Abstract
Increasing activity of the cAMP/protein kinase A (PKA) pathway has often been proposed as an approach to improve memory in various organisms. However, here we demonstrate that single-point mutations, which decrease PKA activity, dramatically improve aversive olfactory memory in Drosophila. These mutations do not affect formation of early memory phases or of protein synthesis-dependent long-term memory but do cause a significant increase in a specific consolidated form of memory, anesthesia-resistant memory. Significantly, heterozygotes of null mutations in PKA are sufficient to cause this memory increase. Expressing a PKA transgene in the mushroom bodies, brain structures critical for memory formation in Drosophila, reduces memory back to wild-type levels. These results indicate that although PKA is critical for formation of several memory phases, it also functions to inhibit at least one memory phase.
Keywords: anesthesia-resistant memory, DC0 gene, learning, behavior
Many different mutations that decrease learning and memory in Drosophila and other experimental organisms have been identified (1). However, mutations that improve memory have been difficult to obtain. In several cases, overexpression of genes important for memory formation has been reported to improve memory, including Notch (2), CREB (3, but also see 4), NMDA receptor subunit 2 (5, 6), the ubiquitin ligase Neuralized (7), and others; but to date, no single-point mutations that improve memory have been identified.
The cAMP/protein kinase A (PKA) pathway has long been known to be essential for memory formation. Mutations in rutabaga, which encodes a Ca2+-dependent adenylyl cyclase, cause significant defects in short-term memory (STM) (8). In addition, severe reductions in expression or activity of DC0, the gene encoding the catalytic subunit of PKA, cause deficits in learning, STM and middle-term memory (MTM) (9–11). amnesiac mutants are defective for neuropeptides proposed to regulate adenylyl cyclase and are defective for MTM (12, 13). The CREB transcription factor is required for long-term memory (LTM) formation, and CREB activity is regulated by PKA (3, 4, 14). These results have prompted researchers to try to improve memory by pharmacologically increasing PKA activity. Indeed, agonists of D1/D5 dopamine receptors that are positively associated with adenylyl cyclase, analogs of cAMP, and rolipram, a cAMP phosphodiesterase inhibitor, have all been reported to enhance memory (15, 16). However, increases in cAMP/PKA activity have also been shown to be detrimental to memory. Mutations in dunce, a cAMP-specific phosphodiesterase, increase cAMP levels and cause a severe STM defect (17). Protein phosphatase 1-mutated flies also have defective learning and memory (18). Increasing PKA expression by using a heat shock promoter or a mushroom body-specific promoter inhibits learning and memory (19, 20), whereas decreasing PKA activity to 24% of wild type inhibits learning (9, 11). Further decreasing PKA activity to 16% of wild-type inhibits both learning and memory retention (11). These results indicate that normal memory formation is highly sensitive to PKA expression levels, and it is not necessarily clear whether increasing or decreasing PKA activity improves memory.
Early phases of memory (STM and MTM) are consolidated into 2 types of longer lasting forms of memory in Drosophila: LTM and anesthesia-resistant memory (ARM) (21). ARM is produced after a single training or multiple repetitive trainings of an aversive association (21). It is gradually produced shortly after training and reaches asymptotic levels between 1 and 2 h after training (21). After multiple trainings, ARM lasts over 24 h and decays within 4 days (21). LTM of an aversive association requires multiple trainings with rest intervals between trainings to be formed and lasts up to 7 days (21). Interestingly, for appetitive memory, both ARM and LTM are produced after a single cycle of training, with ARM again being produced earlier than LTM (22).
LTM requires new protein synthesis to be formed and also requires activity of the CREB transcription factor (4, 14, 21). In contrast, ARM does not require CREB activity and can be formed in the presence of concentrations of protein synthesis inhibitors that inhibit protein synthesis by 50% (21). Although PKA activity is important for CREB activity and LTM formation, the role of PKA in ARM formation has not been extensively studied.
Previously, we determined a situation in which decreasing PKA activity improves memory. Drosophila, similar to other species, suffer a reduction of memory upon aging, known as age-related memory impairment (AMI) (23). Learning is relatively unaffected, but a strong and significant impairment of MTM occurs at old ages. Decreasing PKA activity dramatically delays AMI and allows flies to maintain youthful memory into old ages (20). This suggests that AMI may occur because of an age-dependent increase in cAMP/PKA activity or an age-dependent increase in expression of a PKA substrate that inhibits MTM upon phosphorylation.
In this work, we describe a second situation in which decreasing PKA activity improves memory. While characterizing mutants for amelioration of AMI, we observed that some PKA mutants strongly enhance memory at young ages as well. We show that memory improvement in these PKA mutants consists of a specific increase in ARM. This memory enhancement does not depend on genetic background and can be reverted to normal by expressing PKA in the mushroom bodies (MBs), structures important for memory formation in the Drosophila brain.
Results
Identification of Specific Point Mutations That Improve Memory Retention.
