Abstract
The orosensory responses elicited by nicotine are relevant for the development and maintenance of addiction to tobacco products. However, although nicotine is described as bitter tasting, the molecular and neural substrates encoding the taste of nicotine are unclear. Here, rats and mice were used to determine whether nicotine activates peripheral and central taste pathways via TRPM5-dependent mechanisms, which are essential for responses to other bitter tastants such as quinine, and/or via nicotinic acetylcholine receptors (nAChRs). When compared with wild-type mice, Trpm5−/− mice had reduced, but not abolished, chorda tympani (CT) responses to nicotine. In both genotypes, lingual application of mecamylamine, a nAChR-antagonist, inhibited CT nerve responses to nicotine and reduced behavioral responses of aversion to this stimulus. In accordance with these findings, rats were shown to discriminate between nicotine and quinine presented at intensity-paired concentrations. Moreover, rat gustatory cortex (GC) neural ensemble activity could also discriminate between these two bitter tastants. Mecamylamine reduced both behavioral and GC neural discrimination between nicotine and quinine. In summary, nicotine elicits taste responses through peripheral TRPM5-dependent pathways, common to other bitter tastants, and nAChR-dependent and TRPM5-independent pathways, thus creating a unique sensory representation that contributes to the sensory experience of tobacco products.
Keywords: chorda tympani, gustatory cortex, neurophysiology, preference, discrimination
Nicotine is the main addictive component of tobacco products, mainly because of its effects on the central nervous system (CNS) (1). However, the peripheral sensory impact of inhaled nicotine is also relevant in the regulation of cigarette smoking (2, 3). Nicotine activates multiple sensory systems (4), including gustatory pathways (5), and is described as bitter tasting (6). Although oral somatosensory responses to nicotine occur only at relatively high concentrations (7), taste responses (5, 6) have been described at nicotine concentrations close to those found in the saliva of smokers (8). Several studies suggest that taste is relevant in the context of cigarette smoking. In fact, the ability to sense the bitter taste of phenylthiocarbamide, a trait that is genetically determined by polymorphisms of a taste receptor gene (T2R38) (9), protects from the development of addiction to cigarette smoking (10) and reduces the positive reinforcement from smoking (11). Finally, lesions of the insular cortex that include the primary sensory gustatory cortex (GC), promote smoking cessation (12).
Subpopulations of taste receptor cells (TRCs) express T2R taste receptors that have diverse chemical selectivity for bitter tastants (9) and activate the TRC via a common intracellular signaling pathway (13). Nicotine and other bitter tastants have been shown to activate transducin in vitro (14) but the participation of this taste-related G protein for bitter taste recognition in vivo has not been demonstrated (15). Additionally, a variety of results have been obtained from central and peripheral gustatory neurons regarding whether nicotine can be discriminated from other bitter tastants (16–18). Thus, it is unclear whether taste responses to nicotine depend on pathways common to other bitter tastants. In particular, it is unknown if gustatory responses to nicotine depend on TRPM5, a member of the transient receptor potential (TRP) superfamily of channels (19). TRPM5 is expressed in TRCs (20) and is required for peripheral transduction of bitter, sweet, and umami tastants (13). Furthermore, this channel has been shown to participate in chemosensory detection of nicotine in the nasal cavity (21). Previous work has also suggested the participation of nicotinic acetylcholine receptors (nAChR) (22, 23) in the detection of nicotine by the peripheral taste system. The role of nAChRs in taste responses to nicotine is particularly intriguing because mecamylamine, a broad spectrum nAChR antagonist that has been used as a smoking cessation aid (24), reduces the sensitivity to peripheral sensory stimulation by cigarette smoke (25). However, hexamethonium (22), a different nAChR antagonist, inhibits taste responses to nicotine as well as to other tastants, suggesting that the inhibition resulting from nAChR antagonism may be unspecific. In summary, the mechanisms underlying the detection, discrimination, and neural coding of the taste of nicotine remain unclear.
Here, we aimed to clarify these uncertainties of the physiological mechanisms for peripheral taste transduction and neural representation of nicotine. We measured behavioral and taste neural responses to nicotine and quinine (a standard bitter tastant) and tested the modulation of these responses by targeted genetic and pharmacological manipulations. Neural responses were recorded from the chorda tympani (CT), a peripheral nerve with almost exclusively taste-dependent sensory activity (26), and the GC, an area of the central nervous system where neural responses that discriminate between tastants have been previously described (17, 26, 27). Trpm5−/− (KO) mice lack behavioral and peripheral neural responses to quinine and other prototypical bitter tastants (13). We used KO and wild-type (WT) animals to measure the degree of overlap between peripheral taste transduction pathways for nicotine and quinine. Furthermore, in both mice and rats, mecamylamine was used to clarify the effect of lingual nAChR antagonism in behavioral, and CT or GC neural responses, to nicotine, quinine, and other tastants.
