Abstract
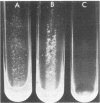
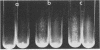
Coaggregation between Cytophaga sp. strain DR2001 and Actinomyces israelii PK16 was partially inhibited by certain N-acetylated amino sugars (N-acetylneuraminic acid, N-acetylgalactosamine, and N-acetylglucosamine) and was completely inhibited by the trisaccharide neuraminin-lactose. The monosaccharides exerted their effect at concentrations between 30 to 100 mM, whereas the trisaccharide was an effective inhibitor at significantly lower concentrations. Outer membrane preparations caused A. israelii PK16 cells to aggregate; however, vesicles released from the cell envelope during growth failed to do so. Adherence studies with a non-coaggregating mutant of the cytophaga suggest that the spheroidal hydroxyapatite attachment sites and coaggregation receptors are separate entities.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwar H., Brown M. R., Cozens R. M., Lambert P. A. Isolation and characterization of the outer and cytoplasmic membranes of Pseudomonas cepacia. J Gen Microbiol. 1983 Feb;129(2):499–507. doi: 10.1099/00221287-129-2-499. [DOI] [PubMed] [Google Scholar]
- Celesk R. A., London J. Attachment of oral Cytophaga species to hydroxyapatite-containing surfaces. Infect Immun. 1980 Aug;29(2):768–777. doi: 10.1128/iai.29.2.768-777.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970 Dec;15(12):1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Kelstrup J., Funder-Nielsen T. D. Aggregation of oral streptococci with Fusobacterium and Actinomyces. J Biol Buccale. 1974 Dec;2(4):347–362. [PubMed] [Google Scholar]
- Kolenbrander P. E., Celesk R. A. Coaggregation of human oral Cytophaga species and Actinomyces israelii. Infect Immun. 1983 Jun;40(3):1178–1185. doi: 10.1128/iai.40.3.1178-1185.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E. Isolation and characterization of coaggregation-defective mutants of Actinomyces viscosus, Actinomyces naeslundii, and Streptococcus sanguis. Infect Immun. 1982 Sep;37(3):1200–1208. doi: 10.1128/iai.37.3.1200-1208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marucha P. T., Keyes P. H., Wittenberger C. L., London J. Rapid method for identification and enumeration of oral Actinomyces. Infect Immun. 1978 Sep;21(3):786–791. doi: 10.1128/iai.21.3.786-791.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Crosby L. K., Barlow J. J., Matta K. L. Structural preferences of beta-galactoside-reactive lectins on Actinomyces viscosus T14V and Actinomyces naeslundii WVU45. Infect Immun. 1983 Aug;41(2):848–850. doi: 10.1128/iai.41.2.848-850.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerkamp A. H., McBride B. C. Characterization of the adherence properties of Streptococcus salivarius. Infect Immun. 1980 Aug;29(2):459–468. doi: 10.1128/iai.29.2.459-468.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]