The findings of this study point to cytoplasmic nucleophosmin as a new marker for distinguishing between acute myeloid leukemia associated with NPM1 mutations and blastic plasmacytoid dendritic cell neoplasm.
Keywords: plasmacytoid dendritic cells, acute myeloid leukemia, NPM1, nucleophosmin, mutations, antibodies, immunohistochemistry
Abstract
Acute myeloid leukemia carrying cytoplasmic mutated nucleophosmin (NPMc+ AML) and blastic plasmacytoid dendritic cell neoplasm have been included as new entities in the 4th edition (2008) WHO classification of myeloid neoplasms. These conditions may show clinical and pathological overlapping features (leukemic and skin involvement, and expression of macrophage markers). In this study, we provide evidence that aberrant cytoplasmic dislocation of nucleophosmin – the immunohistochemical surrogate for NPM1 mutations – allows the two entities to be genetically separated. In fact, nucleophosmin is consistently cytoplasmic in NPMc+ AML (because of the presence of NPM1 mutations), whilst it is nucleus-restricted (predictive of a germline NPM1 gene) in blastic plasmacytoid dendritic cell neoplasm. Our results clearly point cytoplasmic nucleophosmin (a full predictor of NPM1 mutations) as a new marker for distinguishing NPMc+ AML and blastic plasmacytoid dendritic cell neoplasm, further clarify the cell of origin of NPMc+ AML, and justify the inclusion of these pathological conditions as separate entities in the new WHO classification.
Introduction
Acute myeloid leukemia (AML) carrying cytoplasmic mutated nucleophosmin (NPMc+ AML)1 accounts for about one-third of adult de novo AML and has distinctive biological and clinical features.2–4 For these reasons, it has been included as a new provisional entity (named AML with mutated NPM1) in the new WHO classification of myeloid neoplasms.
Among the most compelling evidence that AML with mutated NPM1 represents an entity is that NPM1 mutation – or its immunohistochemical surrogate, cytoplasmic nucleophosmin5 – is specific for AML6 (usually of de novo origin), is very stable during the course of the disease,7 is mutually exclusive of AML carrying recurrent genetic abnormalities8 and associates with distinct gene expression9 and microRNA profiles.10 However, no investigation has, as yet, been carried out into the relationship between NPMc+ AML and the blastic plasmacytoid dendritic cell (BPDC) neoplasm11 (previously known as blastic NK-cell lymphoma or agranular CD4+/CD56+ hematodermic neoplasm), that has been also introduced as a distinct entity in the new WHO classification.11
Distinction between these two pathological conditions is important since they may share some clinical and pathological features (e.g. high frequency of cutaneous and leukemic dissemination, expression of the macrophage-restricted CD68 molecule and CD34-negativity).11 On the other hand, since BPDC is generally associated with genetic abnormalities and dismal clinical evolution, the study of nucleophosmin status in BPDC could be of interest in the process of designation of AML with mutated NPM1 as a distinct clinico-pathological entity.
In this study, we provide evidence that the immunohistochemical study of subcellular distribution of nucleophosmin allows the two entities to be genetically separated. In fact, nucleophosmin is cytoplasmic in NPMc+ AML (because of the presence of NPM1 mutations) but nucleus-restricted in BPDC neoplasm (because of a germline NPM1 gene). Our results, have important diagnostic implications, further clarify the cell of origin of NPMc+ AML, and justify the inclusion of AML with mutated NPM1 and BPDC neoplasm as separate entities in the new WHO classification.
Design and Methods
Pathological samples
The aim of this study was to investigate the presence of NPM1 mutations in a wide spectrum of PDC proliferations. The following pathological samples were studied: n=13 typical BPDC neoplasms, whose phenotypic features are summarized in Table 1; reactive lymph nodes (n=16), myelodysplastic syndromes (n=14), AML (n=9) and myeloid sarcoma (n=5) harboring nodules of mature PDCs. Since no fresh material for molecular analysis was available from these cases, we used immunohistochemistry to detect aberrant cytoplasmic expression of nucleophosmin that is known to be fully predictive of NPM1 mutations.5
Table 1.
Phenotypic features of 13 BPDC neoplasms.
