Abstract
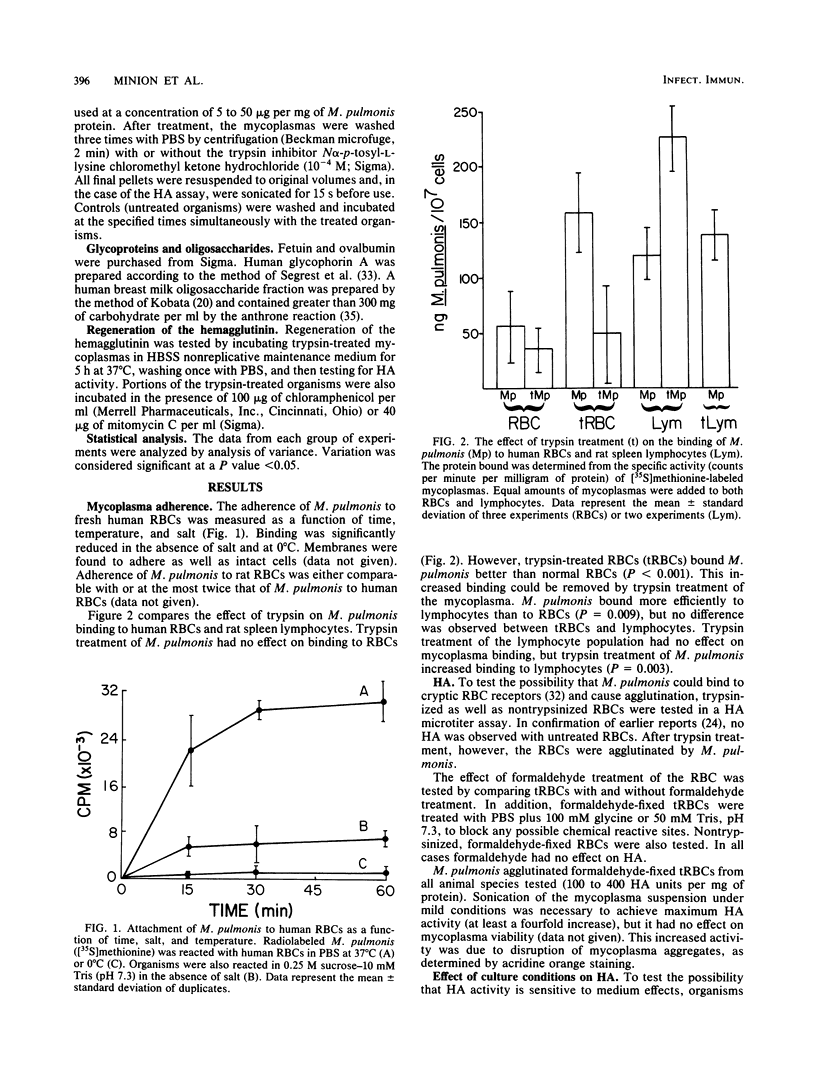
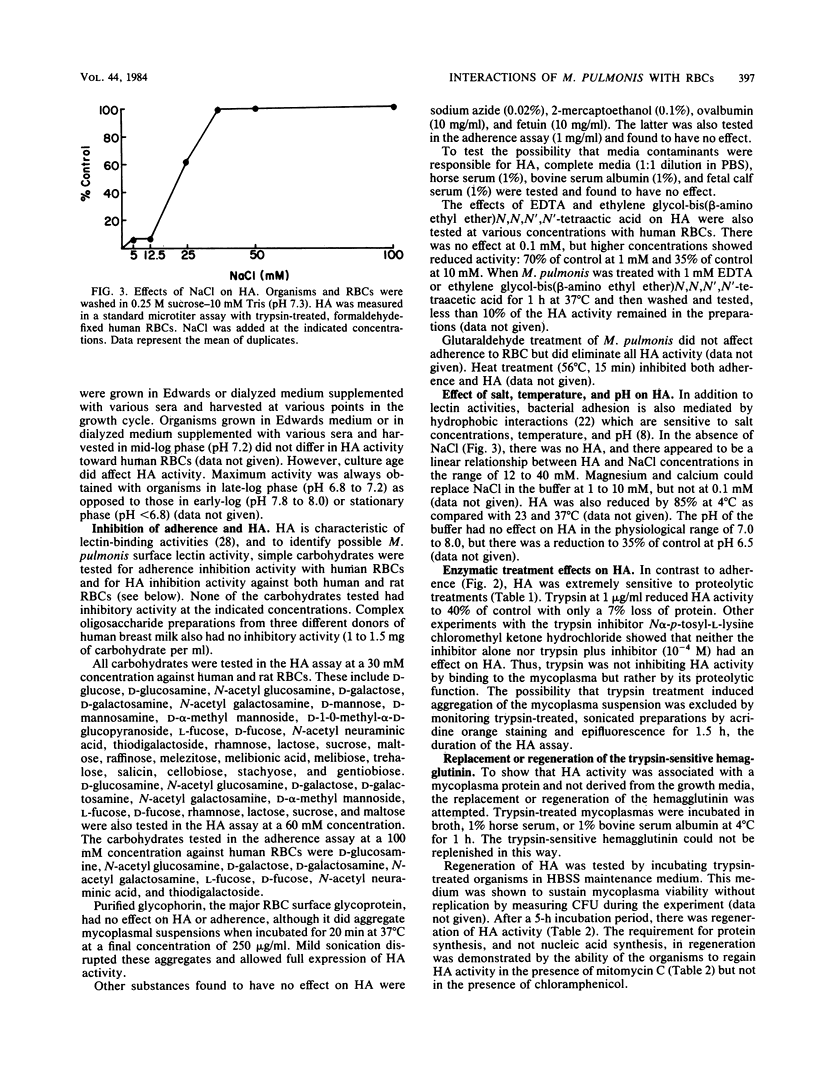
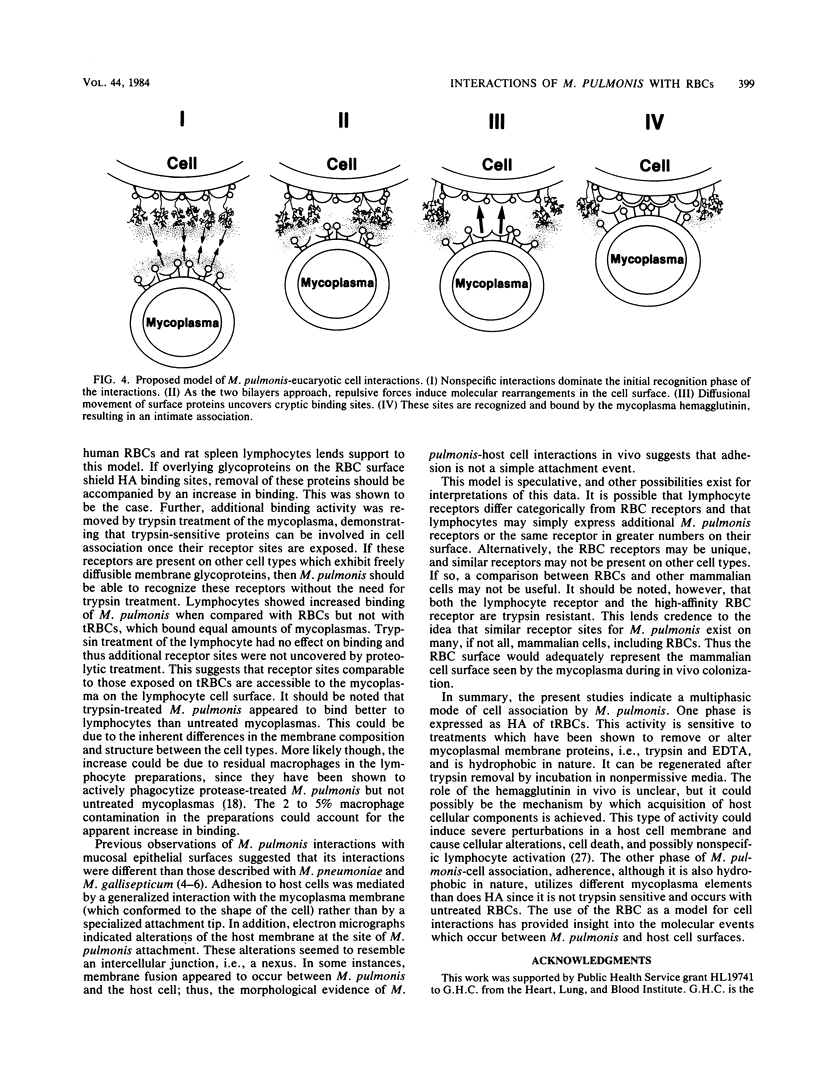
The mechanism(s) of interaction between Mycoplasma pulmonis and eucaryotic cells was studied by adherence to and hemagglutination of erythrocytes. Simple and complex carbohydrates and glycoproteins were unable to inhibit either adherence or hemagglutination, indicating that neither was a lectin activity. Both interactions appeared to be hydrophobic due to their requirement for salt and their sensitivity to temperature. Hemagglutination, but not adherence, was inhibited by both trypsin and glutaraldehyde treatment of the mycoplasma, suggesting that adherence and hemagglutination are qualitatively different. The erythrocyte receptor sites for the two activities were also separable since hemagglutination, but not adherence, required trypsinization of erythrocytes. The hemagglutinin was shown to be an integral mycoplasma component and not a broth contaminant. Once removed, hemagglutinating activity could not be replenished by incubation in serum or broth at 4 degrees C, but could be regenerated during protein synthesis under nonreplicative conditions. Thus, a mycoplasma membrane protein was detected which was capable of interacting with opposing membrane surfaces through hydrophobic interactions. Consequently, a multiphasic model of M. pulmonis-eucaryotic cell interactions was proposed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banai M., Kahane I., Razin S., Bredt W. Adherence of Mycoplasma gallisepticum to human erythrocytes. Infect Immun. 1978 Aug;21(2):365–372. doi: 10.1128/iai.21.2.365-372.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banai M., Razin S., Bredt W., Kahane I. Isolation of binding sites to glycophorin from Mycoplasma pneumoniae membranes. Infect Immun. 