Late relapse (>5 years) of acute myeloid leukemia (AML) is rare.1 Detecting at the time of late relapse the same genetic alteration as at diagnosis strongly suggests it may play a critical role in leukemogenesis. We previously reported in this journal that in AML with mutated nucleophosmin (NPM1), NPM1 mutation is very stable at relapse.2 However, this conclusion was based upon molecular analysis of AML patients in whom the interval between diagnosis and relapse was short (median one year).2 Here, we describe for the first time the clinico-pathological and molecular features of a patient with NPM1-mutated, FLT3-ITD positive AML who, after eight years, relapsed with NPM1-mutated, FLT3-ITD negative AML. These findings provide the most compelling clinical evidence to date that NPM1 mutation is stable, strongly suggesting it is a founder genetic lesion and further supporting the view that AML with mutated NPM13 is a separate entity with distinct biological and clinical features.4,5
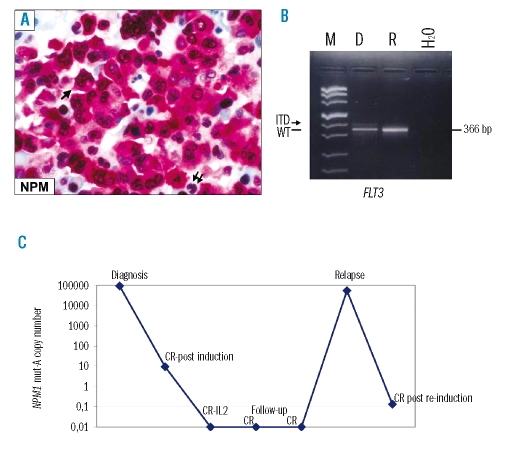
In May 2000, a 19-year old woman was diagnosed with acute myelo-monocytic leukemia (Online Supplementary Figures 1A) at the Institute of Hematology, “La Sapienza” University, Rome. The leukemic cells’ immunophenotype in cell suspension was: CD33+ (95%), myeloperoxidase-positive (55%), CD13+ (23%), CD14+ (25%), CD15+ (46%), CD117+ (1%), HLA-DR+ (1%), CD34+(0%). Results of immunohistochemistry are shown in the Online Supplementary Figures 1B and C. Immunohistochemical staining of bone marrow trephine revealed aberrant nucleophosmin expression in leukemic cell cytoplasm6 (Figure 1A), predicting NPM1 mutation. NPM1 gene sequencing showed a 4 base pair (TCTG) insertion after nucleotide 1018 corresponding to mutation A3. Molecular studies also revealed an internal tandem duplication of the FLT3 gene (FLT3-ITD) (Figure 1B). The number of NPM1 mutant copies assessed by quantitative PCR at diagnosis is shown in Figure 1C. Cytogenetic analysis showed a der(13;14) constitutional Robertsonian recombination associated with trisomy 4 in six metaphases and der(13;14) as the sole finding in five (data not shown).
Figure 1.
(A) NPM subcellular expression at diagnosis. Acute myeloid leukemia cells show aberrant cytoplasmic positivity for nucleophosmin (NPM) (arrow) whilst residual hemopoietic cells exhibit a nucleus-restricted positivity for NPM (double arrows). APAAP technique; × 800; hematoxylin counterstaining. (B) FLT3 gene status at diagnosis and relapse. The arrow shows the presence of a faint extra-band corresponding to the FLT3-ITD expressed at low level at diagnosis but not at relapse. The black line indicates the 366bp product corresponding to the amplification of FLT3 wild-type. M: marker; D: diagnosis; R: relapse; H2O=negative control, water line. C) Retrospective quantification of NPM1 mut-A by real time RT-PCR. Variation in NPM1 mut-A transcript level at diagnosis, complete remission (CR) and relapse is shown. The highest transcript copies number was detected at diagnosis and relapse, whereas the transcript copies number decreased following induction and became undetectable during follow-up. After reinduction therapy a reduction of NPM1 mut-A copies number was detected again. IL2 indicates interleukin-2.
