Abstract
Canine degenerative myelopathy (DM) is a fatal neurodegenerative disease prevalent in several dog breeds. Typically, the initial progressive upper motor neuron spastic and general proprioceptive ataxia in the pelvic limbs occurs at 8 years of age or older. If euthanasia is delayed, the clinical signs will ascend, causing flaccid tetraparesis and other lower motor neuron signs. DNA samples from 38 DM-affected Pembroke Welsh corgi cases and 17 related clinically normal controls were used for genome-wide association mapping, which produced the strongest associations with markers on CFA31 in a region containing the canine SOD1 gene. SOD1 was considered a regional candidate gene because mutations in human SOD1 can cause amyotrophic lateral sclerosis (ALS), an adult-onset fatal paralytic neurodegenerative disease with both upper and lower motor neuron involvement. The resequencing of SOD1 in normal and affected dogs revealed a G to A transition, resulting in an E40K missense mutation. Homozygosity for the A allele was associated with DM in 5 dog breeds: Pembroke Welsh corgi, Boxer, Rhodesian ridgeback, German Shepherd dog, and Chesapeake Bay retriever. Microscopic examination of spinal cords from affected dogs revealed myelin and axon loss affecting the lateral white matter and neuronal cytoplasmic inclusions that bind anti-superoxide dismutase 1 antibodies. These inclusions are similar to those seen in spinal cord sections from ALS patients with SOD1 mutations. Our findings identify canine DM to be the first recognized spontaneously occurring animal model for ALS.
Amyotrophic lateral sclerosis (ALS) refers to a heterogeneous group of adult onset human diseases, in which progressive neurodegeneration affecting both the upper and lower motor neuron systems causes advancing weakness and muscle atrophy, and culminates in paralysis and death. Approximately 5 to 10% of ALS cases are familial; the rest appear to be sporadic (1–3). Mutations in SOD1 account for ≈20% of the familial ALS cases and 1 to 5% of the cases of sporadic ALS (1–4); >120 different SOD1 mutations have been identified in ALS patients (http://alsod.iop.kcl.ac.uk/Als/index.aspx). Elucidation of mechanisms underlying ALS has been hampered by a paucity of biological material from affected individuals in early stages of the disease (5). To our knowledge, there are no previous reports of spontaneously occurring animal models of ALS. Thus, ALS research has relied heavily on transgenic rodents expressing mutant human SOD1 (hSOD1m) to produce a motor neuron disease, which recapitulates many features of ALS (5–7). In contrast, nullizygous SOD1 knockout mice develop normally (8), suggesting that the neurodegeneration in hSOD1m mice and in ALS patients results from a toxic gain of function (1, 5–8). Although the nature of the toxin is unclear, several experiments suggest that the neurodegeneration occurs because conformational changes in the mutant superoxide dismutase 1 protein (SOD1) alter the biological activity and/or promote the formation of intracellular SOD1 aggregates (1, 4, 9, 10).
Canine degenerative myelopathy (DM) has been recognized for >35 years as a spontaneously occurring, adult-onset spinal cord disorder of dogs (11). When pelvic limb hyporeflexia and nerve root involvement were observed, the disease was termed chronic degenerative radiculomyelopathy (12). Initially thought to be specific to German Shepherds, it has also been called German Shepherd dog myelopathy (13). Since these early reports, DM has been diagnosed in several other breeds. The disease is common in certain breeds including the Pembroke Welsh corgi, Boxer, Rhodesian ridgeback, and Chesapeake Bay retriever (14).
With DM, there is no sex predilection. Most dogs are at least 8 years old before the onset of clinical signs (11–18). The initial clinical sign is a spastic and general proprioceptive ataxia in the pelvic limbs. At this stage of the disease, the presence of spinal reflexes indicates an upper motor neuron paresis (11). The asymmetric weakness frequently reported at disease onset progresses to paraplegia (11, 12, 14, 16, 18). Hyporeflexia of the myotatic and withdrawal reflexes occur in the latter disease stage (11, 12, 14, 16, 18). The disease duration can exceed 3 years; however, dog owners usually elect euthanasia within a year of diagnosis when their dogs become paraplegic. If the disease is allowed to progress, clinical signs will ascend to affect the thoracic limbs (11, 14, 16). Because various common acquired compressive spinal cord diseases can mimic DM by compromising the upper motor neuron and general proprioceptive pathways, a definitive diagnosis of DM can only be accomplished postmortem by the histopathologic observation of axonal and myelin degeneration, which can occur at all levels of the spinal cord (16–18) and in all spinal cord funiculi, but are consistently most severe in the dorsal portion of the lateral funiculus within the middle to caudal thoracic region (11, 13–18).
Results
Mapping the DM Locus and Identification of a SOD1 Missense Mutation.