1980 Dec;30(3):628–634. doi: 10.1128/iai.30.3.628-634.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell G. H., Wilborn W. H., Silvers S. H., Minion F. C. Adherence and colonization of Mycoplasma pulmonis to genital epithelium and spermatozoa in rats. Isr J Med Sci. 1981 Jul;17(7):593–598. [PubMed] [Google Scholar]
- Chandler D. K., Collier A. M., Barile M. F. Attachment of Mycoplasma pneumoniae to hamster tracheal organ cultures, tracheal outgrowth monolayers, human erythrocytes, and WiDr human tissue culture cells. Infect Immun. 1982 Mar;35(3):937–942. doi: 10.1128/iai.35.3.937-942.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. K., Delozier K. M., Asa D. K., Minion F. C., Cassell G. H. Interactions between murine alveolar macrophages and Mycoplasma pulmonis in vitro. Infect Immun. 1980 Aug;29(2):590–599. doi: 10.1128/iai.29.2.590-599.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Taylor-Robinson D. Interaction of Mycoplasma pneumoniae with human lung fibroblasts: role of receptor sites. Infect Immun. 1979 Jul;25(1):455–459. doi: 10.1128/iai.25.1.455-459.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner T. K., Williams D. C., Minion F. C., Phillips D. R. Thrombin-induced platelet aggregation is mediated by a platelet plasma membrane-bound lectin. Science. 1978 Jun 16;200(4347):1281–1283. doi: 10.1126/science.663608. [DOI] [PubMed] [Google Scholar]
- Gartner T. K., Williams D. C., Phillips D. R. Platelet plasma membrane lectin activity. Biochem Biophys Res Commun. 1977 Nov 21;79(2):592–599. doi: 10.1016/0006-291x(77)90198-x. [DOI] [PubMed] [Google Scholar]