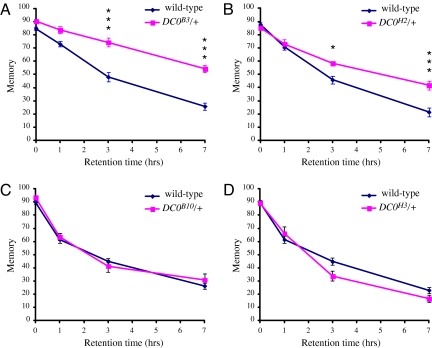
In a previous study, we determined that heterozygotes of lethal point mutations in DC0, the gene encoding the major catalytic subunit of cAMP-dependent protein kinase (PKAc), dramatically delay AMI onset (20). These heterozygotes all reduce PKA activity to ≈50% of wild type and delay AMI onset >2-fold (20, 24). While characterizing these mutants further, we made the startling observation that some of them also improve memory retention in young flies. Wild-type flies have a characteristic memory retention curve after a single cycle of training where memory is initially extremely robust but then gradually decays as the time interval between training and testing increases. Two mutants, DC0B3/+ and DC0H2/+, have dramatically improved memory retention curves compared with wild-type flies, whereas 3 others, DC0B10/+, DC0H3/+, and DC0B12/+ (data not shown) have retention curves similar to wild-type (Fig. 1). Avoidance of naïve flies to the odors and electrical shocks used during training was not significantly different between wild-type and DC0 heterozygous mutants, indicating that the increases in memory we observed are not caused by increased sensitivity to these stimuli [supporting information (SI) Fig. S1].
Fig. 1.
Heterozyotes of null (DC0B3/+) and strong (DC0H2/+) DC0 mutations significantly improve memory retention, whereas heterozygotes of weaker DC0 alleles (DC0B10/+ and DC0H3/+) do not. The DC0B3 mutation truncates PKAc by >50 aa, is genetically and phenotypically indistinguishable from deficiency lines lacking DC0, and is likely to be a null mutation in DC0. DC0H2 is classified as a severe or strong DC0 mutation based on the early lethality of hemizyotes. DC0H3, DC0B10, and DC0B12 (data not shown) are classified as medium and weak alleles based on complementation studies and the lethal phase of hemizygotes. Severity of alleles is based on classification by Lane and Kalderon (24). Note that the null allele improves memory to a greater extent than the strong allele, whereas the medium and weak alleles do not improve memory at all. n ≥6 for all data points. DC0B3/+ and DC0H2/+ curves show significant differences compared with wild type with respect to genotype, retention time, and interaction between genotype and retention time as analyzed by 2-way ANOVA (P < 0.003 in all cases). DC0B10/+ and DC0H3/+ curves show no significant differences from wild-type with respect to genotype. Bonferroni post hoc analyses indicate that DC0B3/+ and DC0H2/+ lines do not show significant differences from wild type at retention times of 0 and 1 h but do show significant differences at 3 and 7 h. *, P < 0.05; ***, P < 0.001.
DC0B3/+ and DC0H2/+ Mutants Have Increased ARM.
Memory retention curves of DC0B3/+ and DC0H2/+ mutants indicate that early forms of memory, including memory tested immediately after training (3-min memory) and short forms of memory (1-h memory) are not greatly affected. However, memory at later time points, 3 h and 7 h after training, progressively increases relative to wild type, such that at 7 h, memory is approximately double that of wild-type. This type of memory improvement suggested to us that short forms of memory are normal, but consolidation from STM to more stable forms of memory is increased in these DC0/+ mutants. The consolidated form of memory produced in Drosophila after a single cycle of olfactory conditioning is ARM (21). Seven hours after a single cycle of training, shorter forms of memory have decayed, leaving ARM as the predominant remaining memory phase (21). The second form of consolidated memory, LTM, forms only after multiple spaced trainings for aversive associations (25).
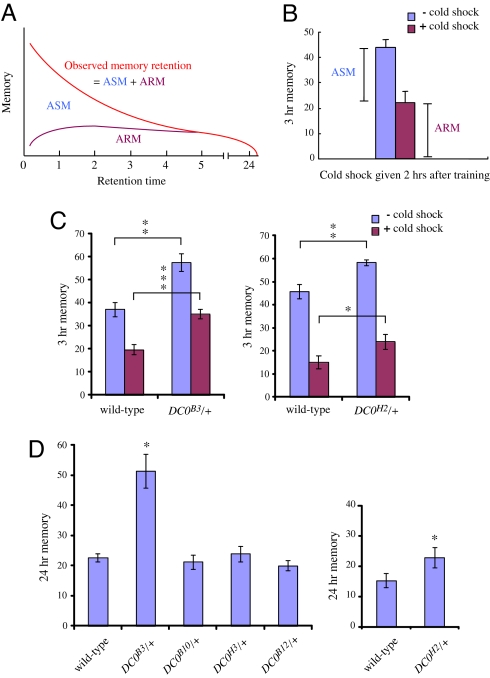
To address whether formation of ARM is specifically enhanced in our DC0B3/+ and DC0H2/+ mutants, we performed cold shock retrograde amnesia experiments (21). Three hours after training, memory consists of anesthesia-sensitive memory (ASM), a combination of STM and MTM, and ARM (Fig. 2A). ARM can be separated from the shorter-lasting, more labile ASM because it is resistant to cold shock anesthesia (21, 26, 27). Thus, if flies are cold shocked by placing them in prechilled vials and submerging them in ice water for 90 s, 1 h before testing, STM and MTM are abolished, leaving only the ARM component of 3-h memory (Fig. 2B). As seen in Fig. 2C, both DC0B3/+ and DC0H2/+ flies have improved 3-h memory in the absence of cold shock, indicating that at least 1 memory component of 3-h memory is increased. When a cold shock is given 2 h after training, and 3-h memory is measured, both lines again show an increase in memory, indicating that cold shock-resistant ARM is increased in these flies. Importantly, the increase in total 3-h memory and the increase in cold shock-resistant 3-h memory are similar (20.35 ± 4.64 and 15.45 ± 3.17, P > 0.3 for DC0B3/+ compared with wild type and 12.55 ± 3.34 and 8.91 ± 4.64, P > 0.5 for DC0H2/+ compared with wild type), indicating that the memory improvement seen in DC0/+ heterozygotes consists of an increase in ARM and not STM and MTM.