Results
Trpm5-KO Mice Respond Behaviorally to Nicotine, but Not to Quinine.
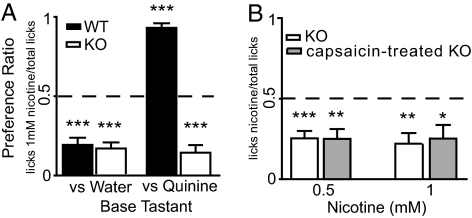
Taste preferences for quinine and nicotine were tested in KO and WT mice using 2-bottle tests. As established in ref. 13, 10 mM quinine was aversive (i.e., preference ratio significantly <0.5) for WT but not for KO mice (Fig. S1A; see preference ratios and statistical details for behavioral tests in Table S1). In separate groups of naïve mice the preference for 1 mM nicotine was also tested vs. water or vs. 10 mM quinine. Nicotine was aversive for both genotypes when tested against water and only in KO mice when tested against quinine. In contrast, WT mice preferred nicotine over quinine (i.e., preference ratio significantly >0.5; Fig. 1A). Results were confirmed with naïve animals using-bottle tests with 0.5 mM nicotine (Fig. S1B) and brief access tests (Fig. S1C). Thus, nicotine is equally aversive for KO and WT mice, showing that behavioral responses to this tastant are modulated by a TRPM5-independent mechanism.
Fig. 1.
Preference for nicotine and quinine in mice. (A) Preference for 1 mM nicotine was measured in 2-bottle tests vs. water (9 KO and 10 WT) and vs. 10 mM quinine (9 KO and 9 WT). With the exception of WT mice, that preferred nicotine to quinine, nicotine was always aversive (***, P < 0.0001, independent 1-sample t tests vs. 0.5, Bonferroni–Holm's; dashed line at the 0.5 indifference ratio). Accordingly, significant effects on preference were found for genotype, reference tastant and their interaction (P < 0.0001 for all; 2-way ANOVA) with differences between genotypes only when nicotine was tested against quinine (P < 0.001, Bonferroni). (B) The preference for 0.5 and 1 mM nicotine vs. water was tested in two bottle tests run in 9 KO and 9 capsaicin-treated KO mice. Nicotine was aversive in both groups of animals (*, P < 0.04; **, P < 0.004; ***, P < 0.0008) and treatment, concentration or their interaction had no effects on preference (P > 0.5, P > 0.2 and P > 0.1; 2-way repeated measures ANOVA).
We hypothesized that behavioral responses to orally delivered nicotine in KO mice could be modulated by somatosensory input or, alternatively, by TRPM5-independent taste pathways. Because nicotine activates capsaicin-sensitive trigeminal neurons (4, 7), the contribution of somatosensory input was tested by measuring behavioral responses to nicotine in adult KO animals that had been injected with capsaicin as neonates. This treatment produces systemic and life-long elimination of the majority of capsaicin-sensitive neurons, causing deficits in chemonociceptive reactivity (28). Although responses to capsaicin solutions confirmed the treatment was effective (Fig. S2), preference for 0.5 and 1 mM nicotine did not differ between untreated and capsaicin-treated KO animals (Fig. 1B). Given that KO animals, irrespective of a reduction in capsaicin-sensitive somatosensory neurons, retained an aversion to nicotine, we concluded that, at these concentrations, alternate TRPM5-independent sensory pathways, presumably taste-related, participate in the detection of nicotine.
Nicotine Elicits Chorda Tympani (CT) Responses Through Pathways That Are Common to Quinine and Others That Are Selective for Nicotine.