Immunohistochemical studies
Paraffin sections from all pathological samples were subjected to antigen retrieval and stained with antibodies directed against fixative-resistant epitopes of the protein nucleophosmin1 and various PDC-associated markers. Nucleophosmin was detected using the monoclonal antibody (mAb) anti-NPM (clone 376)1; PDCs were identified using mAbs anti-CD4 (clone OPD4; DakoCytomation), anti-CD56 (clone 1B6; NovoCastra), anti-CD123 (clone 7G3; BD Pharmingen), anti-TCL1 (clone TCL1A; Upstate), and anti-CLA (clone HECA-452; BD Pharmingen). Four cases were immunostained with an antibody directed against the new PDC marker CD2AP (kindly provided by Dr. Teresa Marafioti, University of Oxford). All samples were also investigated for expression of C23/nucleolin (mAb anti-C23, clone MS-3; Santa Cruz Bio-technology), the CD34 (mAb antibody anti-CD34, clone Qbend/10; DakoCytomation) and CD68 macrophage-restricted molecule (mAb PG-M1 generated in B. Falini’s laboratory).
The antibody-antigen reaction was revealed by immunoalkaline phosphatase (APAAP) or immunoperoxidase, according to standard procedures.1
Results and Discussion
All 13 BPDC neoplasms expressed three or more of the PDC-associated molecules CD4, CD56, CLA, CD123, TCL1 (Table 1; Figures 1 and 2), including the recently described PDC marker CD2AP12 that was investigated in 4 cases (data not shown); all 10 samples investigated were CD34-negative (Table 1). Notably, tumor cells from all specimens showed nucleus-restricted expression of nucleophosmin (Figures 1 and 2) that was the same as C23/nucleolin and was fully predictive of NPM1 gene in a germline configuration. Interestingly, the nucleus-restricted positivity for nucleophosmin was also observed in paraffin sections from bone marrow biopsies of 14 patients with leukemias of ambiguous lineage (Online Supplementary Figure 1S) as defined by WHO, i.e. pathological conditions which share some but not all immunophenotypic features of BPDC neoplasm.11 The nucleophosmin staining pattern in BPDC neoplasm and leukemias of ambiguous lineages clearly differed from the aberrant cytoplasmic NPM positivity found in 10 cases of AML with mutated NPM1 (data not shown) that were used as controls for the immunohistochemical procedures.
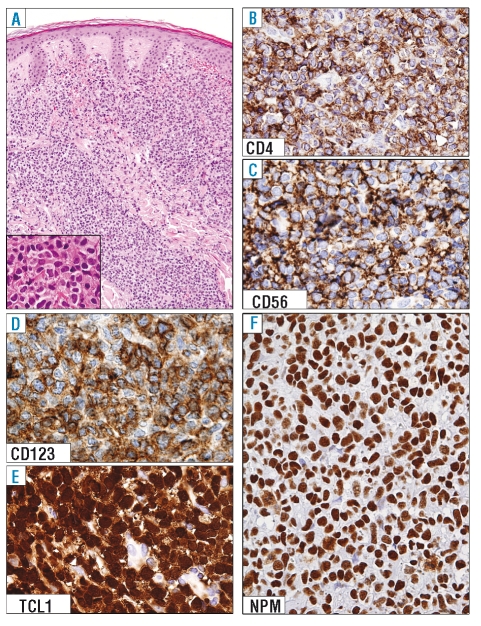
Figure 1.
Expression pattern of nucleophosmin (NPM) in BPDC neoplasm (skin involvement). (A) Dense dermal infiltrate by immature tumor cells, not involving the epidermis (skin biopsy; hematoxylin-eosin; ×200; inset, ×500). (B–E) Leukemic cells express the classical BPDC neoplasm phenotype, that includes CD4, CD56, CD123, and TCL1. (F) Leukemic cells show a nucleus-restricted positivity for nucleophosmin, which is indicative of wild-type NPM1 gene. (B–F): paraffin sections from skin biopsy immunostained with anti-CD4, anti-CD56, anti-CD123, anti-TCL1 and anti-nucleophosmin (monoclonal antibody, clone 376). Immunoperoxidase procedure, hematoxylin counterstain; × 800.
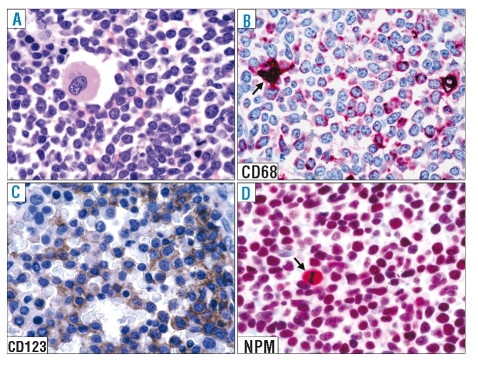
Figure 2.