Fig. 2.
DC0B3/+ and DC0H2/+ increase ARM. (A) Schematic memory retention curve demonstrating that observed memory retention consists of the sum of an early phase of memory, ASM, and a later phase, ARM. ASM consists of STM and MTM and is gradually consolidated into ARM. (B) Schematic demonstrating that 3-h memory consists of an ASM component and an ARM component. The ARM component can be specifically measured by cold-shocking the flies (cold shock anesthesia) 1 h before testing. Doing so specifically erases the ASM component of memory. ASM can be calculated by subtracting ARM from total 3-h memory. (C) DC0B3/+ and DC0H2/+ significantly improve total 3-h memory (P < 0.01 as assayed by t test). They also improve the cold shock-resistant ARM component of 3-h memory to the same extent (P < 0.05 as assayed by t test). Thus, these DC0 mutations increase ARM and not ASM. n ≥6 for all data points. (D) ARM can also be measured as 24-h memory after 10 massed trainings. ARM after massed training is significantly increased relative to wild type in DC0B3/+ (P < 0.05 as assayed by 1-way ANOVA) and DC0H2/+ (P < 0.05 as assayed by t test), whereas it remains unchanged in DC0B10/+, DC0H3/+, and DC0B12/+ lines. Two separate panels are shown in D because these experiments were performed at different times. Although there is some variability in 24-h memory measured in wild-type flies, both DC0B3/+ and DC0H2/+ always show improved ARM relative to wild-type. n = 5–7. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
ARM can also be measured by using a second training regimen. Ten massed trainings (10 trainings repeated one after another with no rest intervals) produce memory that lasts >24 h (21). This memory is not inhibited by feeding protein synthesis inhibitors and consists strictly of ARM. DC0B3/+ and DC0H2/+ both show increased 24-h memory after massed training, again indicating that increased ARM production is responsible for the increase in memory observed in these flies (Fig. 2D).
DC0/+ Mutations Do Not Affect LTM and Enhance ARM Independently of Genetic Background.
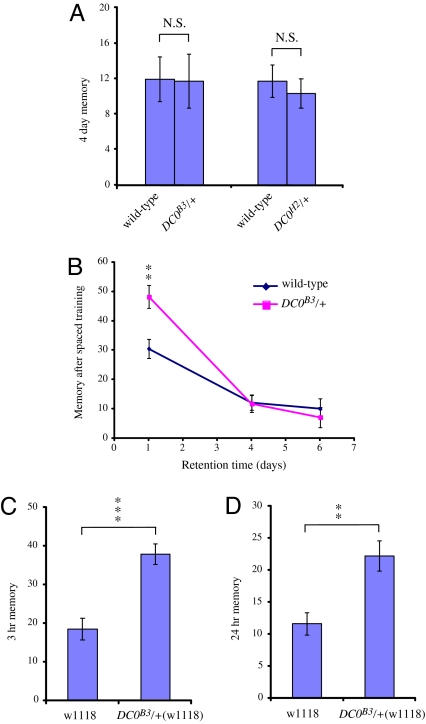
Because DC0/+ mutations can increase ARM, we next wanted to determine whether they could also increase the second form of consolidated memory, LTM. Massed training produces only ARM, whereas spaced training (10 trainings with 15-min rest intervals interspersed between trainings) produces both ARM and LTM (28). ARM decays to baseline within 4 days after spaced training, whereas LTM lasts at least 7 days (21). Thus, to determine whether reducing PKAc activity also improves LTM, we measured 4-day memory after spaced training in wild-type, DC0B3/+ and DC0H2/+ flies. As seen in Fig. 3A, 4-day memory is identical in these lines, suggesting that reductions in PKAc do not increase LTM.
Fig. 3.
