Abstract
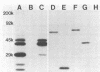
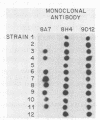
We have isolated plasma cell hybridomas which secrete monoclonal antibodies directed against Haemophilus ducreyi. Two of these monoclonal antibodies recognize all strains of H. ducreyi tested to date and are capable of detecting the presence of H. ducreyi in skin lesions produced by this pathogen in experimental animals. These monoclonal antibodies which react with apparently all strains of H. ducreyi have the potential to be developed into a rapid immunodiagnostic test for chancroid.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Denys G. A., Chapel T. A., Jeffries C. D. An indirect fluorescent antibody technique for Haemophilus ducreyi. Health Lab Sci. 1978 Jul;15(3):128–132. [PubMed] [Google Scholar]
- Fast M. V., Nsanze H., D'Costa L. J., Plummer F. A., Karasira P., Maclean I. W., Ronald A. R. Treatment of chancroid by clavulanic acid with amoxycillin in patients with beta-lactamase-positive Haemophilus ducreyi infection. Lancet. 1982 Sep 4;2(8297):509–511. doi: 10.1016/s0140-6736(82)90597-9. [DOI] [PubMed] [Google Scholar]
- Gaisin A., Heaton C. L. Chancroid: alias the soft chancre. Int J Dermatol. 1975 Apr;14(3):188–197. doi: 10.1111/ijd.1975.14.3.188. [DOI] [PubMed] [Google Scholar]
- Gulig P. A., Frisch C. F., Hansen E. J. A set of two monoclonal antibodies specific for the cell surface-exposed 39K major outer membrane protein of Haemophilus influenzae type b defines all strains of this pathogen. Infect Immun. 1983 Nov;42(2):516–524. doi: 10.1128/iai.42.2.516-524.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Frisch C. F., Johnston K. H., Hansen E. J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982 Jul;37(1):82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafiz S., Kinghorn G. R., McEntegart M. G. Chancroid in Sheffield. A report of 22 cases diagnosed by isolating Haemophilus ducreyi in a modified medium. Br J Vener Dis. 1981 Dec;57(6):382–386. doi: 10.1136/sti.57.6.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafiz S., Kinghorn G. R., McEntegart M. G. Starch aggregation as a presumptive test for Haemophilus ducreyi. Lancet. 1982 Oct 16;2(8303):872–872. doi: 10.1016/s0140-6736(82)90830-3. [DOI] [PubMed] [Google Scholar]
- Hammond G. W., Lian C. J., Wilt J. C., Ronald A. R. Antimicrobial susceptibility of Haemophilus ducreyi. Antimicrob Agents Chemother. 1978 Apr;13(4):608–612. doi: 10.1128/aac.13.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. W., Lian C. J., Wilt J. C., Ronald A. R. Comparison of specimen collection and laboratory techniques for isolation of Haemophilus ducreyi. J Clin Microbiol. 1978 Jan;7(1):39–43. doi: 10.1128/jcm.7.1.39-43.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning U., Schwarz H., Chen R. Radioimmunological screening method for specific membrane proteins. Anal Biochem. 1979 Aug;97(1):153–157. doi: 10.1016/0003-2697(79)90339-7. [DOI] [PubMed] [Google Scholar]
- Kinghorn G. R., Hafiz S., McEntegart M. G. Modified haemin-containing medium for isolation of Haemophilus ducreyi. Lancet. 1982 Feb 13;1(8268):393–394. doi: 10.1016/s0140-6736(82)91417-9. [DOI] [PubMed] [Google Scholar]
- Messing M., Sottnek F. O., Biddle J. W., Schlater L. K., Kramer M. A., Kraus S. J. Isolation of Haemophilus species from the genital tract. Sex Transm Dis. 1983 Apr-Jun;10(2):56–61. doi: 10.1097/00007435-198304000-00002. [DOI] [PubMed] [Google Scholar]
- Nayyar K. C., Stolz E., Michel M. F. Rising incidence of chancroid in Rotterdam. Epidemiological, clinical, diagnostic, and therapeutic aspects. Br J Vener Dis. 1979 Dec;55(6):439–441. doi: 10.1136/sti.55.6.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nsanze H., Fast M. V., D'Costa L. J., Tukei P., Curran J., Ronald A. Genital ulcers in Kenya. Clinical and laboratory study. Br J Vener Dis. 1981 Dec;57(6):378–381. doi: 10.1136/sti.57.6.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhofer T. R., Back A. E. Isolation and cultivation of Haemophilus ducreyi. J Clin Microbiol. 1982 Apr;15(4):625–629. doi: 10.1128/jcm.15.4.625-629.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Dondero D. V., Gallo D., Devlin V., Woodie J. D. Serological analysis of herpes simplex virus types 1 and 2 with monoclonal antibodies. Infect Immun. 1982 Jan;35(1):363–367. doi: 10.1128/iai.35.1.363-367.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S. M., Frisch C. F., Gulig P. A., Kettman J. R., Johnston K. H., Hansen E. J. Monoclonal antibodies directed against a cell surface-exposed outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1982 Apr;36(1):80–88. doi: 10.1128/iai.36.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S. M., Kettman J. R., Miller J. N., Norgard M. V. Murine monoclonal antibodies specific for virulent Treponema pallidum (Nichols). Infect Immun. 1982 Jun;36(3):1076–1085. doi: 10.1128/iai.36.3.1076-1085.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sng E. H., Lim A. L., Rajan V. S., Goh A. J. Characteristics of Haemophilus ducreyi. A study. Br J Vener Dis. 1982 Aug;58(4):239–242. doi: 10.1136/sti.58.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottnek F. O., Biddle J. W., Kraus S. J., Weaver R. E., Stewart J. A. Isolation and identification of Haemophilus ducreyi in a clinical study. J Clin Microbiol. 1980 Aug;12(2):170–174. doi: 10.1128/jcm.12.2.170-174.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Tam M. R., Kuo C. C., Nowinski R. C. Monoclonal antibodies to Chlamydia trachomatis: antibody specificities and antigen characterization. J Immunol. 1982 Mar;128(3):1083–1089. [PubMed] [Google Scholar]
- Yolken R. H., Leister F. J., Whitcomb L. S., Santosham M. Enzyme immunoassays for the detection of bacterial antigens utilizing biotin-labeled antibody and peroxidase biotin--avidin complex. J Immunol Methods. 1983 Feb 11;56(3):319–327. doi: 10.1016/s0022-1759(83)80021-0. [DOI] [PubMed] [Google Scholar]