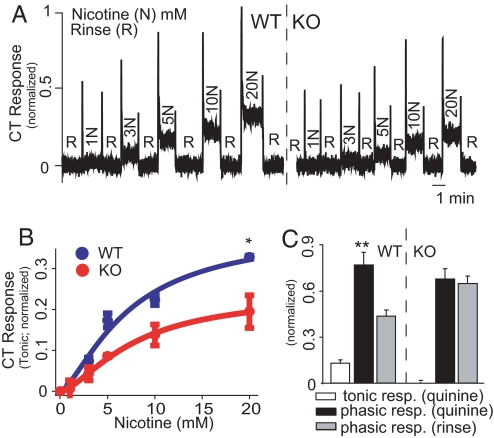
To confirm the presence of TRPM5-independent responses to nicotine in the peripheral taste system, we measured CT activity when nicotine was delivered to the tongue of anesthetized WT or KO mice. In both genotypes, nicotine elicited a concentration-dependent CT response consisting of distinct phasic and tonic components (Fig. 2A). Half-maximal phasic and tonic responses were observed at 3.2 and 7.2 mM for WT, and 2.3 and 7.8 mM for KO, with 40% lower tonic responses in KO than in WT mice (Fig. 2B). In accordance with the findings in ref. 13, CT responses to 10 mM quinine in KO mice were not different from those obtained with water (Fig. 2C and Fig. S3A). It follows that nicotine elicits both TRPM5-dependent and -independent taste responses.
Fig. 2.
Chorda Tympani (CT) responses to nicotine and quinine in mice. (A) Phasic and tonic CT responses to nicotine (N) were of lower magnitude in a KO (right trace) than in a WT (left trace) mouse. In all CT recordings, water was applied between stimuli as rinse solution (R) and responses were normalized to the mean tonic response obtained with 300 mM NH4Cl. (B) Tonic responses to nicotine were compared in 3 WT (blue curve, n = 1.44, EC50 = 7.2 mM, R2 = 0.96) and 3 KO (red curve, n = 1.5, EC50 = 7.8 mM, R2 = 0.99) mice. Significant differences were found for genotype (P < 0.002), nicotine concentration and their interaction (P < 0.0001 for both, 2-way repeated measures ANOVA). Genotype-dependent differences were significant at 20 mM (*, P < 0.05; Bonferroni). (C) Tonic responses to 10 mM quinine were observed in 3 WT mice (Left) but not in 3 KO mice (Right). In KO mice, phasic responses were not different from those obtained with rinse (P > 0.3), whereas, in WT mice, they were significantly higher (**, P < 0.002; paired 2-sample t test).
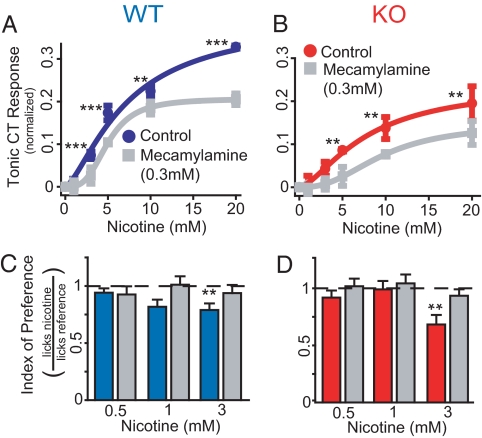
CT responses to nicotine were investigated further by testing the effect of mecamylamine. At 0.3 mM, this nAChR antagonist significantly inhibited tonic CT response to nicotine 47.5% in WT mice (Fig. 3A and Fig. S4A) and 28.6% in KO mice (Fig. 3B and Fig. S4B). Thus, CT responses to nicotine are partially nAChR-dependent, and this component of the response is conserved in the absence of TRPM5-dependent input. To verify if nAChR-dependent responses are behaviorally relevant, naïve WT and KO mice were tested in brief access tests with nicotine (0.5, 1 and 3 mM), in the presence or absence of 0.3 mM mecamylamine. In both genotypes, mecamylamine significantly reduced the aversive effects of nicotine (Fig. 3 C and D).
Fig. 3.
