Abstract
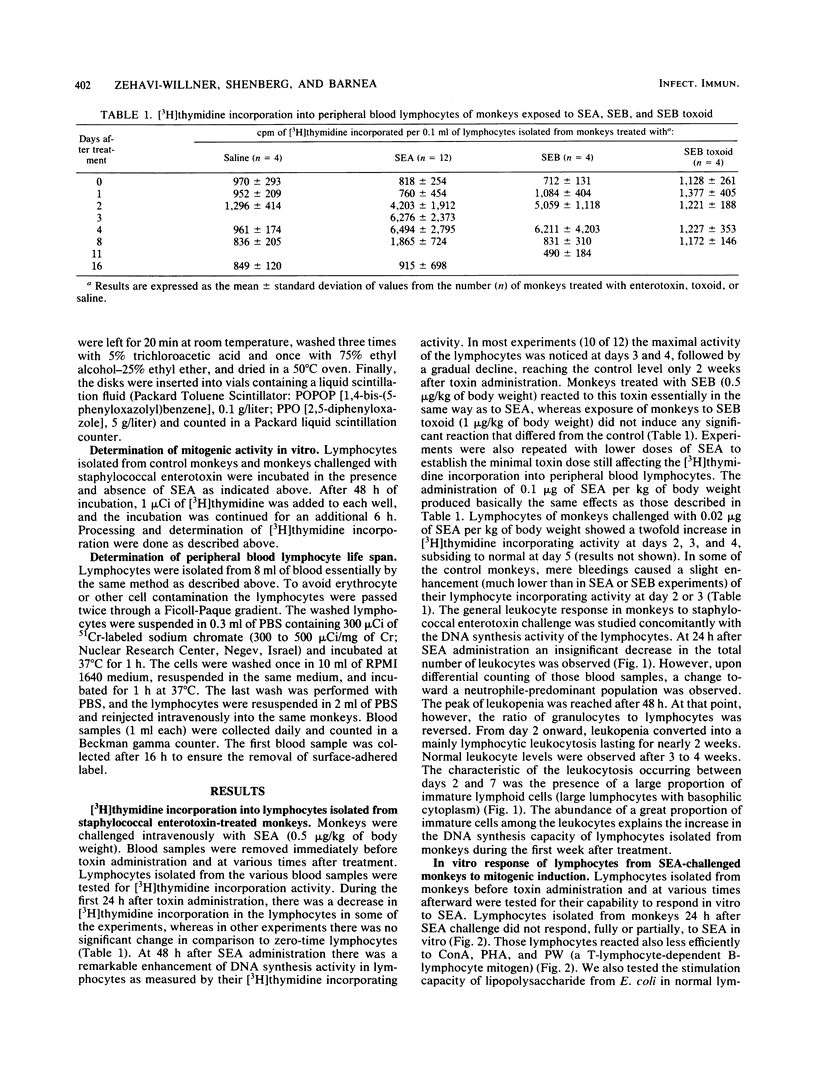
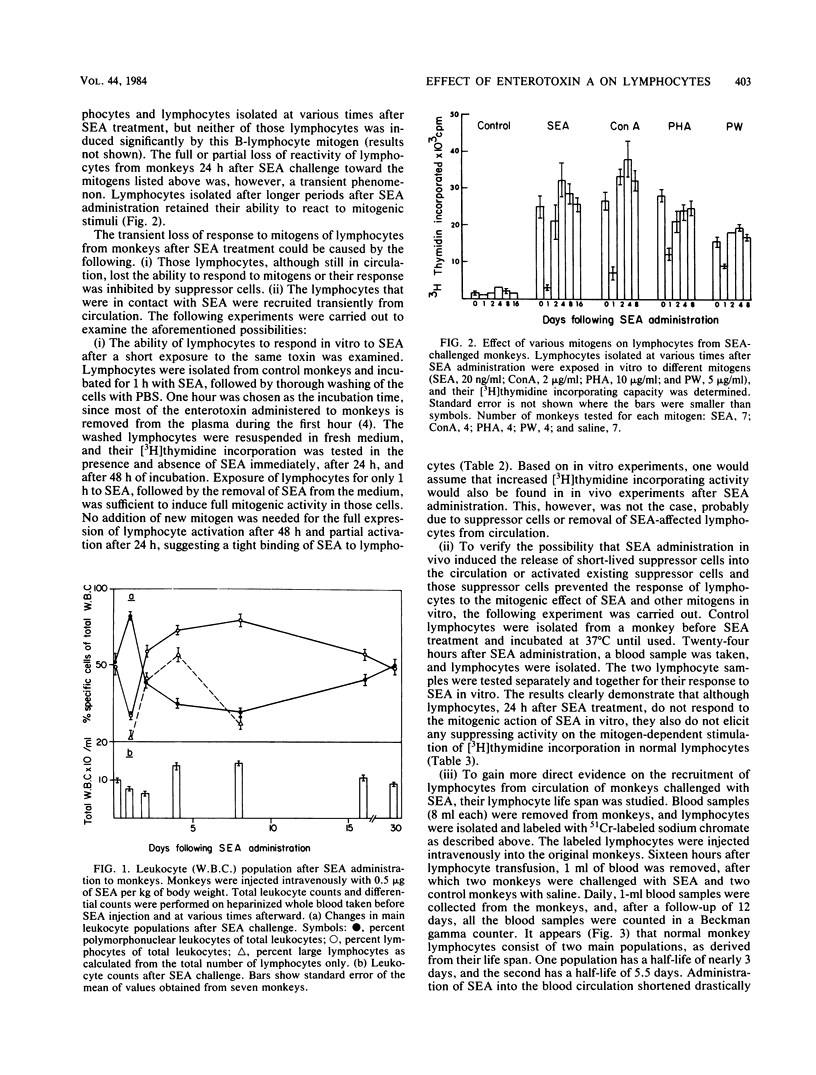
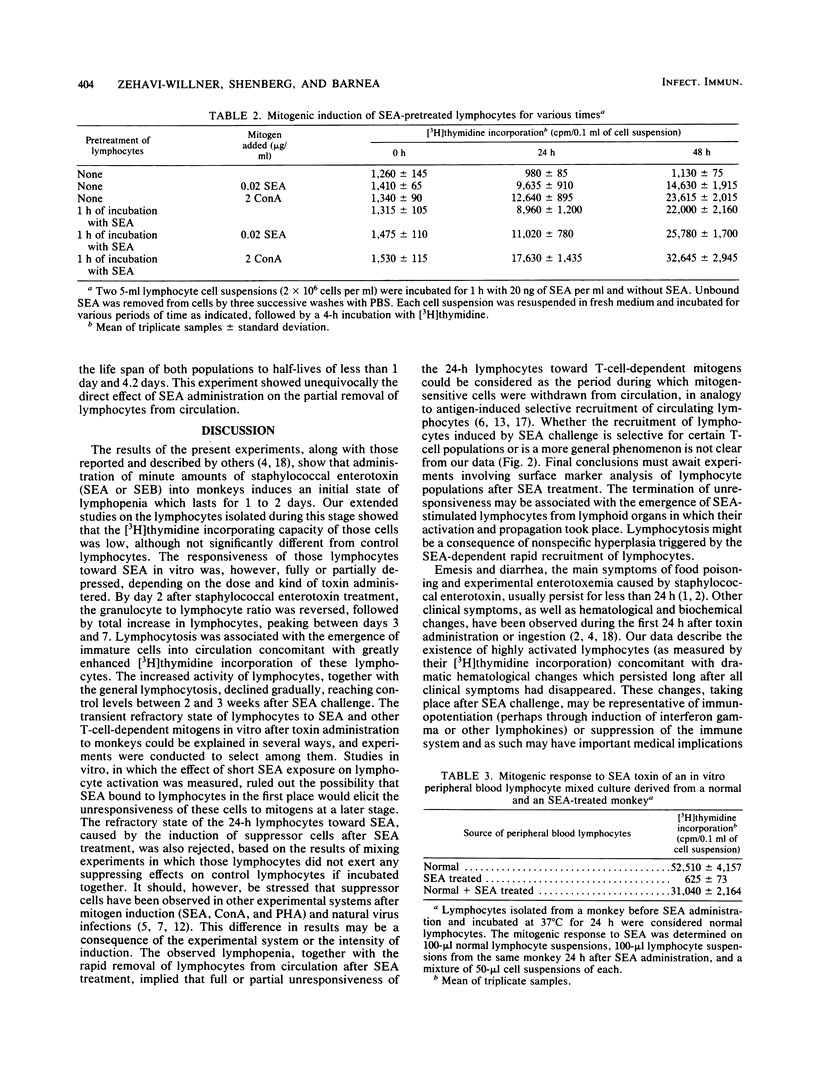
Staphylococcal enterotoxin A (SEA) administration to monkeys produced an initial lymphocytic leukopenia lasting approximately 24 h. Lymphocytes isolated from blood circulation (PBL) during this stage had normal or decreased [3H]thymidine incorporating activity. After 48 h, however, a significant increase (five- to sixfold) in [3H]thymidine incorporating activity into PBL was apparent. The peak of incorporating activity (seven- to eightfold) was reached 3 to 4 days after SEA administration, followed by a gradual decline, reaching the baseline after 2 weeks. The increased levels of [3H] thymidine incorporation in PBL were concomitant with the conversion of lymphopenia into lymphocytosis, accompanied by the release of many immature cells into the circulation. Lymphocytes isolated 24 h after SEA administration in vivo did not respond to the mitogenic action of SEA in vitro. Lymphocytes isolated at later stages after SEA challenge were fully activated by toxin. From a series of studies, it was concluded that SEA administered to monkeys caused, during the initial 24 h, the removal of a great proportion of lymphocytes from the circulation, followed by the release of new immature cells with augmented DNA synthesis activity. The lymphocytic leukocytosis state declined gradually and reached normal levels between 3 and 4 weeks after the SEA challenge. The biological implications of the hematological changes occurring after SEA challenge in vivo are discussed.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böyum A. Isolation of leucocytes from human blood. Further observations. Methylcellulose, dextran, and ficoll as erythrocyteaggregating agents. Scand J Clin Lab Invest Suppl. 1968;97:31–50. [PubMed] [Google Scholar]