Expression pattern of nucleophosmin (NPM) in BPDC neoplasm (bone marrow involvement). (A) Diffuse marrow infiltration by blast cells. A residual megakaryocyte is present (bone marrow biopsy; hematoxylineosin; ×800). (B) Leukemic cells express the macrophage-restricted form of the CD68 antigen with a dot-like pattern; the arrow indicates a histiocyte. (C) Most leukemic cells are strongly positive for the CD123 molecule. (D) Leukemic cells show a nucleus-restricted positivity for nucleophosmin which is indicative of wild-type NPM1 gene. A mitotic figure (arrow) shows the expected nucleophosmin cytoplasmic positivity. (B–D) Paraffin sections from bone marrow trephines immunostained with monoclonal antibodies against CD68 (PG-M1), CD123, and nucleophosmin (clone 376). (B) and (D), immunophosphatase alkaline (APAAP) technique; hematoxylin counterstain; ×800; (C): immunoperoxidase procedure hematoxylin counterstain; ×800.
Similarly to tumor cells of BPDC neoplasm, nodules of mature PDCs in reactive lymph nodes (n=16), myelodysplastic syndromes (n=14), AML (n=9) and myeloid sarcoma (n=5) showed nucleus-restricted positivity for NPM (data not shown). Interestingly, a variable percentage of mature PDCs was negative for nucleophosmin, which is consistent with previous findings that NPM1 is down-regulated during differentiation (e.g. NPM protein levels are much lower in normoblasts or neutrophils than in the more immature erythroid or myeloid precursors).2
Our immunohistological results clearly indicate that cytoplasmic expression of nucleophosmin (the surrogate marker for NPM1 mutation)5 is mutually exclusive of BPDC neoplasm and other PDC proliferations. These findings concur with reports that 10–20% of BPDC neoplasms are associated with, or develop into AML, which can evolve from underlying myelodysplasia,11 and that PDC nodules are not infrequently present in myelodysplastic syndromes. NPM1 mutation, on the contrary, appears to be characteristic of de novo AML13 and mutually exclusive of myelodysplastic syndromes.14 Cytogenetically, the two conditions are also quite different. Most cases of NPMc+ AML are associated with normal karyotype,1 while about two-thirds of BPDC neoplasms have an abnormal karyotype,11 which is usually characterized by complex, albeit not specific, chromosome aberrations. Mutual exclusion of NPM1 mutation and BPDC neoplasm may reflect not only different underlying genetic backgrounds but also diverse cells of origin. BPDC is thought to derive from the precursors of plasmacytoid dendritic cells, a specialized subset of dendritic cells that are also known as professional type-1 interferon producing cells or plasmacytoid monocytes.15 A multilineage potential in some cases of BPDC neoplasm is suggested by immunophenotypic heterogeneity with regards to TdT, association with myeloid disorders and expression of lymphoid markers (such as CD2, CD5 and, more rarely, CD3).11 Although multilineage involvement is also a feature of NPMc+ AML,16 it is restricted to the compartment of myeloid cells since NPM1 mutations do not target lymphoid cells.17 Our results suggest that NPM1 mutations may also spare the yet poorly understood pathways leading to PDC generation in bone marrow.18 The results presented herein also have implications in the differential diagnosis between BPDC neoplasm and myelomonocytic (M4) or monoblastic/monocytic (M5) leukemia.19 This may represent a major diagnostic dilemma, because all these pathological conditions, in addition to bone marrow infiltration, may show skin or lymph node involvement, negativity for CD34 and expression of the macrophage-restricted CD68 molecule. Immunohistochemical detection in paraffin sections of several PDC-associated molecules, including CD123 (interleukin-3 α-chain receptor), TCL1, CD2AP,12 CD56 and CD4 is a valid diagnostic assay. However, none of these markers is specific for BPDC neoplasm, as AML cells may express TCL1, or CD123, as well as CD56, associated or not with CD4. Immunostaining for nucleophosmin can be of help in distinguishing AML or myeloid sarcoma with mutated NPM1, especially the subset with MPO−/CD4+/CD56+ phenotype, which is characterized by aberrant cytoplasmic NPM,20 from the BPDC neoplasm which shows nucleus-restricted NPM positivity. The distinction between BPDC neoplasm and NPMc+ AML is clinically elevant since BPDC is usually very aggressive,11 with a median survival of 12–14 months, whilst NPMc+ AML, in the absence of a concomitant internal tandem duplication of FLT3 (FLT3 -ITD), has a favorable prognosis.2 In conclusion, our results point to subcellular nucleophosmin expression as another marker to be added to the battery of morphological, immunohistochemical and cytogenetic studies which are required in the differential diagnosis between BPDC neoplasm and AML. Anti-NPM immunohistochemistry appears to be particularly useful since, given the rarity of BPDC neoplasm and the small size of skin biopsies (the most frequently involved site), fresh material for cytogenetic and molecular studies more often is not available. Moreover, our results provide additional information on the NPMc+ AML cell of origin and justify the inclusion of AML with mutated NPM1 and BPDC neoplasm as separate entities in the new WHO classification of myeloid neoplasms.11,21
Footnotes
Authorship and Disclosures
BF had the original idea of the study and wrote the manuscript. FF and SAP performed immunohistochemical studies and helped write the manuscript. CA, MPM, MP, AV and MFM provided and analyzed samples.