Effects of mecamylamine on mouse CT responses to nicotine. (A) In 3 WT mice, tonic CT responses to nicotine alone (dark blue curve, see Fig. 2B) were of higher magnitude than when mecamylamine was added to stimuli (gray curve, n = 3.2, EC50 = 5 mM, R2 = 1). The effects of mecamylamine were significant (P < 0.0001 for mecamylamine, nicotine concentration and interaction; 2-way repeated measures ANOVA), particularly >1 mM (***, P < 0.001; **, P < 0.01). (B) In 3 KO mice, the effects of mecamylamine on the tonic CT response to nicotine were similar to those in WT [nicotine alone: red curve (see Fig. 2B); nicotine + mecamylamine: gray curve, n = 2.5, EC50 = 9.6 mM, R2 = 0.99; P < 0.0001 for mecamylamine and nicotine concentration and P < 0.03 for interaction, 2-way repeated measures ANOVA; **, P < 0.01]. (C) Twelve WT mice were tested in brief-access tests with 0.5, 1, and 3 mM nicotine. An index of preference for each stimulus was calculated from lick ratios (number of licks for each stimulus normalized to the number of licks for water). All stimuli and water were presented in counterbalanced days with or without 0.3 mM mecamylamine. Nicotine was aversive (i.e., lick ratio significantly <1) at 3 mM when dissolved in water (**, P < 0.005; 1-sample t tests vs. 1, Bonferroni–Holm's; dashed line at the 1 indifference index). Significant main effects on index of preference were found for mecamylamine (P < 0.04) but not nicotine concentration or their interaction (P > 0.5 and P > 0.2 respectively; 2-way, repeated measures ANOVA). (D) Nine KO mice were tested in brief-access tests as described in C. Findings were similar to those described for WT (**, P < 0.006; significant main effects were found for mecamylamine, P < 0.03; and nicotine concentration, P < 0.02; but not their interaction, P > 0.3; 2-way, repeated measures ANOVA).
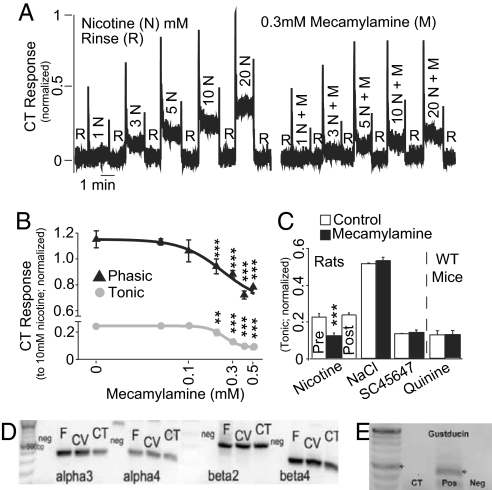
In rat CT recordings, results were similar to those obtained in mice (Fig. 4A and Fig. S4 C and D). Additionally, mecamylamine inhibited both phasic and tonic responses to 10 mM nicotine in a concentration-dependent manner, with 50% inhibition of responses occurring at 0.23 mM mecamylamine (Fig. 4B). Furthermore, even at the highest concentration of mecamylamine tested (0.5 mM), CT responses to nicotine were higher than those observed for water alone (data not shown), demonstrating that a component of the chemosensory response is resistant to mecamylamine inhibition. The effects of 0.3 mM mecamylamine on CT responses to nicotine were reversible, showing that this effect is not due to nAChR desensitization (Fig. 4C). Finally, nAChR antagonism had no effect on tonic responses to 100 mM NaCl and 5 mM SC45647 [a potent artificial sweetener (15)] in rats (Fig. 4C), whereas, in WT mice, it did not affect either CT or behavioral responses to 10 mM quinine (Fig. 4C and Fig. S3 B and C). These data indicate that the effects of nAChR antagonism are quite specific for nicotine.
Fig. 4.
nAChR-dependent taste responses in rats. (A) CT responses to nicotine (N) in the presence and absence of 0.3 mM mecamylamine (M) (also see Fig. S4 C and D). (B) In 3 rats, a lingual perfusion chamber was used to reduce variability in the phasic component of CT responses, and the inhibitory effects of mecamylamine on the phasic and tonic CT responses to 10 mM nicotine were shown to be dose-dependent. Responses at each mecamylamine concentration were fitted to sigmoidal response-inhibition curves (phasic, black curve: n = −4.9, EC50 = 0.23 mM, R2 = 0.96; tonic, gray curve: n = −1.9, EC50 = 0.23 mM, R2 = 0.99). Response inhibition relative to nicotine alone (0 mM mecamylamine) was, in both cases, significant at concentrations >0.1 mM (***, P < 0.001; **, P < 0.01; Bonferroni). (C) In 3 other rats, 0.3 mM mecamylamine inhibited the responses to nicotine (***, P < 0.0002), but the responses to nicotine alone after exposure to mecamylamine (post) were not different from the responses obtained before use of the antagonist (pre; P > 0.3, paired-sample t tests). In these animals, 0.3 mM mecamylamine had no effect on the tonic responses to 100 mM NaCl or 5 mM SC45647 (P > 0.3 for both). In 3 WT mice the responses to 10 mM quinine were also resistant to inhibition by 0.3 mM mecamylamine (P > 0.9). (D) A cDNA library from rat fungiform (FF) and circumvallate (CV) taste buds and the chorda tympani nerve (CT) was screened for α-3, α-4, β-2 and β-4 subunits of the nAChR. All subunits were found with bands of expected size (DNA ladder on Left side). Negative control (neg) was milliQ water. (E) Control RT-PCR experiments demonstrated that α-gustducin was not found in CT tissue. As expected, it is expressed in FF, CV (also see Fig. S5), and in an additional sample from isolated circumvallate taste buds (pos).