- Glasgow L. R., Hill R. L. Interaction of Mycoplasma gallisepticum with sialyl glycoproteins. Infect Immun. 1980 Nov;30(2):353–361. doi: 10.1128/iai.30.2.353-361.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratzer W. B. The red cell membrane and its cytoskeleton. Biochem J. 1981 Jul 15;198(1):1–8. doi: 10.1042/bj1980001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Hu P. C., Cole R. M., Huang Y. S., Graham J. A., Gardner D. E., Collier A. M., Clyde W. A., Jr Mycoplasma pneumoniae infection: role of a surface protein in the attachment organelle. Science. 1982 Apr 16;216(4543):313–315. doi: 10.1126/science.6801766. [DOI] [PubMed] [Google Scholar]
- Jackson R. L., Segrest J. P., Kahane I., Marchesi V. T. Studies on the major sialoglycoprotein of the human red cell membrane. Isolation and characterization of tryptic glycopeptides. Biochemistry. 1973 Jul 31;12(16):3131–3138. doi: 10.1021/bi00740a600. [DOI] [PubMed] [Google Scholar]
- Jones T. C., Yeh S., Hirsch J. G. Studies on attachment and ingestion phases of phagocytosis of Mycoplasma pulmonis by mouse peritoneal macrophages. Proc Soc Exp Biol Med. 1972 Feb;139(2):464–470. doi: 10.3181/00379727-139-36165. [DOI] [PubMed] [Google Scholar]
- Kahane I., Pnini S., Banai M., Baseman J. B., Cassell G. H., Bredt W. Attachment of mycoplasmas to erythrocytes: a model to study mycoplasma attachment to the epithelium of the host respiratory tract. Isr J Med Sci. 1981 Jul;17(7):589–592. [PubMed] [Google Scholar]
- Kobata A., Ginsburg V. Oligosaccharides of human milk. II. Isolation and characterization of a new pentasaccharide, lacto-N-fucopentaose 3. J Biol Chem. 1969 Oct 25;244(20):5496–5502. [PubMed] [Google Scholar]
- Magnusson K. E., Davies J., Grundström T., Kihlström E., Normark S. Surface charge and hydrophobicity of Salmonella, E. coli, Gonococci in relation to their tendency to associate with animal cells. Scand J Infect Dis Suppl. 1980;Suppl 24:135–140. [PubMed] [Google Scholar]
