Abstract
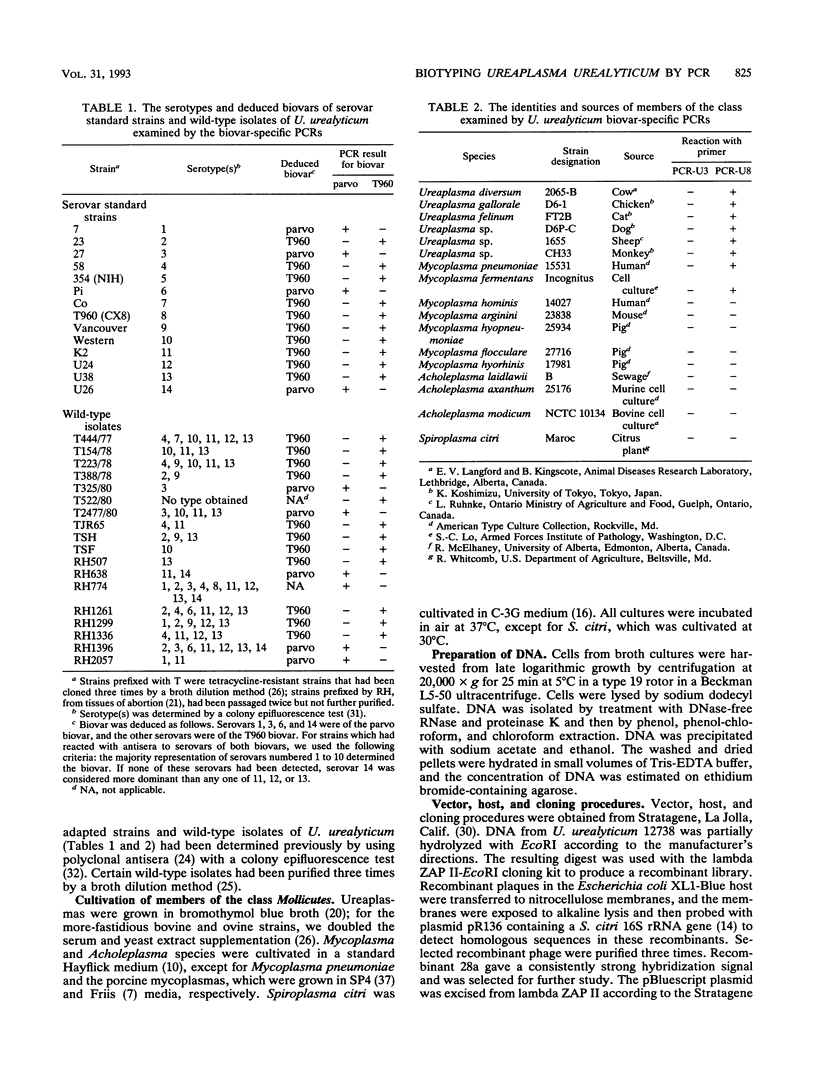
Several fundamental phenotypic and genotypic differences have separated strains of the genital mycoplasma Ureaplasma urealyticum into two clusters or biovars. However, the lack of an easily performed and unambiguous test to discriminate between them has hampered investigation of the relationship between these biovars and disease. We determined the 16S rRNA nucleotide sequence of U. urealyticum 27, the serovar 3 standard and representative of the parvo biovar (serovars 1, 3, 6, and 14). This sequence was compared with the published sequence of U. urealyticum T960, which is the type strain and the serovar 8 standard and is representative of the T960 biovar which is composed of the 10 intervening serovars. Homology between the two sequences was 98.8%; differences were exploited to provide primers for biovar-specific polymerase chain reactions (PCRs). The results of these reactions placed all 14 serovar standard strains into the correct biovar. The PCRs were also applied to 10 cloned and 8 noncloned isolates that had been serotyped earlier. For 16 of them, we deduced their biovars from the serotyping data and then confirmed them by PCR. One unpredictable isolate and one nonserotypeable isolate were also classified as to biovar. Thus, we have developed a method for biotyping U. urealyticum that is applicable to both laboratory-adapted strains and wild-type isolates and that is appropriate for testing large numbers of clinical isolates. The amplification by the T960 biovar PCR protocol of DNAs from ureaplasmas of animals and certain Mycoplasma species suggested that the parvo biovar has diverged from the mainstream of the evolution of this clade.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crăcea E., Botez D., Constantinescu S., Georgescu-Brăila M. Ureaplasma urealyticum serotypes isolated from cases of female sterility. Zentralbl Bakteriol Mikrobiol Hyg A. 1982 Sep;252(4):535–539. [PubMed] [Google Scholar]
- Deng S., Hiruki C., Robertson J. A., Stemke G. W. Detection by PCR and differentiation by restriction fragment length polymorphism of Acholeplasma, Spiroplasma, Mycoplasma, and Ureaplasma, based upon 16S rRNA genes. PCR Methods Appl. 1992 Feb;1(3):202–204. doi: 10.1101/gr.1.3.202. [DOI] [PubMed] [Google Scholar]
- Ford D. K. Relationships between mycoplasma and the etiology of nongonococcal urethritis and Reiter's syndrome. Ann N Y Acad Sci. 1967 Jul 28;143(1):501–504. doi: 10.1111/j.1749-6632.1967.tb27694.x. [DOI] [PubMed] [Google Scholar]
- Friis N. F. Some recommendations concerning primary isolation of Mycoplasma suipneumoniae and Mycoplasma flocculare a survey. Nord Vet Med. 1975 Jun;27(6):337–339. [PubMed] [Google Scholar]
- Harasawa R., Imada Y., Ito M., Koshimizu K., Cassell G. H., Barile M. F. Ureaplasma felinum sp. nov. and Ureaplasma cati sp. nov. isolated from the oral cavities of cats. Int J Syst Bacteriol. 1990 Jan;40(1):45–51. doi: 10.1099/00207713-40-1-45. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Kakulphimp J., Finch L. R., Robertson J. A. Genome sizes of mammalian and avian Ureaplasmas. Int J Syst Bacteriol. 1991 Apr;41(2):326–327. doi: 10.1099/00207713-41-2-326. [DOI] [PubMed] [Google Scholar]
- Lane D. J., Pace B., Olsen G. J., Stahl D. A., Sogin M. L., Pace N. R. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6955–6959. doi: 10.1073/pnas.82.20.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouches C., Taylor-Robinson D., Stipkovits L., Bove J. M. Comparison of human and animal Ureaplasmas by one- and two-dimensional protein analysis on polyacrylamide slab gel. Ann Microbiol (Paris) 1981 Sep-Oct;132B(2):171–196. [PubMed] [Google Scholar]