The authors reported no potential conflicts of interest.
The online version of this paper contains a supplementary appendix. Funding: this work was supported by the Associazione Italiana per la Ricerca sul Cancro (A.I.R.C.) (to MFM and BF) and Fondazione Berlucchi (to FF). Acknowledgments: we thank Roberta Pacini, Manola Carini, Simona Righi, and Wilma Pellegrini for performing the immunohistochemical stainings, Mrs. Claudia Tibidò for the secretarial assistance and Dr G A Boyd for English language editing.
References
- 1.Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352:254–66. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 2.Falini B, Nicoletti I, Martelli MF, Mecucci C. Acute myeloid leukemia carrying cytoplasmic/mutated nucleophosmin (NPMc+ AML): biologic and clinical features. Blood. 2007;109:874–85. doi: 10.1182/blood-2006-07-012252. [DOI] [PubMed] [Google Scholar]
- 3.Falini B, Nicoletti I, Bolli N, Martelli MP, Liso A, Gorello P, et al. Translocations and mutations involving the nucleophosmin (NPM1) gene in lymphomas and leukemias. Haematologica. 2007;92:519–32. doi: 10.3324/haematol.11007. [DOI] [PubMed] [Google Scholar]
- 4.Liso A, Castiglione F, Cappuccio A, Stracci F, Schlenk RF, Amadori S, et al. A one-mutation mathematical model can explain the age incidence of acute myeloid leukemia with mutated nucleophosmin (NPM1) Haematologica. 2008;93:1219–26. doi: 10.3324/haematol.13209. [DOI] [PubMed] [Google Scholar]
- 5.Falini B, Martelli MP, Bolli N, Bonasso R, Ghia E, Pallotta MT, et al. Immunohistochemistry predicts nucleophosmin (NPM) mutations in acute myeloid leukemia. Blood. 2006;108:1999–2005. doi: 10.1182/blood-2006-03-007013. [DOI] [PubMed] [Google Scholar]
- 6.Liso A, Bogliolo A, Freschi V, Martelli MP, Pileri SA, Santodirocco M, et al. In human genome, generation of a nuclear export signal through duplication appears unique to nucleophosmin (NPM1) mutations and is restricted to AML. Leukemia. 2008;22:1285–9. doi: 10.1038/sj.leu.2405045. [DOI] [PubMed] [Google Scholar]
- 7.Falini B, Martelli MP, Mecucci C, Liso A, Bolli N, Bigerna B, et al. Cytoplasmic mutated nucleophosmin is stable in primary leukemic cells and in a xenotransplant model of NPMc+ acute myeloid leukemia in SCID mice. Haematologica. 2008;93:775–9. doi: 10.3324/haematol.12225. [DOI] [PubMed] [Google Scholar]
- 8.Falini B, Mecucci C, Saglio G, Lo Coco F, Diverio D, Brown P, et al. NPM1 mutations and cytoplasmic nucleophosmin are mutually exclusive of recurrent genetic abnormalities: a comparative analysis of 2562 patients with acute myeloid leukemia. Haematologica. 2008;93:439–42. doi: 10.3324/haematol.12153. [DOI] [PubMed] [Google Scholar]
- 9.Alcalay M, Tiacci E, Bergomas R, Bigerna B, Venturini E, Minardi SP, et al. Acute myeloid leukemia bearing cytoplasmic nucleophosmin (NPMc+ AML) shows a distinct gene expression profile characterized by up-regulation of genes involved in stem-cell maintenance. Blood. 2005;106:899–902. doi: 10.1182/blood-2005-02-0560. [DOI] [PubMed] [Google Scholar]