- Magnusson K. E. Hydrophobic interaction--a mechanism of bacterial binding. Scand J Infect Dis Suppl. 1982;33:32–36. [PubMed] [Google Scholar]
- Manchee R. J., Taylor-Robinson D. Haemadsorption and haemagglutination by mycoplasmas. J Gen Microbiol. 1968 Mar;50(3):465–478. doi: 10.1099/00221287-50-3-465. [DOI] [PubMed] [Google Scholar]
- Manchee R. J., Taylor-Robinson D. Studies on the nature of receptors involved in attachment of tissue culture cells to mycoplasmas. Br J Exp Pathol. 1969 Feb;50(1):66–75. [PMC free article] [PubMed] [Google Scholar]
- Manchee R. J., Taylor-Robinson D. Utilization of neuraminic acid receptors by mycoplasmas. J Bacteriol. 1969 Jun;98(3):914–919. doi: 10.1128/jb.98.3.914-919.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naot Y., Tully J. G., Ginsburg H. Lymphocyte activation by various Mycoplasma strains and species. Infect Immun. 1977 Nov;18(2):310–317. doi: 10.1128/iai.18.2.310-317.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock M. E., Bonner S. V. Comparison of undefined medium and its dialyzable fraction for growth of Mycoplasma. J Bacteriol. 1969 Feb;97(2):522–525. doi: 10.1128/jb.97.2.522-525.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand R. P. Interacting phospholipid bilayers: measured forces and induced structural changes. Annu Rev Biophys Bioeng. 1981;10:277–314. doi: 10.1146/annurev.bb.10.060181.001425. [DOI] [PubMed] [Google Scholar]
- Reitherman R. W., Rosen S. D., Barondes S. H. Lectin purification using formalinised erythrocytes as a general affinity adsorbant. Nature. 1974 Apr 12;248(449):599–600. doi: 10.1038/248599a0. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Wilkinson T. M., Sheng L. Isolation of glycophorin with deoxycholate. Biochim Biophys Acta. 1979 Jul 5;554(2):533–537. doi: 10.1016/0005-2736(79)90389-4. [DOI] [PubMed] [Google Scholar]