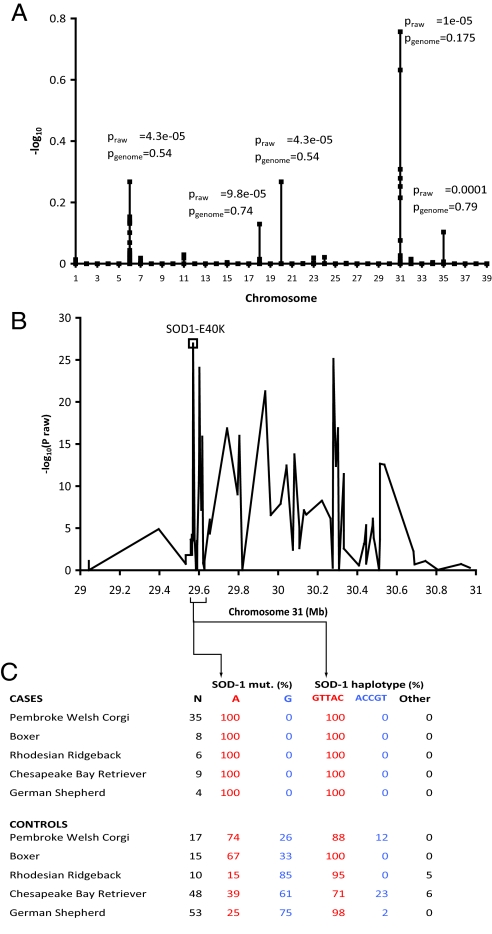
Genome-wide association (GWA) mapping of DM was performed with 38 cases and 17 controls older than 6 years of age (mean age = 9.4 years) from the Pembroke Welsh corgi breed by using the Affymetrix Canine Genome 2.0 Array. The strongest association was detected on CFA31 (praw = 1 × 10−5; pgenome = 0.18), with weaker signals on 4 other chromosomes, suggesting modifiers or population substructure (Fig. 1A). Within the associated CFA31 region, all affected dogs were homozygous for a common haplotype from 28.91 to 29.67 Mb (CanFam2.0), which contains 3 genes: SOD1, TIAM1, and SFRS15. Clinical similarities between DM and ALS made SOD1 a viable candidate gene. Resequencing SOD1 from normal and DM-affected dogs revealed a G to A transition in exon 2 that predicts an E40K missense mutation. The 55 corgi DNA samples were genotyped for the SOD1:c.118G>A polymorphism. All 38 samples from affected corgis were homozygous for the A allele, whereas the 17-sample asymptomatic control group consisted of 10 A/A homozygotes, 6 A/G heterozygotes and 1 G/G homozygote. To verify our localization of the DM mutation, we fine mapped 90 SNPs across a 1.9-Mb region from 29.04 to 30.97 Mb in 5 breeds, which segregate for DM (Fig. 1B). Affected dogs from all 5 breeds share a 5-SNP haplotype (maximum 195 kb in size), which contains the E40K mutation (Fig. 1C). This haplotype is also present in dogs that do not have the mutation. No other SNP or haplotype in the region is both shared across all breeds and concordant with recessive inheritance. Thus, the significant proportion of A/A mutant homozygotes among the controls and the presence of E40K mutation on an ancestral haplotype still present in the population may explain the relatively weak GWA to this region. Nonetheless, the presented genetic data strongly links the E40K mutation with the disease.
Fig. 1.
Mapping of a major DM locus. (A) GWA of 49,663 SNPs by using 38 cases (phenotypic stringencies 1 to 4) and 17 controls from Pembroke Welsh corgis identified a major locus on CFA31 (pgenome = 0.18) and weaker signals on other chromosomes by using 10,000 permutations in PLINK (38). (B) The CFA31 region of association spans ≈1.5 Mb and includes SOD1. P values from fine-mapping with 90 SNPs in 63 cases (phenotypic stringency levels 1–3) and 144 controls from 5 breeds (Boxer, 8/15 [Case/Control]; Chesapeake Bay retriever, 9/48; German Shepherd dog, 4/54; Pembroke Welsh corgi, 35/17; and Rhodesian ridgeback, 7/8) are shown as well as the association for the missense mutation, which was separately assayed. (C) Fine-mapping data shows that a 195-kb haplotype surrounding the SOD1 mutation is associated in all 5 breeds and that this haplotype is older than the SOD1 mutation.
Additional Genotyping Confirms an Association Between DM and Homozygosity for the SOD1 Missense Mutation.
The Pembroke Welsh corgis used for GWA, plus an additional 64 Pembroke Welsh corgis and 418 representatives from 4 other breeds, were genotyped for the SOD1:c.118G>A polymorphism. Across all breeds, 100 of the samples were from dogs diagnosed with DM; however, these diagnoses were not all based on equally stringent criteria (see Methods). Table 1 shows the distribution of genotypes for all 537 representatives of the 5 breeds by diagnostic class. Significant associations between the DM phenotype and homozygosity for the A allele were detected when all 5 affected breeds were jointly analyzed (P = 2.93E-19) and when each breed was analyzed individually (Table 1). The frequency of the A allele in a separate “other-breeds” control group, consisting of samples from dog breeds in which DM is rarely diagnosed, was significantly lower than in the controls from the affected breeds (Table 1). The 4 dogs in the study that were classified as affected, but were not A/A homozygotes, were all diagnosed by using the least stringent criteria and may be phenocopies.