- Naessens A., Foulon W., Breynaert J., Lauwers S. Serotypes of Ureaplasma urealyticum isolated from normal pregnant women and patients with pregnancy complications. J Clin Microbiol. 1988 Feb;26(2):319–322. doi: 10.1128/jcm.26.2.319-322.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. A. Bromothymol blue broth: improved medium for detection of Ureaplasma urealyticum (T-strain mycoplasma). J Clin Microbiol. 1978 Feb;7(2):127–132. doi: 10.1128/jcm.7.2.127-132.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. A., Chen M. H. Effects of manganese on the growth and morphology of Ureaplasma urealyticum. J Clin Microbiol. 1984 Jun;19(6):857–864. doi: 10.1128/jcm.19.6.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. A., Honore L. H., Stemke G. W. Serotypes of Ureaplasma urealyticum in spontaneous abortion. Pediatr Infect Dis. 1986 Nov-Dec;5(6 Suppl):S270–S272. doi: 10.1097/00006454-198611010-00014. [DOI] [PubMed] [Google Scholar]
- Robertson J. A., Pyle L. E., Stemke G. W., Finch L. R. Human ureaplasmas show diverse genome sizes by pulsed-field electrophoresis. Nucleic Acids Res. 1990 Mar 25;18(6):1451–1455. doi: 10.1093/nar/18.6.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. A., Stemke G. W., Maclellan S. G., Taylor D. E. Characterization of tetracycline-resistant strains of Ureaplasma urealyticum. J Antimicrob Chemother. 1988 Mar;21(3):319–332. doi: 10.1093/jac/21.3.319. [DOI] [PubMed] [Google Scholar]
- Robertson J. A., Stemke G. W. Modified metabolic inhibition test for serotyping strains of Ureaplasma urealyticum (T-strain Mycoplasma). J Clin Microbiol. 1979 Jun;9(6):673–676. doi: 10.1128/jcm.9.6.673-676.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard M. C., Howard D. R. Identification of "T" mycoplasmas in primary agar cultures by means of a direct test for urease. Ann N Y Acad Sci. 1970 Oct 30;174(2):809–819. doi: 10.1111/j.1749-6632.1970.tb45598.x. [DOI] [PubMed] [Google Scholar]
- Shepard M. C., Lunceford C. D. Serological typing of Ureaplasma urealyticum isolates from urethritis patients by an agar growth inhibition method. J Clin Microbiol. 1978 Nov;8(5):566–574. doi: 10.1128/jcm.8.5.566-574.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemke G. W., Laigret F., Grau O., Bové J. M. Phylogenetic relationships of three porcine mycoplasmas, Mycoplasma hyopneumoniae, Mycoplasma flocculare, and Mycoplasma hyorhinis, and complete 16S rRNA sequence of M. flocculare. Int J Syst Bacteriol. 1992 Apr;42(2):220–225. doi: 10.1099/00207713-42-2-220. [DOI] [PubMed] [Google Scholar]
- Stemke G. W., Robertson J. A. Modified colony indirect epifluorescence test for serotyping Ureaplasma urealyticum and an adaptation to detect common antigenic specificity. J Clin Microbiol. 1981 Nov;14(5):582–584. doi: 10.1128/jcm.14.5.582-584.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemke G. W., Robertson J. A. Problems associated with serotyping strains of Ureaplasma urealyticum. Diagn Microbiol Infect Dis. 1985 Jul;3(4):311–320. doi: 10.1016/0732-8893(85)90005-7. [DOI] [PubMed] [Google Scholar]
- Stemke G. W., Stemler M. E., Robertson J. A. Growth characteristics of ureaplasmas from animal and human sources. Isr J Med Sci. 1984 Oct;20(10):935–937. [PubMed] [Google Scholar]
- Stemler M. E., Stemke G. W., Robertson J. A. ATP measurements obtained by luminometry provide rapid estimation of Ureaplasma urealyticum growth. J Clin Microbiol. 1987 Feb;25(2):427–429. doi: 10.1128/jcm.25.2.427-429.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully J. G., Whitcomb R. F., Clark H. F., Williamson D. L. Pathogenic mycoplasmas: cultivation and vertebrate pathogenicity of a new spiroplasma. Science. 1977 Mar 4;195(4281):892–894. doi: 10.1126/science.841314. [DOI] [PubMed] [Google Scholar]
- Weisburg W. G., Tully J. G., Rose D. L., Petzel J. P., Oyaizu H., Yang D., Mandelco L., Sechrest J., Lawrence T. G., Van Etten J. A phylogenetic analysis of the mycoplasmas: basis for their classification. J Bacteriol. 1989 Dec;171(12):6455–6467. doi: 10.1128/jb.171.12.6455-6467.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]