DC0/+ mutants do not increase LTM although they do increase ARM in a different genetic background. (A) LTM, a second consolidated form of memory that forms after 10 spaced trainings, is not increased in DC0/+ mutants. LTM measured 4 days after spaced training remains unchanged relative to wild-type in DC0B3/+ and DC0H2/+ flies (P = 0.9629 and P = 0.5730 as assayed by t test). n = 10 and 11, respectively. (B) Memory retention curves of wild-type and DC0B3/+ flies after spaced training. Memory is improved 1 day after training but returns to normal 4 days after training in DC0B3/+ flies. Two-way ANOVA demonstrates significant differences caused by retention time (P < 0.0001) and interaction between genotype and retention time (P = 0.0142). Bonferroni post hoc analyses indicate that DC0B3/+ shows significant differences from wild type at a retention time of 1 day (P < 0.01) but not at 4 and 6 days (P > 0.05). n = 5–10 for each data point. (C and D) Improvement of memory in DC0B3/+ mutants is not caused by specific background effects. Both 3-h memory after single-cycle training (C) and 24-h memory after massed training (D) are improved by the DC0B3/+ mutation in a w1118 background. n ≥ 6. **, P < 0.01; ***, P < 0.001. N.S., not significant.
Fig. 3B shows memory retention in days after spaced training in wild-type and DC0B3/+ flies. Memory in DC0B3/+ flies is enhanced 1 day after spaced training and is normal at 4 and 6 days after training. Overall, our data indicate that memory enhancement begins between 1 and 3 h after single cycle training and reverts to normal within 4 days after spaced training. These results are entirely consistent with a model where memory enhancement in DC0B3/+ and DC0H2/+ flies consists of a specific increase in ARM (25).
Because DC0B3/+ and DC0H2/+ are the first single-point mutations identified that significantly improve memory retention, we were concerned that they might function by complementing an existing defect present in our w(CS) wild-type line rather than improving memory in general. To address this possibility, we outcrossed our DC0B3/+ line to a different wild-type line, w1118, for 6 generations and tested for both 3-h memory after single cycle training and 24-h memory after massed training. As seen in Fig. 3 C and D, DC0B3/+ improves ARM in this line as well. Thus, we believe that improvement of ARM by reducing PKA activity is a general effect unrelated to genetic background.
A General Reduction in PKAc Activity Is Responsible for the Increase in ARM.
All DCO alleles assayed in this article, DC0B3, DC0H2, DC0H3, DCOB10, and DC0B12, are homozygous-lethal, and PKA assays of head extracts of heterozygotes of all 5 alleles show a reduction of PKA activity to ≈50% of wild-type activity as assayed biochemically from crude extracts (20, 24). However, previous studies have revealed notable differences between these different alleles. DC0B3 and DC0H2 have been characterized as null and strong alleles, respectively, whereas DC0H3, DC0B10, and DC0B12 have been classified as weaker alleles (24). The DC0B3 allele contains a nonsense mutation that truncates the last 54 aa of PKAc, whereas the DC0H2 allele contains a missense mutation that changes a nonpolar amino acid to a charged residue (Gly203 → Asp). The weaker alleles all contain less drastic missense mutations: DC0H3 changes Thr200 to Ala, DC0B10 changes Asn219 to Ile, and DC0B12 changes Gly128 to Ser. The lethality of homozygotes of the weaker alleles can be rescued by 1 copy of a DC0 transgene that expresses at ≈10% of wild-type levels, whereas the stronger alleles require 2 copies for appreciable rescue (24). In addition, strong and weak DC0 alleles can be separated based on the stage at which lethality occurs in hemizygotes and the limited ability of weaker alleles to complement each other for viability (24). Furthermore, at least 1 group has demonstrated that a heterozygote of 1 of the weaker alleles, DC0B10, retains more PKA activity than a heterozyote of a deficiency encompassing the DC0 gene (29). Thus, there are clearly small but significant differences in activity between different alleles that have biological relevance. We reasoned that memory improvement in DC0B3/+ and DC0H2/+ mutants could be caused by two possibilities. Improvement could be caused by these minor differences in PKA activity, or improvement could be an allele-specific effect where defective proteins encoded by DC0B3 and DC0H2 could be inhibiting specific PKA complexes that repress memory retention.
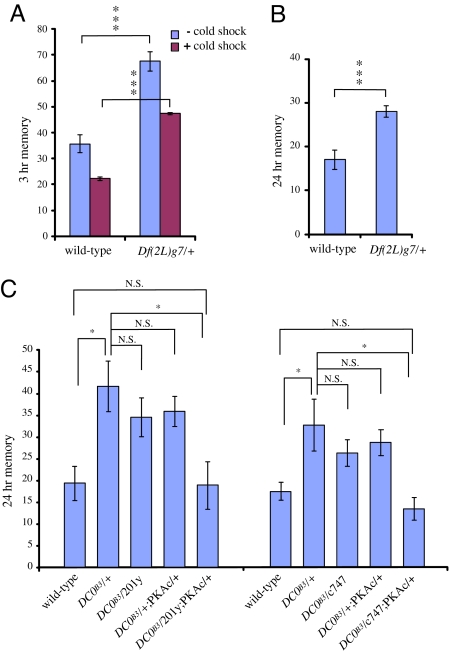
To distinguish between these two possibilities, we next measured memory in heterozygotes of a deficiency line, Df(2L)γ7, that removes the entire PKA coding region. Similar to DC0B3/+ and DC0H2/+ flies, Df(2L)γ7/+ flies have increased total and cold shock-resistant 3-h memory after a single cycle of training (Fig. 4A). In addition, they have significantly increased 7-h memory (data not shown) and significantly increased 24-h memory after massed training (Fig. 4B) compared with wild-type, indicating that an overall reduction in PKA activity to a certain level, rather than allele-specific effects, causes improvement of memory in Drosophila.