- Crawley G. J., Gray I., Leblang W. A., Blanchard J. W. Blood binding, distribution and excretion of staphylococcal enterotoxin in monkeys. J Infect Dis. 1966 Feb;116(1):48–56. doi: 10.1093/infdis/116.1.48. [DOI] [PubMed] [Google Scholar]
- Egan H. S., Ekstedt R. D. Suppression of a thymus dependent hymoral response in mice by concanavalin A in vivo. Cell Immunol. 1975 Aug;18(2):365–374. doi: 10.1016/0008-8749(75)90065-9. [DOI] [PubMed] [Google Scholar]
- Geha R. S., Jonsen M. E., Ault B. H., Yunis E., Broff M. D. Macrophage T cell interaction in man: binding of antigen-specific human proliferating and helper T cells to antigen-pulsed macrophages. J Immunol. 1981 Feb;126(2):781–786. [PubMed] [Google Scholar]
- Johnson H. M., Stanton G. J., Baron S. Relative ability of mitogens to stimulate production of interferon by lymphoid cells and to induce suppression of the in vitro immune response. Proc Soc Exp Biol Med. 1977 Jan;154(1):138–141. [PubMed] [Google Scholar]
- Langford M. P., Stanton G. J., Johnson H. M. Biological effects of staphylococcal enterotoxin A on human peripheral lymphocytes. Infect Immun. 1978 Oct;22(1):62–68. doi: 10.1128/iai.22.1.62-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavy D. L., Adler W. H., Smith R. T. The mitogenic effects of endotoxin and staphylococcal enterotoxin B on mouse spleen cells and human peripheral lymphocytes. J Immunol. 1970 Dec;105(6):1453–1458. [PubMed] [Google Scholar]
- Pinto M., Torten M., Birnbaum S. C. Suppression of the in vivo humoral and cellular immune response by staphylococcal enterotoxin B (SEB). Transplantation. 1978 Jun;25(6):320–323. doi: 10.1097/00007890-197806000-00008. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., O'Brien C., Rosenthal P., Schlossman S. F. The cellular basis for viral-induced immunodeficiency: analysis by monoclonal antibodies. J Immunol. 1980 Sep;125(3):1269–1274. [PubMed] [Google Scholar]