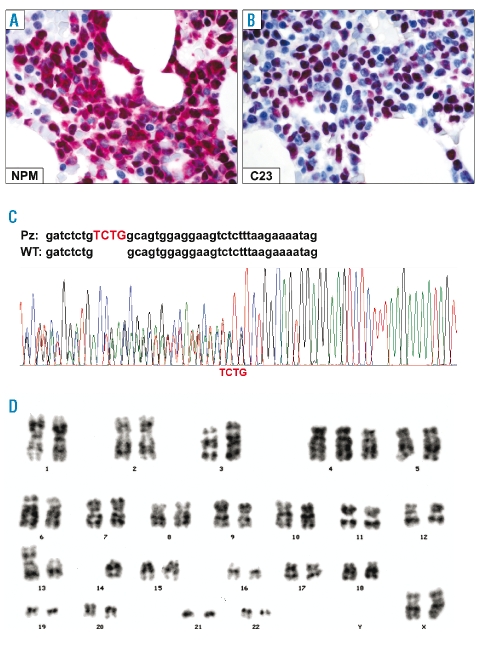
The patient was treated under the Gruppo Italiano Malattie EMatologiche dell’Adulto (GIMEMA)/EORTC AML12 protocol (Online Supplementary Appendix). Complete hematologic remission, first documented in June 2000, continued until May 2008 when the patient relapsed. Immunohistochemical and molecular studies confirmed nucleophosmin was dislocated into leukemic cell cytoplasm (C23/nucleolin was nucleus-restricted) (Figure 2A, B) and NPM1 mutation A was present (Figure 2C). FLT3 gene analysis revealed no ITD or D835 mutations (Figure 1B). Cytogenetic investigation again found a 46,XX, der(13;14) (q10;q10), +4 karyotype in 4/9 metaphases (Figure 2D). Rescue therapy was immediately started with ARA-C, idarubicin and mylotarg (Online Supplementary Appendix). At present (September 2008), the patient is in complete hematological remission.
Figure 2.
NPM status and cytogenetics at relapse. (A) Leukemic cells retain the aberrant cytoplasmic expression of nucleophosmin (NPM). (B) AML cells show the expected nucleus-restricted positivity for nucleolin/C23. A and B, immuno-alkaline phosphatase anti-alkaline phosphatase (APAAP) technique; × 800; hematoxylin counterstaining. (C) Electropherogram of NPM1 mutation A at relapse. (D) G-banded karyotype at relapse showing 46,XX, der(13;14) (q10;q10), +4.
Our results raise interesting questions about the significance and stability of NPM1 and FLT3 mutations in AML. We previously reported an NPMc+ (cytoplasmic-positive) myeloid sarcoma occurring 20 years after diagnosis of AML7, but no information was available on cytogenetics or NPM1 gene status of AML at presentation. In the present report, retention of NPM1 mutation-A and aberrant nucleophosmin expression in leukemic cell cytoplasm after eight years is a remarkable example of NPM1 mutation stability, which has otherwise been observed to date only in a NOD-SCID mouse model2, and strongly suggests the NPM1 mutation is needed for leukemia growth and survival. Interestingly, FLT3-ITD had disappeared at relapse, in keeping with evidence that FLT3-ITD is a secondary genetic alteration8 that is not stable over the course of disease.9,10
Clinical findings in this case of NPM1-mutated AML are also quite peculiar. Despite a concomitant FLT3-ITD, which usually antagonizes the favorable prognostic impact of the NPM1 mutation,4 the patient achieved a long-lasting complete hematologic remission that was associated with an undetectable number of NPM1 mutant A copies at quantitative PCR (Figure 1C). This may be because a low burden of leukemic cells carried FLT3-ITD at diagnosis (Figure 1B). On the contrary increasing FLT3-ITD mutant levels appear to associate with a highly significant trend for worsening relapse risk and overall survival.11 The reasons for the very late relapse are poorly understood but, as FLT3-ITD was absent at relapse, other underlying biological factors may have come into play such as, for example, trisomy 4 which was found at diagnosis and relapse.