Table 1.
Distribution of SOD1:c.118G>A genotypes among DM-affected and control dogs
| Breed | DM stringency 1AA/AG/GG | DM stringencies 1 and 2AA/AG/GG | DM stringencies 1 to 3AA/AG/GG | DM stringencies 1 to 4AA/AG/GG | Affected breed controls AA/AG/GG | Other breed controls AA/AG/GG |
|---|---|---|---|---|---|---|
| Boxer | 9/0/0* | 10/0/0* | 12/0/0* | 22/0/0** | 86/57/14 | — |
| Chesapeake Bay retriever | 7/0/0** | 9/0/0** | 9/0/0** | 10/0/0** | 7/25/21 | — |
| German shepherd dog | 2/0/0* | 4/0/0** | 4/0/0** | 4/0/1** | 7/30/83 | — |
| Pembroke Welsh corgi | 25/0/0** | 30/0/0** | 35/0/0** | 50/1/1** | 44/14/9 | — |
| Rhodesian ridgeback | 3/0/0* | 6/0/0** | 6/0/0** | 10/1/0** | 4/15/21 | — |
| All affected breeds | 46/0/0*** | 59/0/0*** | 66/0/0*** | 96/2/2*** | 148/141/148 | 0/5/115*** |
*, Different than breed-specific controls at P < 0.01;
**, different than breed-specific controls at P < 0.001;
***, different than all affected breed controls at P < 0.00001.
Dogs with DM Exhibit Symptoms and Histopathologic and Immunohistopathologic Lesions Similar to Those in ALS Patients.
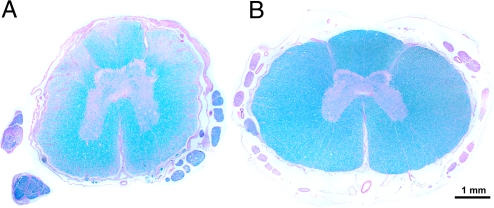
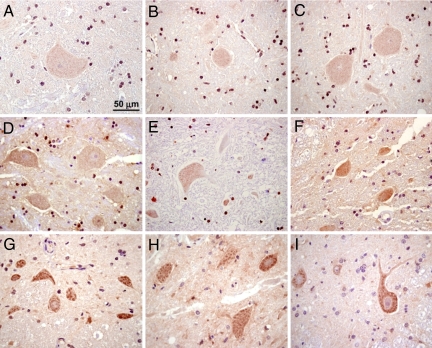
The diagnosis of DM was confirmed by histopathologic examination of spinal cord sections in 46 dogs. Affected dogs had lesions in the posterior and lateral columns (Fig. 2). Surviving spinal cord neurons from 7 DM-affected dogs and 10 similarly aged asymptomatic control dogs were examined by immunohistopathology. All 7 of the DM-affected dogs were A/A homozygotes, and all contained cytoplasmic inclusions, which, when stained with anti-SOD1 antibodies, appeared as well-defined dark clumps. In contrast, no staining or diffuse light staining similar to the background staining was found in cells in the spinal cords from all 5 of the control dogs with the G/G genotype and in 3 of the 5 A/G heterozygous controls. Intermediate levels of cytoplasmic staining with anti-SOD1 antibodies were observed in the spinal cords from the remaining 2 heterozygous control dogs [Fig. 3; supporting information (SI) Table S1].
Fig. 2.
Spinal cord histopathology. (A) Luxol fast blue-periodic acid Schiff staining of a thoracic spinal cord cross-section from a DM-affected 13-year-old Pembroke Welsh corgi. The white matter degeneration is depicted by regions of pallor where there has been loss of nerve fibers. (B) A similarly stained spinal cord cross section from an unaffected 13-year-old Labrador retriever. Note there is no evidence of nerve fiber loss. The bar in the lower right of the photomicrograph indicates the magnification.
Fig. 3.
Immunohistochemical staining with anti-SOD1 antibody in representative sections from the spinal cords from 3 G/G homozygous asymptomatic control dogs (A–C), 3 A/G heterozygous asymptomatic control dogs (D–F), and 3 A/A homozygous dogs with a confirmed diagnosis of DM (G–I). The samples were from a 13-year-old Rhodesian ridgeback (A), an 8-year-old Labrador retriever (B), a 13-year-old Labrador retriever (C), an 8-year-old Australian Shepherd (D), a 13-year-old Tibetan terrier (E), an 8-year-old German Shepherd dog (F), an 8-year-old Rhodesian ridgeback (G), a 13-year-old Pembroke Welsh corgi (H), and a 10-year-old Boxer (I). The bar in A indicates the magnification for all spinal cord cross-sections.