Fig. 4.
A heterozygote of a deficiency line lacking 1 copy of DC0 improves ARM. Expressing PKA in the MBs restores normal memory to a DC0/+ mutant. (A) Total and cold shock-resistant 3-h memory are both increased by similar amounts in a Df(2L)γ7/+ line, which lacks 1 copy of DC0, compared with wild type (P < 0.0001 as assayed by t test). n = 6. (B) Twenty-four-hour memory after massed training is also significantly increased in Df(2L)γ7/+ flies (P = 0.0004 as assayed by t test). n = 14. (C) Expressing a DC0 transgene specifically in the MBs reverts the improved memory of DC0B3/+ back to the wild type. DC0B3/201y and DC0B3/c747 are a DC0B3/+ lines containing the specified MB drivers, 201y and c747, alone. DC0B3/+;PKAc/+ is a DC0B3/+ line containing a DC0 transgene in the absence of a driver, and DC0B3/201y;PKAc/+ and DC0B3/c747;PKAc/+ express the DC0 transgene under 201y and c747 control. One-way ANOVA demonstrates significant differences in 24-h memory caused by genotype in both experiments (P = 0.0045 and P = 0.0002). Bonferroni post hoc analyses show that there are no significant differences between DC0B3/+, DC0B3/201y, DC0B3/c747, and DC0B3/+;PKAc/+ lines (P > 0.05), whereas there are significant differences between DC0B3/+ and wild-type, DC0B3/201y;PKAc/+, and DC0B3/c747;PKAc/+ lines (P < 0.05). n ≥6. *, P < 0.05; ***, P < 0.001; N.S., not significant.
Expression of DC0 in the MBs Suppresses DC0B3/+ Memory Enhancement.
If reduced PKAc expression causes increased memory in DC0/+ mutant flies, memory improvement should be suppressed if we express a DC0 transgene in these mutants. In the Drosophila brain, DC0 is mainly expressed in the MBs, structures known to be critical for olfactory learning and memory (9, 30). Thus, we expressed a UAS-DC0 transgene under control of 2 drivers, 201y and c747, which predominantly drive expression in the MBs. As seen in Fig. 4C, the presence of the 201y or c747 drivers alone or the UAS-DC0 transgene alone does not significantly alter 24-h memory after massed training in a DC0B3/+ background. However, expressing DC0 under 201y or c747 control in a DC0B3/+ background reduces memory back to the wild-type level.
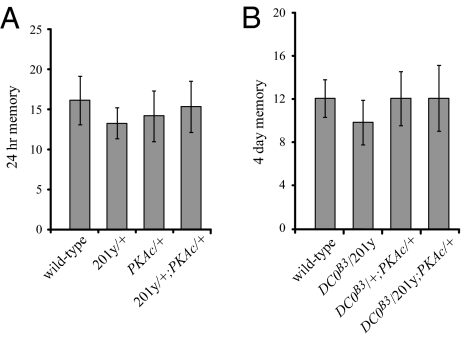
Because strong overexpression of PKAc on its own can decrease learning and memory in a wild-type background (19), it remained possible that the apparent complementation of memory enhancement that we observed is not the result of rescue but rather an unrelated effect caused by overexpression. If this is the case, we would expect (i) that PKAc expression using our MB drivers would inhibit ARM in a wild-type background and (ii) that PKAc expression using these drivers would not be specific to ARM but would also inhibit LTM. Indeed, expression of PKAc using a c309 MB driver abolishes 24-h memory after massed training in both a wild-type and a DC0B3/+ background (data not shown). However, expression of PKAc using the 201y driver has no effect on ARM in a wild-type background (Fig. 5A), whereas it reduces ARM back to wild-type levels in a DC0B3/+ background (Fig. 4C). Furthermore, expression of PKAc using a 201y driver does not affect LTM, as assayed by 4-day memory after spaced training, in a DC0B3/+ background. Taken together, these results indicate that enhancement of ARM depends on reduced PKAc expression in the MBs.
Fig. 5.
Expressing PKAc under 201y control does not (A) inhibit ARM in a wild-type background and (B) reduce LTM in a DC0B3/+ background. ARM was measured as 24-h memory after massed training, and LTM was measured as 4-day memory after spaced training. These results indicate that restoration of normal ARM in DC0B3/+ upon 201y-dependent PKAc expression results from complementation of the DC0B3/+ memory phenotype rather than nonspecific memory inhibition because of PKAc overexpression. In both A and B, no significant differences between genotypes were demonstrated when assayed by 1-way ANOVA (P = 0.8983 and P = 0.8516, respectively). n ≥8.