Finally, the favorable leukemic genotype (NPM1-mutated/FLT3-ITD negative) at relapse raises the question of the most appropriate therapy. Should this young woman receive an allogeneic stem cell transplantation or should she be exempted since the AML relapse is very late and the genotype is favorable¿12 In weighing up the pros and cons, one realizes that the outcome of clinical decisions in patients such as this may help improve our understanding of the biology and clinical behavior of AML with mutated NPM1.
Acknowledgments
Supported by the Associazione Italiana per Ricerca sul Cancro.
References
- 1.Medeiros BC, Minden MD, Schuh AC, Schimmer AD, Yee K, Lipton JH, et al. Characteristics and outcomes of acute myelogenous leukemia patients with very late relapse (>5 years) Leuk Lymphoma. 2007;48:65–71. doi: 10.1080/10428190601043252. [DOI] [PubMed] [Google Scholar]
- 2.Falini B, Martelli MP, Mecucci C, Liso A, Bolli N, Bigerna B, et al. Cytoplasmic mutated nucleophosmin is stable in primary leukemic cells and in a xenotransplant model of NPMc+ acute myeloid leukemia in SCID mice. Haematologica. 2008;93:775–9. doi: 10.3324/haematol.12225. [DOI] [PubMed] [Google Scholar]
- 3.Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352:254–66. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 4.Falini B, Nicoletti I, Martelli MF, Mecucci C. Acute myeloid leukemia carrying cytoplasmic/mutated nucleophosmin (NPMc+ AML): biologic and clinical features. Blood. 2007;109:874–85. doi: 10.1182/blood-2006-07-012252. [DOI] [PubMed] [Google Scholar]
- 5.Garzon R, Garofalo M, Martelli MP, Briesewitz R, Wang L, Fernandez-Cymering C, et al. Distinctive microRNA signature of acute myeloid leukemia bearing cytoplasmic mutated nucleophosmin. Proc Natl Acad Sci USA. 2008;105:3945–50. doi: 10.1073/pnas.0800135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falini B, Martelli MP, Bolli N, Bonasso R, Ghia E, Pallotta MT, et al. Immunohistochemistry predicts nucleophosmin (NPM) mutations in acute myeloid leukemia. Blood. 2006;108:1999–2005. doi: 10.1182/blood-2006-03-007013. [DOI] [PubMed] [Google Scholar]
- 7.Bolli N, Galimberti S, Martelli MP, Tabarrini A, Roti G, Mecucci C, et al. Cytoplasmic nucleophosmin in myeloid sarcoma occurring 20 years after diagnosis of acute myeloid leukaemia. Lancet Oncol. 2006;7:350–2. doi: 10.1016/S1470-2045(06)70661-1. [DOI] [PubMed] [Google Scholar]
- 8.Liso A, Castiglione F, Cappuccio A, Stracci F, Schlenk RF, Amadori S, et al. A one-mutation mathematical model can explain the age incidence of acute myeloid leukemia with mutated nucleophosmin (NPM1) Haematologica. 2008;93:1219–26. doi: 10.3324/haematol.13209. [DOI] [PubMed] [Google Scholar]
- 9.Chou WC, Tang JL, Lin LI, Yao M, Tsay W, Chen CY, et al. Nucleophosmin mutations in de novo acute myeloid leukemia: the age-dependent incidences and the stability during disease evolution. Cancer Res. 2006;66:3310–6. doi: 10.1158/0008-5472.CAN-05-4316. [DOI] [PubMed] [Google Scholar]
- 10.Palmisano M, Grafone T, Ottaviani E, Testoni N, Baccarani M, Martinelli G. NPM1 mutations are more stable than FLT3 mutations during the course of disease in patients with acute myeloid leukemia. Haematologica. 2007;92:1268–9. doi: 10.3324/haematol.11202. [DOI] [PubMed] [Google Scholar]
- 11.Gale RE, Green C, Allen C, Mead AJ, Burnett AK, Hills RK, et al. The impact of FLT3 internal tandem duplication mutant level, number, size and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008;111:2776–84. doi: 10.1182/blood-2007-08-109090. [DOI] [PubMed] [Google Scholar]
- 12.Schlenk RF, Dohner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–18. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]