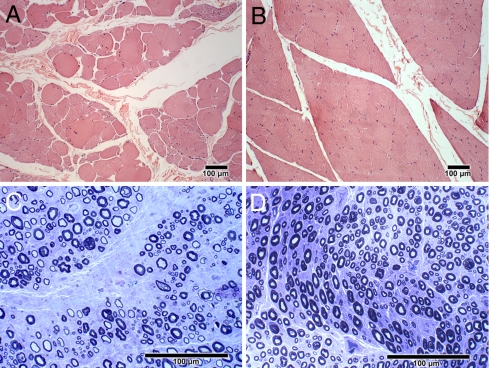
Although most dogs with DM are euthanized at an early stage of the disease when upper motor neuron pathology predominates, the owners of some dogs in the study elected to maintain their dogs until the disease was more advanced. Dogs with advanced DM exhibited clinical signs of lower motor neuron disease, including ascending flaccid tetraparesis, generalized muscle atrophy, and hyporeflexia in all limbs. One DM affected Pembroke Welsh corgi was euthanized 48 months after the onset of clinical signs due to swallowing difficulties, which suggests that the disease can progress to bulbar signs. In Movie S1, we show dogs with DM at various stages of disease progression. In the early disease stage, no spontaneous activity was detected by electromyography (EMG), and nerve conduction velocities were within normal limits. In the late disease stage, EMG revealed multifocal spontaneous activity in the distal appendicular musculature. Fibrillation potentials and sharp waves were the most common waveforms recorded. Compared with canine-specific reference ranges (19), compound muscle action potentials (M waves) recorded in the tibial and ulnar nerves showed temporal dispersion and decreases in amplitudes, and motor nerve conduction velocities were decreased (Fig. S1). Muscle specimens from dogs with advanced DM showed excessive variability in myofiber size with large and small groups of atrophic fibers typical of denervation (Fig. 4A). Peripheral nerve specimens from these dogs showed nerve fiber loss as indicated by axonal degeneration, endoneurial fibrosis, numerous inappropriately thinly myelinated fibers, and secondary demyelination (Fig. 4C).
Fig. 4.
Skeletal muscle and peripheral nerve histopathology in advanced DM. (A) H&E stained paraffin sections of the gastrocnemius muscle from a 13-year-old DM-affected Pembroke Welsh corgi showed excessive variability in myofiber size with large and small groups of atrophic fibers consistent with denervation. (B) For comparison, a similarly stained gastrocnemius muscle from an age-matched control dog. (C) Toluidine blue stained resin embedded sections of the peroneal nerve from the same Pembroke Welsh corgi showed substantial myelinated fiber loss, endoneurial fibrosis and secondary demyelination. (D) For comparison, a similarly stained peroneal nerve from an age-matched control dog. Bars in the lower right of all figures indicate the magnification.
Age-Related Incomplete Penetrance.
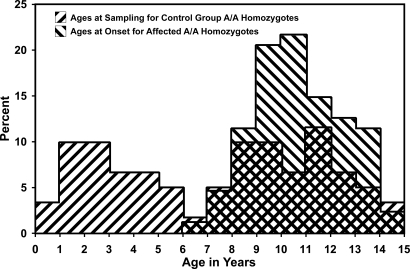
Many of the 148 A/A homozygotes in the “affected-breeds” control group were younger when sampled than the typical age at onset of clinical signs of DM (Fig. 5). Some of these dogs may develop DM when they grow older. Nonetheless, the considerable number of A/A homozygotes among the older affected-breed controls that exhibited no clinical signs of DM (Fig. 5) indicates that the penetrance among A/A homozygotes is incomplete, possibly due to modifier loci, environmental factors, and/or because the A/A homozygotes die from other causes before the clinical signs become apparent.
Fig. 5.
Distributions of the ages at sampling of the A/A homozygotes in the affected-breed control group and the ages at onset of clinical signs in the DM-affected dogs.
Discussion
Dogs with Advanced DM Have Both Upper and Lower Motor Neuron Disease.
DM is a common neurodegenerative disease of dogs. Several past studies have described the distribution of histopathologic changes (11–18), and many have indicated that the pathology is limited to nerve fiber loss in the thoracolumbar spinal cord (11–13, 15). Thus, DM has been commonly believed to be a disease of the upper motor neuron system. Owners of dogs with DM usually elect euthanasia at 6 months to a year after diagnosis when the dogs can no longer support weight with their pelvic limbs, whereas people with ALS typically progress to the state of complete paralysis and succumb to respiratory failure. We have now had an opportunity to examine dogs with DM that were maintained long past the point of paraplegia. These dogs exhibited clear clinical, electrophysiologic, and histopathologic evidence of lower motor neuron involvement. Clinical evidence of lower motor neuron disease included generalized and severe muscle atrophy, hyporeflexia, and flaccid weakness. Electromyographic changes and decreased M wave amplitudes were indicative of denervation and motor axonopathy. Decreased motor nerve conduction velocity was evidence of peripheral demyelination (Fig. S1). Neuromuscular histopathologic findings included denervation atrophy in skeletal muscles and demyelination in peripheral nerves (Fig. 4). These findings are consistent with previous clinical reports of ascending tetraparesis, flaccid paralysis, and widespread muscle atrophy in dogs with advanced DM (14, 16), and further characterize the peripheral nerve involvement. The progression of the disease and the distribution of lesions are similar to those reported for the upper motor neuron dominant onset form of ALS (20, 21).