Discussion
In this work, we report the surprising finding that optimal cAMP/PKA activity for maximal memory retention of an olfactory associative task does not occur at the wild-type level but rather at a reduced level of ≈50% of wild type. Our results demonstrate that heterozygous DC0 mutants fall into 2 classes: those that increase ARM and those that do not. Interestingly, in terms of activity measured from head extracts, both types of mutants reduce PKA activity approximately equally (20, 24), suggesting that either minor changes (≈10–20%) in PKA activity can have major effects on ARM or that the 3 weaker mutants that do not improve ARM maintain a specific PKA function that inhibits ARM. In a previous study, Lane and Kalderon (24) classified PKA mutants as null, strong, medium, and weak based on survivorship and egg development in complementation studies with weak PKA mutants. The 3 mutants that improve ARM all fall into the null and strong PKA mutant classes, whereas the 3 mutants that fail to improve ARM fall into the medium and weak classes, suggesting that the weak PKA mutants retain some activity that functions to inhibit ARM. It will be of great interest in the future to identify differences between weak and strong PKA alleles to identify potential PKA complexes or substrates involved in inhibiting ARM.
Although many groups have demonstrated that decreasing PKA activity inhibits learning and memory (9–11, 19), our data are not necessarily inconsistent with these previous results. Studies in which PKA activity has been measured indicate that strong reductions to 24% or 20% of wild-type activity are necessary to reduce learning strongly (9, 11), and a further reduction to 16% is necessary to observe further defects in MTM (11). Moderate reductions to ≈60% of wild-type activity have minor or no effects on learning (9, 11). In addition, previous work has focused on the role of PKA in learning and short forms of memory, and specific studies on consolidated ARM have not been reported. Our data indicate that there is only a brief window of PKA activity in which memory improvement occurs. Memory is normal when PKA activity is between 100% and ≈60% of wild-type levels. Below this, an improvement in ARM can be observed; but as activity decreases to less than ≈24% and 16%, reductions in learning and MTM predominate and may mask improvements in ARM.
The molecular basis through which ARM is formed is unknown. ARM formation has been proposed to be independent of new protein synthesis because its formation does not depend on activity of the CREB transcription factor (14) and is not prevented when protein synthesis is inhibited by 50% (21). Thus, ARM may be regulated by posttranslational alterations of already existing proteins. Consistent with this idea, excess production of PKMζ, a persistently active truncated atypical protein kinase C, shortly after training, enhances ARM (31). We believe that ARM may be positively regulated by atypical PKC-dependent phosphorylation and negatively regulated by PKA-dependent phosphorylation. Interestingly, one ARM-specific memory mutant, radish, has been identified (27). The radish gene has been cloned, and, although its function is unknown, the putative radish gene product has 23 potential PKA sites and 14 potential PKC sites, suggesting that it may be regulated by phosphorylation (32).
Overall memory retention consists of the summation of various different memory phases (33). Our data suggest that these different phases are differentially affected by PKA such that increasing PKA activity may have positive effects on learning, STM, and LTM, whereas decreasing PKA activity has positive effects on ARM. Similarly, in mammalian systems, various memory types, including hippocampus-dependent memory, are enhanced by increasing cAMP/PKA activity, whereas a different type of memory, working memory, is inhibited by kinases including PKA (34, 35).
Although ARM and working memory are functionally distinct (working memory is a temporary memory form used to keep in mind information that needs to be monitored to make a particular decision, and ARM is a consolidated memory that forms over 2 h and lasts several days), it is intriguing to note that both forms of memory are enhanced by lowering PKA activity, and both may have antagonistic functions with other memory types (34, 36, 37).
DC0 mutations are able to improve ARM in young flies and delay AMI in aged flies. Expression of DC0 in the MBs suppresses both of these effects, suggesting that PKA substrates that cause AMI and inhibit ARM are both expressed in the MBs. However, we believe that suppression of AMI and enhancement of ARM may be 2 separable and distinct effects. AMI consists of a specific reduction in MTM, whereas ARM is unaffected by aging in flies (20, 38). In addition, the DC0 mutations that improve ARM and the mutations that delay AMI are not identical. The weaker DC0 mutants, DC0B10/+, DC0B12/+, and DC0H3/+, are able to delay AMI (20) but are not able to improve ARM. Furthermore, AMI can be delayed by expressing a PKA inhibitor, PKI, in the MBs of Drosophila, but this same treatment is unable to improve ARM (J.H., unpublished observations). This finding suggests that the amounts of PKA inhibition required to delay AMI and enhance ARM are different and potential PKA substrates that are phosphorylated to inhibit ARM and cause AMI may be distinct.
Materials and Methods
Fly Stocks.
All fly lines used in this work except for Df(2L)γ7/+ were outcrossed to our wild-type control line w(CS) (39) for at least 6 generations before use. Our DC0/+ lines do not contain any selectable markers at the mutation site, so flies containing each DC0 mutation were identified after each outcross by using genomic PCR. The specific end points of Df(2L)γ7 are not known, so this line was outcrossed to w(CS) containing a second chromosomal balancer, w(CS) CyO/Sp. At the final outcross to obtain heterozygotes, the deficiency was mated to w(CS) flies. Thus, our Df(2L)γ7/+ line contains 1 w(CS) second chromosome and 1 unoutcrossed second chromosome containing the deficiency. To obtain DC0B3/+ in a w1118 background, DC0B3/+(w1118), DC0B3/+ from the w(CS) background was outcrossed to w1118 for 6 generations before use. All fly stocks were maintained at 25 ± 2 °C at a humidity of 60 ± 10% under a 12-h/12-h light dark cycle under standard conditions.