- Rowley D. A., Gowans J. L., Atkins R. C., Ford W. L., Smith M. E. The specific selection of recirculating lymphocytes by antigen in normal and preimmunized rats. J Exp Med. 1972 Sep 1;136(3):499–513. doi: 10.1084/jem.136.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz E. J., Roessler W. G., Woodburn M. J., Lynch J. M., Jacoby H. M., Silverman S. J., Gorman J. C., Spero L. Purification and some chemical and physical properties of staphylococcal enterotoxin A. Biochemistry. 1972 Feb 1;11(3):360–366. doi: 10.1021/bi00753a009. [DOI] [PubMed] [Google Scholar]
- Silverman S. J., Espeseth D. A., Schantz E. J. Effect of formaldehyde on the immunochemical and biological activity of staphylococcal enterotoxin B. J Bacteriol. 1969 May;98(2):437–442. doi: 10.1128/jb.98.2.437-442.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Miller J. F., Mitchell G. F. Antigen-induced selective recruitment of circulating lymphocytes. Cell Immunol. 1971 Apr;2(2):171–181. doi: 10.1016/0008-8749(71)90036-0. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., McKissic E. M. Leukocytic response in monkeys challenged with staphylococcal enterotoxin. J Bacteriol. 1966 Aug;92(2):349–352. doi: 10.1128/jb.92.2.349-352.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelestam M., Möllby R., Wadström T. Effects of staphylococcal alpha-, beta-, delta-, and gamma-hemolysins on human diploid fibroblasts and HeLa cells: evaluation of a new quantitative as say for measuring cell damage. Infect Immun. 1973 Dec;8(6):938–946. doi: 10.1128/iai.8.6.938-946.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