A Missense Mutation and the Structure of the SOD1 Protein.
Although DM has been diagnosed in many dog breeds, we focused on the 5 breeds for which we had most samples. These breeds were Boxer, Pembroke Welsh corgi, German Shepherd dog, Chesapeake Bay retriever, and Rhodesian ridgeback. We used Pembroke Welsh corgi cases and controls to map DM to a region of CFA31, which contains SOD1 and identified a SOD1:c.118G>A polymorphism in exon 2 of SOD1. In the 5 breeds we studied, homozygosity for the A allele was strongly associated with DM: 96 of 100 dogs (96%) diagnosed with DM were A/A homozygotes, whereas only 148 of 437 control dogs (34%) in these 5 breeds were A/A homozygotes. Neither imaging using MRI or myelography nor spinal cord histopathology was used to confirm the diagnoses of DM in the 4 dogs that were not A/A homozygotes. These 4 dogs may have been misdiagnosed.
The SOD1:c.118G>A transition predicts an E40K missense mutation in SOD1. SOD1 functions as a homodimer, which converts superoxide radicals to hydrogen peroxide and molecular oxygen. Active sites in each subunit contain 1 copper ion and 1 zinc ion within an 8-stranded antiparallel β-barrel. In canine SOD1, amino acid position 40 lies within the short “Greek key,” connecting loop that stabilizes the alignment between the 2 sets of β-strands that comprise the β-barrel (22, 23). The codon for amino acid position 40 of human SOD1 lies within a region that contains a cluster of missense mutations that are associated with human ALS, including E40G at the position homologous to the canine E40K mutation (24, 25). The canine E40K mutation, like the human E40G mutation and several other ALS-associated SOD1 mutations, reduces the net negative charge of the predicted protein product. The SOD1 isoforms with reduced net negative charge may be prone to aggregation because of reduced repulsive Coulombic forces or because of increased interaction with anionic membrane surfaces (10, 25). A glutamate at a position corresponding to amino acid position 40 in canine SOD1 is conserved in 19 of 20 mammals identified in a Blastp query of the nonredundant protein sequences in the National Center for Biotechnology Information database (Fig. S2). The only exception is equine SOD1, which, like the mutant canine allele, has a lysine at the homologous position. The unusual lysine at this position may be tolerated because of compensatory amino acid substitutions elsewhere in equine SOD1.
SOD1-Containing Cytoplasmic Inclusions.
Spinal cords from some patients with ALS contain cytoplasmic inclusions known as Lewy body-like hyaline inclusions (LBHIs) if in neurons, or astrocytic hyaline inclusions (Ast-HIs) if in astrocytes (5). Both LBHIs and Ast-HIs contain SOD1 antigen (5, 26, 27). Although we have not observed LBHIs or Ast-HIs in hematoxylin and eosin (H&E)-stained spinal cord sections, even from dogs with advanced DM, spinal cords from DM-affected dogs consistently contained cytoplasmic inclusions that stained with anti-SOD1 antibodies. These inclusions were similar to those found in ALS patients with SOD1 mutations and in hSOD1m rodent models (1, 5–7, 26–28). We believe it is significant that spinal cord sections from some of the asymptomatic A/G heterozygotes had lightly staining SOD1-containing inclusions, whereas the inclusions were consistently absent from spinal cord sections from dogs that were homozygous for the wild-type G allele.
Mode of Inheritance of DM.
All of the strictly diagnosed DM-affected dogs were A/A homozygotes; however, several of the aged A/A homozygotes were symptom free. Thus, DM appears to be an incompletely penetrant autosomal recessive disease, whereas most human SOD1 mutations cause dominant forms of ALS. However, the N90A SOD1 isoform is associated with a recessively inherited form of ALS in some families, but with a dominant form in others (29, 30). The natural history of the disease in the families with recessive inheritance resembles canine DM in that onset is invariably in the lower limbs followed by a slow disease progression, whereas the sites of onset and the rates of progression of ALS in heterozygous N90A patients are much more variable (29, 30). Among the families segregating for the dominant form of ALS, rare patients with 2 copies of the mutant SOD1 allele had much earlier ages at disease onset than patients inheriting only a single copy (31, 32). Also, hSOD1m mice with higher transgene copy numbers exhibit earlier disease onset (5, 33), and the disease also occurs much earlier in homozygous hSOD1m mice than in the corresponding heterozygotes (34). With DM, the intermediate levels of staining for SOD1 inclusions observed in 2 of the 5 aged heterozygotes (Fig. 3 E and F) suggests that pathological processes are underway in these dogs even though no clinical signs are apparent. The pathology in SOD1:c.118G>A heterozygotes may develop too slowly to become clinically apparent within the usual canine life span. In this case, only A/A homozygotes would exhibit clinical signs and the mode of inheritance would appear to be recessive even if the pathogenesis, like that of ALS, involves a toxic gain of function.
Dogs with DM Are Models for ALS.