Single-Cycle Training and Memory Assays.
Training and testing of a Pavlovian olfactory association have been described in ref. 8. Briefly, ≈100 flies were placed in a training chamber where they could be exposed simultaneously to an odor and electrical shocks. For single-cycle training, flies were exposed sequentially to 2 odors (3-octanol and 4-methylcyclohexanol) for 1 min each with a rest interval of 45 s between odors. One of the odors was paired with electrical shocks (60 V 1.5-s pulses every 5 s), whereas the other was not. Testing was performed at various time intervals after training by placing the flies at a choice point between the 2 odors and allowing the flies to choose between the 2 odors for 1.5 min. Memory was measured as a performance index (PI), defined as 100 × [2 × (no. of flies choosing the unpaired odor)/(total no. of flies) − 1]. Individual PIs are always the average of 2 experiments where the shock-paired odor was alternated. In all experiments, memory scores are the average of at least 6 independent individual PIs.
Odor Acuity and Shock Reactivity.
Peripheral control experiments including odor acuity and shock reactivity assays were performed as described in ref. 8 to verify that sensitivity to odors and electrical shock were unaffected in our mutants. Approximately 100 naïve flies were placed at the choice point of a T maze where they had to choose between an odor (3-octanol or 4-methylcyclohexanol) and mineral oil (olfactory acuity) or between electrical shocks and nonshocked conditions (shock reactivity). A PI was calculated as described above.
Massed and Spaced Training.
Massed and spaced training were performed by using an automated system where a computer controlled both electric shock and odor application to the flies. Massed training consisted of 10 single-cycle trainings repeated without rest intervals between trainings, and spaced training consisted of 10 single-cycle trainings repeated with 15-min rest intervals between each training. Twenty-four-hour, 4-day, and 6-day memory were measured manually as described above.
Cold Shock.
For cold shock experiments to measure ARM, flies were transferred into prechilled vials that were then placed in ice water for 90 s, 2 h after training. Memory was tested 3 h after training. The 1 h between cold shock and testing allows the flies sufficient time to recover from the cold shock.
Statistics.
All data are expressed as means ± SEM. Statistical analyses were performed by using Prism version 4.01 (GraphPad). P values <0.05 were judged as statistically significant.
Supplementary Material
Acknowledgments.
We thank Daniel Kalderon and members of the Saitoe laboratory for helpful discussion. This work was supported by Grants-in-Aid for Scientific Research (B) (19300137), and for Scientific Research on Priority Areas-Integrative Brain Research (20019039) from the Ministry of Education, Culture, Sports, Science and Technology of Japan awarded to M.S. and a grant from the Takeda Science Foundation, awarded to M.S.
Footnotes
The authors declare no conflicts of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810119105/DCSupplemental.
References
- 1.McGuire SE, Deshazer M, Davis RL. Thirty years of olfactory learning and memory research in Drosophila melanogaster. Prog Neurobiol. 2005;76:328–347. doi: 10.1016/j.pneurobio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Ge X, et al. Notch signaling in Drosophila long-term memory formation. Proc Natl Acad Sci USA. 2004;101:10172–10176. doi: 10.1073/pnas.0403497101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin JC, Del Vecchio M, Zhou H, Tully T. CREB as a memory modulator: Induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995;81:107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]
- 4.Perazzona B, Isabel G, Preat T, Davis RL. The role of cAMP response element-binding protein in Drosophila long-term memory. J Neurosci. 2004;24:8823–8828. doi: 10.1523/JNEUROSCI.4542-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang YP, et al. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 6.Wu CL, et al. Specific requirement of NMDA receptors for long-term memory consolidation in Drosophila ellipsoid body. Nat Neurosci. 2007;10:1578–1586. doi: 10.1038/nn2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavlopoulos E, Anezaki M, Skoulakis EM. Neuralized is expressed in the α/β lobes of adult Drosophila mushroom bodies and facilitates olfactory long-term memory formation. Proc Natl Acad Sci USA. 2008;105:14674–14679. doi: 10.1073/pnas.0801605105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 9.Skoulakis EM, Kalderon D, Davis RL. Preferential expression in mushroom bodies of the catalytic subunit of protein kinase A and its role in learning and memory. Neuron. 1993;11:197–208. doi: 10.1016/0896-6273(93)90178-t. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin SF, et al. Defective learning in mutants of the Drosophila gene for a regulatory subunit of cAMP-dependent protein kinase. J Neurosci. 1997;17:8817–8827. doi: 10.1523/JNEUROSCI.17-22-08817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, Tully T, Kalderon D. Effects of a conditional Drosophila PKA mutant on olfactory learning and memory. Learn Mem. 1996;2:320–333. doi: 10.1101/lm.2.6.320. [DOI] [PubMed] [Google Scholar]