- 10.Garzon R, Garofalo M, Martelli MP, Briesewitz R, Wang L, Fernandez-Cymering C, et al. Distinctive microRNA signature of acute myeloid leukemia bearing cytoplasmic mutated nucleophosmin. Proc Natl Acad Sci USA. 2008;105:3945–50. doi: 10.1073/pnas.0800135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Facchetti F, Jones D, Petrella T. Blastic plasmacytoid dendritic cell neoplasm. In: Swerdlow SH, et al., editors. WHO Classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC press; 2008. pp. 145–7. [Google Scholar]
- 12.Marafioti T, Paterson JC, Ballabio E, Reichard KK, Tedoldi S, Hollowood K, et al. Novel markers of normal and neoplastic human plasmacytoid dendritic cells. Blood. 2008;111:3778–92. doi: 10.1182/blood-2007-10-117531. [DOI] [PubMed] [Google Scholar]
- 13.Pasqualucci L, Li S, Meloni G, Schnittger S, Gattenlohner S, Liso A, et al. NPM1-mutated acute myeloid leukaemia occurring in JAK2-V617F+ primary myelofibrosis: de-novo origin¿. Leukemia. 2008;22:1459–63. doi: 10.1038/sj.leu.2405093. [DOI] [PubMed] [Google Scholar]
- 14.Shiseki M, Kitagawa Y, Wang YH, Yoshinaga K, Kondo T, Kuroiwa H, et al. Lack of nucleophosmin mutation in patients with myelodysplastic syndrome and acute myeloid leukemia with chromosome 5 abnormalities. Leuk Lymphoma. 2007;48:2141–4. doi: 10.1080/10428190701615900. [DOI] [PubMed] [Google Scholar]
- 15.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–23. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 16.Pasqualucci L, Liso A, Martelli MP, Bolli N, Pacini R, Tabarrini A, et al. Mutated nucleophosmin detects clonal multilineage involvement in acute myeloid leukemia: Impact on WHO classification. Blood. 2006;108:4146–55. doi: 10.1182/blood-2006-06-026716. [DOI] [PubMed] [Google Scholar]
- 17.Martelli MP, Manes N, Pettirossi V, Liso A, Pacini R, Mannucci R, et al. Absence of nucleophosmin leukaemic mutants in B and T cells from AML with NPM1 mutations: implications for the cell of origin of NPMc+ AML. Leukemia. 2008;22:195–8. doi: 10.1038/sj.leu.2404857. [DOI] [PubMed] [Google Scholar]
- 18.Comeau MR, Van der Vuurst de Vries AR, Maliszewski CR, Galibert L. CD123bright plasmacytoid pre-dendritic cells: progenitors undergoing cell fate conversion¿. J Immunol. 2002;169:75–83. doi: 10.4049/jimmunol.169.1.75. [DOI] [PubMed] [Google Scholar]
- 19.Dijkman R, van Doorn R, Szuhai K, Willemze R, Vermeer MH, Tensen CP. Gene-expression profiling and array-based CGH classify CD4+CD56+ hematodermic neoplasm and cutaneous myelomonocytic leukemia as distinct disease entities. Blood. 2007;109:1720–7. doi: 10.1182/blood-2006-04-018143. [DOI] [PubMed] [Google Scholar]
- 20.Falini B, Lenze D, Hasserjian R, Coupland S, Jaehne D, Soupir C, et al. Cytoplasmic mutated nucleophosmin (NPM) defines the molecular status of a significant fraction of myeloid sarcomas. Leukemia. 2007;21:1566–70. doi: 10.1038/sj.leu.2404699. [DOI] [PubMed] [Google Scholar]
- 21.Arber DA, Brunning RD, Le Beau MM, Falini B, Vardiman JW, Porwit A, et al. Acute myeloid leukemia with recurrent genetic abnormalities. In: Swerdlow SH, et al., editors. WHO Classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC press; 2008. pp. 110–23. [Google Scholar]