The discovery that homozygosity for E40K is a major genetic risk factor underlying DM should lead to marker-based long-term dog-breeding strategies for avoiding future generations of dogs at risk for developing DM. Nonetheless, the high frequency of the mutation in some breeds such as the Boxer and Pembroke Welsh corgi suggests that strict avoidance of this mutation could severely reduce the effective population sizes of these breeds. The potential of identifying modifier loci and altering their frequency may offer an alternative route to breed improvement. Even with a DM marker test, many thousands of privately owned dogs, born before the availability of the test, will continue to develop clinical signs, and it should take at least a decade before marker-based breeding can substantially reduce the incidence of this late-onset disease. In the mean time, DM-affected dogs are potential animal models for ALS. These dogs could be used to investigate the processes underlying the motor neuron degeneration in DM and ALS, to map modifier loci, and to identify environmental factors that exacerbate or ameliorate disease severity. The common euthanasia of affected dogs early in the progression of the disease should provide necropsy material at a disease stage that is rarely accessible with human patients. Also, the canine model may prove to be particularly valuable for evaluating therapeutic interventions. A wide variety of potential therapeutic agents have been found to influence the age-at-onset and/or the rate of disease progression in hSOD1m rodent models; however, these agents have seldom performed well in human clinical trials (35, 36). Compared with the hSOD1m rodent models, dogs with DM are more similar to people in size, in the structure and complexity of their nervous systems, and in the duration of the disease. Also, they are unlikely to possess the very high levels of mutant SOD1 expression, which occur in many of the hSOD1m rodent models (5), and which may induce pathologic processes distinct from those affecting ALS patients. Thus, the results from clinical trials conducted with DM-affected dogs may better predict the efficacies of therapeutic interventions for treating ALS.
Methods
Sources of Canine DNA Samples.
Individual DNA samples from normal and DM-affected dogs were obtained from the Canine Health Information Center (CHIC), DNA Repository (www.caninehealthinfo.org/), and from DNA collections at the University of Missouri, the Broad Institute of Harvard and Massachusetts Institute of Technology, and the University of Pennsylvania. The other-breeds control group consisted of 2 randomly selected and unrelated individuals from 60 dog breeds, in which DM is rarely, if ever, reported.
Diagnosis of DM.
The DM cases involved privately owned dogs that were referred to one of the participating Colleges of Veterinary Medicine. Depending on the availability of tissues and clinical information, diagnoses of DM were made on the basis of 4 sets of criteria of varying stringency. We considered confirmation of DM by histopathology to be the most stringent criterion for DM diagnosis (stringency level 1); however, spinal cords were not available from all dogs in the study. The diagnoses of DM at stringency levels 2 and 3 were based on the presence of typical clinical signs and the absence of a compressive lesion detectable by MRI (level 2) or myelography (level 3). The least stringent diagnoses (level 4) were based solely on suggestive clinical signs, which included progressive upper motor neuron paresis and general proprioceptive ataxia.
Association Mapping.
GWA analysis was undertaken by using the Affymetrix Canine Genome 2.0 Array “Platinum Panel” containing 49,663 SNP markers in 38 cases (diagnostic classification: score 1 n = 21, 2 n = 5, 3 n = 2, 4 n = 10) and 17 controls. SNP genotypes were obtained following the human 500K array protocol, but with a smaller hybridization volume to allow for the smaller surface area of the canine array as described elsewhere (37). Detailed information on the arrays is available at http://www.broad.mit.edu/node/456. Case-control GWA mapping was evaluated by using PLINK (38), followed by the identification of a region of homozygosity in affected individuals based on SNP genotypes. Fine mapping was performed by using MassARRAY (Sequenom) assays for 63 SNPs in 207 samples from 5 breeds as previously described (37). Haplotype analysis was performed with Haploview (39). The sources of samples used for fine mapping are identified in Fig. 1C.
Resequencing and Genotyping.
Exons 2 to 5 of canine SOD1 were resequenced after PCR amplification of genomic DNA from DM-affected and normal dogs. Table S2 contains the sequences of the oligonucleotide primers, designed from sequences flanking these exons from build 2.1 of the canine genome reference sequence (www.ncbi.nlm.nih.gov/projects/mapview/map_search.cgi?taxid=9615). Because exon 1 of SOD1 is not represented in build 2.1, we used RT-PCR to amplify exon 1-containing RNA segments in total RNA extracted from blood from DM-affected and normal dogs with the PAXgene Blood RNA Kit (Qiagen). The RT-PCR primers (Table S2) were designed from the consensus sequence produced from an alignment of all canine exon 1-containing expressed sequence tags in GenBank. Purified PCR and RT-PCR amplicons were sequenced with an Applied Biosystems 3730xl DNA analyzer. Canine DNA samples were genotyped at the SOD1:c.118G>A locus by pyrosequencing with a PSQ 96 Pyrosequencer. The PCR primers were 5′-biotinyl-AGTGGGCCTGTTGTGGTATCA with CTCCAAACTGATGGACGTGGAAT, and AATCCATGCTCGCCTT was used for the sequencing primer. Genotype distributions for affected and control samples were compared by using 2 × 2 contingency tables, in which A/G and G/G genotypes were pooled under the assumption of autosomal recessive inheritance. Fisher's exact 1-tailed test was used to test for the independence of the genotype classes between the case and control samples.
