat. 2003;43:533–542. doi: 10.1016/s0197-0186(03)00044-5. [DOI] [PubMed] [Google Scholar]
- Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ. Health Perspect. 2003;111:1249–1252. doi: 10.1289/ehp.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnachie LA, Mohar I, Hudson FN, Ware CB, Ladiges WC, Fernandez C, Chatterton-Kirchmeier S, White CC, Pierce RH, Kavanagh TJ. Glutamate cysteine ligase modifier subunit deficiency and gender as determinants of acetominophen-induced hepatotoxicity in mice. Toxicol. Sci. 2007;99:628–636. doi: 10.1093/toxsci/kfm165. [DOI] [PubMed] [Google Scholar]
- McDonald TA. Polybrominated diphenylether levels among United States residents: daily intake and risk of harm to the developing brain and reproductive organs. Integr. Environ. Assess. Manag. 2005;1:343–354. [PubMed] [Google Scholar]
- Nakamura S, Kugiyama K, Sugiyama S, Miyamoto S, Koide S, Fukushima H, Honda O, Yoshimura M, Ogawa H. Polymorphism in the 5’-flanking region of human glutamate-cysteine ligase modifier subunit gene is associated with myocardial infarction. Circulation. 2002;105:2968–2973. doi: 10.1161/01.cir.0000019739.66514.1e. [DOI] [PubMed] [Google Scholar]
- Reistad T, Mariussen E. A commercial mixture of the brominated flame retardant pentabrominated diphenyl ether (DE-71) induces respiratory burst in human neutrophil granulocytes in vitro. Toxicol. Sci. 2005;87:57–65. doi: 10.1093/toxsci/kfi222. [DOI] [PubMed] [Google Scholar]
- Reistad T, Fonnum F, Mariussen E. Neurotoxicity of the pentabrominated diphenyl ether mixture, DE-71, and hexabromocyclododecane (HBCD) in rat cerebellar granule cells in vitro. Arch. Toxicol. 2006;80:785–796. doi: 10.1007/s00204-006-0099-8. [DOI] [PubMed] [Google Scholar]
- Rice DC, Reeve EA, Herlihy A, Zoeller RT, Thompson WD, Markowski VP. Developmental delays and locomotor activity in the C57BL6/J mouse following neonatal exposure to the fully brominated PBDE, decabromodiphenyl ether. Neurotoxicol. Teratol. 2007;29:511–520. doi: 10.1016/j.ntt.2007.03.061. [DOI] [PubMed] [Google Scholar]
- Sanders JM, Burka LT, Smith CS, Black W, James R, Cunningham ML. Differential expression of CYP1A, 2B, and 3A genes in the F344 rat following exposure to a polybrominated diphenyl ether mixture or individual components. Toxicol. Sci. 2005;88:127–133. doi: 10.1093/toxsci/kfi288. [DOI] [PubMed] [Google Scholar]
- Schecter A, Papke O, Tung KC, Joseph J, Harris TR, Dahlgreen J. Polybrominated diphenyl ether flame retardants in the U.S. population: current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. J. Occup. Environ. Med. 2005;47:199–211. doi: 10.1097/01.jom.0000158704.27536.d2. [DOI] [PubMed] [Google Scholar]
- Schecter A, Papke O, Harris TR, Tung KC, Musumba A, Olson J, Birnbaum L. Polybrominated diphenyl ether (PBDE) levels in expanded market basket survey of U.S. food and estimated PBDE dietary intake by age and sex. Environ. Health Perspect. 2006;114:1515–1520. doi: 10.1289/ehp.9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Harris TR, Shah N, Musumba A, Papke O. Brominated flame retardants in US foods. Mol. Nutr. Food Res. 2008;52:266–272. doi: 10.1002/mnfr.200700166. [DOI] [PubMed] [Google Scholar]
- Shao J, White CC, Dabrowski MJ, Kavanagh TJ, Eckert ML, Gallagher EP. The role of mitochondrial and oxidative injury in BDE 47 toxicity in human fetal liver hematopoietic stem cells. Toxicol. Sci. 2008;101:81–90. doi: 10.1093/toxsci/kfm256. [DOI] [PubMed] [Google Scholar]