- 12.Feany MB, Quinn WG. A neuropeptide gene defined by the Drosophila memory mutant amnesiac. Science. 1995;268:869–873. doi: 10.1126/science.7754370. [DOI] [PubMed] [Google Scholar]
- 13.Keene AC, et al. Diverse odor-conditioned memories require uniquely timed dorsal paired medial neuron output. Neuron. 2004;44:521–533. doi: 10.1016/j.neuron.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Yin JC, et al. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- 15.Bach ME, et al. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci USA. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci USA. 1998;95:15020–15025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dudai Y, Jan YN, Byers D, Quinn WG, Benzer S. dunce, a mutant of Drosophila deficient in learning. Proc Natl Acad Sci USA. 1976;73:1684–1688. doi: 10.1073/pnas.73.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asztalos Z, et al. Protein phosphatase 1-deficient mutant Drosophila is affected in habituation and associative learning. J Neurosci. 1993;13:924–930. doi: 10.1523/JNEUROSCI.13-03-00924.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drain P, Folkers E, Quinn WG. cAMP-dependent protein kinase and the disruption of learning in transgenic flies. Neuron. 1991;6:71–82. doi: 10.1016/0896-6273(91)90123-h. [DOI] [PubMed] [Google Scholar]
- 20.Yamazaki D, et al. The Drosophila DCO mutation suppresses age-related memory impairment without affecting life span. Nat Neurosci. 2007;10:478–484. doi: 10.1038/nn1863. [DOI] [PubMed] [Google Scholar]
- 21.Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 22.Krashes MJ, Waddell S. Rapid consolidation to a radish and protein synthesis-dependent long-term memory after single-session appetitive olfactory conditioning in Drosophila. J Neurosci. 2008;28:3103–3113. doi: 10.1523/JNEUROSCI.5333-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamura T, et al. Aging specifically impairs amnesiac-dependent memory in Drosophila. Neuron. 2003;40:1003–1011. doi: 10.1016/s0896-6273(03)00732-3. [DOI] [PubMed] [Google Scholar]
- 24.Lane ME, Kalderon D. Genetic investigation of cAMP-dependent protein kinase function in Drosophila development. Genes Dev. 1993;7:1229–1243. doi: 10.1101/gad.7.7a.1229. [DOI] [PubMed] [Google Scholar]
- 25.Tully T, Cambiazo V, Kruse L. Memory through metamorphosis in normal and mutant Drosophila. J Neurosci. 1994;14:68–74. doi: 10.1523/JNEUROSCI.14-01-00068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tully T, et al. Genetic dissection of memory formation in Drosophila melanogaster. Cold Spring Harbor Symp Quant Biol. 1990;55:203–211. doi: 10.1101/sqb.1990.055.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Folkers E, Drain P, Quinn WG. Radish, a Drosophila mutant deficient in consolidated memory. Proc Natl Acad Sci USA. 1993;90:8123–8127. doi: 10.1073/pnas.90.17.8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeZazzo J, Tully T. Dissection of memory formation: From behavioral pharmacology to molecular genetics. Trends Neurosci. 1995;18:212–218. doi: 10.1016/0166-2236(95)93905-d. [DOI] [PubMed] [Google Scholar]
- 29.Majercak J, Kalderon D, Edery E. Drosophila melanogaster deficient in protein kinase A manifests behavior-specific arrhythmia but normal clock function. Mol Cell Biol. 1997;17:5915–5922. doi: 10.1128/mcb.17.10.5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heisenberg M. Mushroom body memoir: From maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 31.Drier EA, et al. Memory enhancement and formation by atypical PKM activity in Drosophila melanogaster. Nat Neurosci. 2002;5:316–324. doi: 10.1038/nn820. [DOI] [PubMed] [Google Scholar]
- 32.Folkers E, Waddell S, Quinn WG. The Drosophila radish gene encodes a protein required for anesthesia-resistant memory. Proc Natl Acad Sci USA. 2006;103:17496–17500. doi: 10.1073/pnas.0608377103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Margulies C, Tully T, Dubnau J. Deconstructing memory in Drosophila. Curr Biol. 2005;15:R700–R713. doi: 10.1016/j.cub.2005.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dash PK, Moore AN, Kobori N, Runyan JD. Molecular activity underlying working memory. Learn Mem. 2007;14:554–563. doi: 10.1101/lm.558707. [DOI] [PubMed] [Google Scholar]
- 35.Ramos BP, et al. Dysregulation of protein kinase A signaling in the aged prefrontal cortex: New strategy for treating age-related cognitive decline. Neuron. 2003;40:835–845. doi: 10.1016/s0896-6273(03)00694-9. [DOI] [PubMed] [Google Scholar]
- 36.Isabel G, Pascual A, Preat T. Exclusive consolidated memory phases in Drosophila. Science. 2004;304:1024–1027. doi: 10.1126/science.1094932. [DOI] [PubMed] [Google Scholar]
- 37.Mery F, Pont J, Preat T, Kawecki TJ. Experimental evolution of olfactory memory in Drosophila melanogaster. Physiol Biochem Zool. 2007;80:399–405. doi: 10.1086/518014. [DOI] [PubMed] [Google Scholar]
- 38.Mery F. Aging and its differential effects on consolidated memory forms in Drosophila. Exp Gerontol. 2007;42:99–101. doi: 10.1016/j.exger.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Dura JM, Preat T, Tully T. Identification of linotte, a new gene affecting learning and memory in Drosophila melanogaster. J Neurogenet. 1993;9:1–14. doi: 10.3109/01677069309167272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.