Histopathology, Immunohistopathology, and Electrodiagnostic Testing.
Standard procedures were used for histopathology, immunohistopathology, and electrodiagnostic testing as detained in SI Methods. Samples used for SOD1 immunohistochemistry were coded, and micrographs of spinal cord motor neurons were obtained in a masked manner. A second masked evaluator classified the neurons in the micrographs according to the presence and appearance of SOD1-positive inclusions based on the following categories: well-defined dark staining clumps, well-defined light staining clumps, poorly defined light staining regions, and no staining or diffuse light staining similar to the background staining; 6 to 9 sections from each cord were examined.
Supplementary Material
Acknowledgments.
We thank Dr. Alexander de Lahunta for helpful critique of the manuscript. This work was supported by American Kennel Club Canine Health Foundation Grants 821 and 732, a Research to Prevent Blindness, Inc. unrestricted grant, the Swedish Research Council, and the European Science Foundation.
Footnotes
Conflict of interest statement: The University of Missouri has applied for a patent covering the use of markers for the SOD1 mutation for the diagnosis of DM or for marker-assisted selective breeding of dogs. G.S.J., J.R.C., K.L.-T., and C.M.W. are listed as inventors in this application.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812297106/DCSupplemental.
References
- 1.Boillee S, Velde CV, Cleveland DW. ALS: A disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Schymick JC, Talbot K, Traynor BJ. Genetics of sporadic amyotrophic lateral sclerosis. Hum Mol Genet. 2008;17:768–774. doi: 10.1093/hmg/ddm215. [DOI] [PubMed] [Google Scholar]
- 3.Rosen DR, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–61. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 4.Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: Insights from genetics. Nat Rev Neurosci. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- 5.Kato S. Amyotrophic lateral sclerosis models and human neuropathology: Similarities and differences. Acta Neuropathol. 2008;115:97–114. doi: 10.1007/s00401-007-0308-4. [DOI] [PubMed] [Google Scholar]
- 6.Gurney ME, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 7.Nagai M, et al. Rats expressing human cytosolic copper-zinc superoxide dismutase transgenes with amyotrophic lateral sclerosis: Associated mutations develop motor neuron disease. J Neurosci. 2001;21:9246–9254. doi: 10.1523/JNEUROSCI.21-23-09246.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reaume AG, et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nature. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- 9.Rakhit R, Chakrabartty A. Structure, folding, and misfolding of Cu,Zn superoxide dismutase in amyotrophic lateral sclerosis. Biochim Biophys Acta. 2006;1762:1025–1037. doi: 10.1016/j.bbadis.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Shaw BF, Valentine JS. How do ALS-associated mutations in superoxide dismutase 1 promote aggregation of the protein? Trends Biochem Sci. 2007;32:78–85. doi: 10.1016/j.tibs.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Averill DR. Degenerative myelopathy in the aging German Shepherd dog: Clinical and pathologic findings. J Am Vet Med Assoc. 1973;162:1045–1051. [PubMed] [Google Scholar]
- 12.Griffiths IR, Duncan ID. Chronic degenerative radiculomyelopathy in the dog. J Sm Anim Pract. 1975;16:461–471. doi: 10.1111/j.1748-5827.1975.tb05773.x. [DOI] [PubMed] [Google Scholar]
- 13.Braund KG, Vandevelde M. German Shepherd dog myelopathy–a morphologic and morphometric study. Am J Vet Res. 1978;39:1309–1315. [PubMed] [Google Scholar]
- 14.Coates JR, et al. Clinical characterization of a familial degenerative myelopathy in Pembroke Welsh corgi dogs. J Vet Intern Med. 2007;21:1323–1331. doi: 10.1892/07-059.1. [DOI] [PubMed] [Google Scholar]
- 15.Johnston PE, Barrie JA, McCulloch MC, Anderson TJ, Griffiths IR. Central nervous system pathology in 25 dogs with chronic degenerative radiculomyelopathy. Vet Rec. 2000;146:629–633. doi: 10.1136/vr.146.22.629. [DOI] [PubMed] [Google Scholar]
- 16.Matthews NS, de Lahunta A. Degenerative myelopathy in an adult miniature poodle. J Am Vet Med Assoc. 1985;186:1213–1215. [PubMed] [Google Scholar]
- 17.March P, et al. Degenerative myelopathy in 18 Pembroke Welsh corgi dogs. Vet Pathol. 2009 doi: 10.1354/vp.46-2-241. in press. [DOI] [PubMed] [Google Scholar]
- 18.Bichsel P, Vandevelde M, Lang J, Kull-Hächler S. Degenerative myelopathy in a family of Siberian Husky dogs. J Am Vet Med Assoc. 1983;183:998–1000. [PubMed] [Google Scholar]