- Sjodin A, Hagmar L, Klasson-Wehler E, Kronholm-Diab K, Jakobsson E, Bergman A. Flame retardant exposure: polybrominated diphenyl ethers in blood from Swedish workers. Environ. Health Perspect. 1999;107:643–648. doi: 10.1289/ehp.107-1566483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin A, Jones RS, Focant JF, Lapeza C, Wang RY, McGahee EE, Zhang Y, Turner WE, Slazyk B, Needham LL, Patterson DG. Retrospective time-trend study of polybrominated diphenyl ether and polybrominated and polychlorinated biphenyl levels in human serum from the United States. Environ. Health Perspect. 2004;112:654–658. doi: 10.1289/ehp.112-1241957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarman E, Darnerud PO, Ohrvik H, Oskarsson A. Reduced thyroxine levels in mice perinatally exposed to polybrominated diphenyl ethers. Environ. Toxicol. Pharmacol. 2005;19:273–281. doi: 10.1016/j.etap.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Sola A, Rogido MR, Deulofeut R. Oxygen as a neonatal health hazard: call for detente in clinical practice. Acta Paediatrica. 2007;96:801–812. doi: 10.1111/j.1651-2227.2007.00287.x. [DOI] [PubMed] [Google Scholar]
- Stringari J, Nunes AK, Franco JL, Bohrer D, Garcia SC, Dafre AL, Milatovic D, Souza DO, Rocha JB, Aschner M, Farina M. Prenatal methylmercury exposure hampers glutathione antioxidant system ontogenesis and causes long-lasting oxidative stress in the mouse brain. Toxicol. Appl. Pharmacol. 2008;227:147–154. doi: 10.1016/j.taap.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen C, Lundanes E, Becher G. Brominated flame retardants in archived serum samples from Norway: a study on temporal trends and the role of age. Environ. Sci. Technol. 2002;36:1414–1418. doi: 10.1021/es0102282. [DOI] [PubMed] [Google Scholar]
- Tu Z, Anders MW. Expression and characterization of human glutamate-cysteine ligase. Arch. Biochem. Biophys. 1998;354:247–254. doi: 10.1006/abbi.1998.0676. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behavior, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice. Toxicol. Appl. Pharmacol. 2003;192:95–106. doi: 10.1016/s0041-008x(03)00217-5. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to the brominated flame retardant 2,2’,4,4’,5-pentabromodiphenyl ether decreases cholinergic nicotinic receptors in hippocampus and affects spontaneous behavior in the adult mouse. Environ. Toxicol. Pharmacol. 2004;17:61–65. doi: 10.1016/j.etap.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Viberg H, Johansson N, Fredriksson A, Eriksson J, Marsh G, Eriksson P. Neonatal exposure to higher polybromintaed diphenyl ethers, hepta-, octa-, or nonabromodiphenyl ether, impairs spontaneous behavior and learning and memory functions in adult mice. Toxicol. Sci. 2006;92:211–218. doi: 10.1093/toxsci/kfj196. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jiang G, Lam PKS, Li A. Polybrominated diphenyl ether in the East Asian environment: a critical review. Environ. Int. 2007;33:963–973. doi: 10.1016/j.envint.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Wilford BH, Shoeib M, Harner T, Zhu J, Jones KC. Polybrominated diphenyl ethers in indoor dust in Ottawa, Canada: implications for sources and exposure. Environ. Sci. Technol. 2005;39:7027–7035. doi: 10.1021/es050759g. [DOI] [PubMed] [Google Scholar]
- Yang Y, Dieter MZ, Che Y, Shetzer HG, Nebert DW, Dalton TP. Initial characterization of the glutamate-cysteine ligase modifier subunit Gclm (−/−) knockout mouse. Novel model system for compromised stress response. J. Biol. Chem. 2002;277:49446–49452. doi: 10.1074/jbc.M209372200. [DOI] [PubMed] [Google Scholar]
- Zhou T, Taylor MM, DeVito MJ, Crofton KM. Developmental exposure to polybrominated diphenyl ethers results in thyroid hormone disruption. Toxicol. Sci. 2002;66:105–116. doi: 10.1093/toxsci/66.1.105. [DOI] [PubMed] [Google Scholar]
- Zuurbier M, Leijs M, Schoeteres G, Tusscher GT, Koppe JG. Children’s exposure to polybrominated dephenyl ethers. Acta Pediatrica. 2006;95 Suppl. 453:65–70. doi: 10.1080/08035320600886299. 30. [DOI] [PubMed] [Google Scholar]