- 19.Walker TL, Redding RW, Braund KG. Motor nerve conduction velocity and latency in the dog. Am J Vet Res. 1979;40:1433–1439. [PubMed] [Google Scholar]
- 20.Engel WK, Kurland LT, Klatzo I. An inherited disease similar to amyotrophic lateral sclerosis with a pattern of posterior column involvement. An intermediate form? Brain. 1965;82:203–220. doi: 10.1093/brain/82.2.203. [DOI] [PubMed] [Google Scholar]
- 21.Hirano A, Kurland LT, Sayre GP. Familial amyotrophic lateral sclerosis: A subgroup characterized by posterior and spinocerebellar tract involvement and hyaline inclusions in the anterior horn cells. Arch Neurol. 1967;16:232–243. doi: 10.1001/archneur.1967.00470210008002. [DOI] [PubMed] [Google Scholar]
- 22.Green SL, et al. Structure, chromosomal location, and analysis of the canine Cu/Zn superoxide dismutase (SOD1) gene. J Hered. 2002;93:119–124. doi: 10.1093/jhered/93.2.119. [DOI] [PubMed] [Google Scholar]
- 23.Boissinot M, et al. Function of the Greek key connection analyzed using permutants of superoxide dismutase. EMBO J. 1997;16:2171–2178. doi: 10.1093/emboj/16.9.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng HX, et al. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science. 1993;261:1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- 25.Sandelin E, Nordlund A, Andersen PM, Marklund SS, Oliveberg M. Amyotrophic lateral sclerosis-associated copper/zinc superoxide dismutase mutations preferentially reduce the repulsive charge of the proteins. J Biol Chem. 2007;282:21230–21236. doi: 10.1074/jbc.M700765200. [DOI] [PubMed] [Google Scholar]
- 26.Shibata N, et al. Intense superoxide dismutase-1 immunoreactivity in intracytoplasmic hyaline inclusions of familial amyotrophic lateral sclerosis with posterior column involvement. J Neuropathol Exp Neurol. 1996;55:481–490. doi: 10.1097/00005072-199604000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Kato S, et al. Pathological characterization of astrocytic hyaline inclusions in familial amyotrophic lateral sclerosis. Am J Pathol. 1997;151:611–620. [PMC free article] [PubMed] [Google Scholar]
- 28.Bruijn LI, et al. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science. 1998;281:1851–1854. doi: 10.1126/science.281.5384.1851. [DOI] [PubMed] [Google Scholar]
- 29.Andersen PM, et al. Autosomal recessive adult-onset amyotrophic lateral sclerosis associated with homozygosity for Asp90Ala CuZn-superoxide dismutase mutation. A clinical and genealogical study of 36 patients. Brain. 1996;119:1153–1172. doi: 10.1093/brain/119.4.1153. [DOI] [PubMed] [Google Scholar]
- 30.Khoris J, et al. Coexistence of dominant and recessive familial amyotrophic lateral sclerosis with the D90A Cu,Zn superoxide dismutase mutation within the same country. Eur J Neurol. 2000;7:207–211. doi: 10.1046/j.1468-1331.2000.00028.x. [DOI] [PubMed] [Google Scholar]
- 31.Hayward C, Brock DJ, Minns RA, Swingler RJ. Homozygosity for Asn86Ser mutation in the CuZn-superoxide dismutase gene produces a severe clinical phenotype in a juvenile onset case of familial amyotrophic lateral sclerosis. J Med Genet. 1998;35:174. doi: 10.1136/jmg.35.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marucci G, et al. Amyotrophic lateral sclerosis with mutation of the Cu/Zn superoxide dismutase gene (SOD1) in a patient with Down syndrome. Neuromuscul Disord. 2007;17:673–676. doi: 10.1016/j.nmd.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Dal Canto MC, Gurney ME. Neuropathological changes in two lines of mice carrying a transgene for mutant human Cu,Zn SOD, and in mice overexpressing wild type human SOD: A model of familial amyotrophic lateral sclerosis (FALS) Brain Res. 1995;676:25–40. doi: 10.1016/0006-8993(95)00063-v. [DOI] [PubMed] [Google Scholar]
- 34.Jonsson PA, et al. Minute quantities of misfolded mutant superoxide dismutase-1 cause amyotrophic lateral sclerosis. Brain. 2004;127:73–88. doi: 10.1093/brain/awh005. [DOI] [PubMed] [Google Scholar]
- 35.DiBernardo AB, Cudkowicz ME. Translating preclinical insights into effective human trials in ALS. Biochim Biophys Acta. 2006;1762:1139–1149. doi: 10.1016/j.bbadis.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Benatar M. Lost in translation: Treatment trials in the SOD1 mouse and in human ALS. Neurobiol Dis. 2007;26:1–13. doi: 10.1016/j.nbd.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 37.Karlsson EK, et al. Efficient mapping of mendelian traits in dogs through genome-wide association. Nat Genet. 2007;39:1321–1328. doi: 10.1038/ng.2007.10. [DOI] [PubMed] [Google Scholar]
- 38.Purcell S, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.