CT responses suggested the presence of nAChRs in TRCs. Using RT-PCR, we confirmed the expression of α-3, α-4, β-2, and β-4 nAChR subunits in taste buds (from fungiform and circumvallate papillae) and CT nerve (Fig. 4D). In a control experiment, we found α-gustducin in taste buds but not in the CT (Fig. 4E and Fig. S5), suggesting that nAChR subunits found in taste buds are expressed in TRCs.
Rats Discriminate Between Nicotine and Quinine.
Because nicotine activates taste pathways that are not common to quinine, we hypothesized that the two tastants can be discriminated. This hypothesis was tested in rats using a two alternative choice test (29) where animals were exposed to either nicotine or quinine and were required to report, by pressing one of two levers, which tastant had been presented. When the correct lever was pressed, access to a water reward was granted, whereas if the incorrect lever was chosen, the animal was punished with 30 s of timeout. To avoid the use of intensity cues in discrimination, 3 isopreferred concentrations of each tastant were used (0.1, 0.2, and 0.3 mM for quinine and 0.3, 1, and 3 mM for nicotine; see Fig. S6 A and B). Seven rats learned this protocol and responded correctly above chance for both nicotine (70 ± 6%, P < 0.02) and quinine (76 ± 2%, P < 0.0001; one sample t tests vs. 50%, Bonferroni–Holm's; Fig. S6C). These responses resulted from orosensory discrimination of the two tastants because the use of water, rather than nicotine and quinine, as the probe tastant, resulted in overall correct responses at chance level (51 ± 2%; P > 0.7).
We also tested if mecamylamine would disrupt the discrimination between nicotine and quinine. Rats were retested, in counterbalanced order, under two different conditions: all nicotine solutions were replaced by either water (control session), or nicotine and mecamylamine (0.3 mM) (test session) (29). In both cases, to avoid intrasession learning, access to water rewards was not contingent upon the animal's response but was delivered with a fixed 0.8 probability. Overall correct discrimination was compared with that obtained in baseline sessions (77 ± 3% choices) and was significantly lower in the test (66 ± 4%; P < 0.05) but not in the control sessions (72 ± 3%; P > 0.05; Newman–Keuls; Fig. S6D). We conclude that the taste of nicotine and mecamylamine mixtures is more similar to the taste of quinine than that of nicotine alone.
Neural Ensembles in the Gustatory Cortex Discriminate Between Nicotine and Quinine.
To determine how nicotine and quinine are represented in the GC, we recorded the activity of 112 single GC neurons across 12 ensembles obtained from 8 rats. Neural ensemble size ranged from 3 to 16 neurons (average = 9.3 neurons). In each recording session, animals were exposed to multiple concentrations of 5 tastants (sucrose, NaCl, MSG, quinine and nicotine) and water, in a total of 10 to 12 stimuli. One or 2 isopreferred concentrations of nicotine and quinine (see Fig. S6 A and B) were presented. Stimuli (i.e., tastants at particular concentrations), were delivered on every fifth lick, with 4 dry licks between each reinforced lick (FR5 schedule; see Fig. S6E).
Neural responses in this behavioral paradigm were similar to those described in ref. 30. Neuronal firing activity after stimulus delivery was generally greater than that resulting from dry licks and these responses usually occurred within the span of a single lick (≈0.15 sec). 46 neurons were chemosensory and broadly tuned, 9 were fluid sensitive but did not discriminate between tastants and 35 were tastant-modulated (30). Responses to tastants in a representative neuron are illustrated in Fig. 5A.
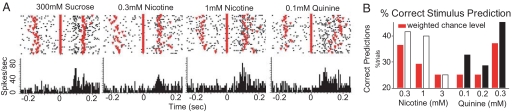
Fig. 5.
Neural discrimination between nicotine and quinine in the GC. (A) Representative example of a broadly tuned chemosensory GC neuron that responded to nicotine, quinine, and sucrose. Shown are the responses to particular stimuli, with raster plots of action potentials and peri-stimulus time histograms depicted respectively in the upper and lower sections. Reinforced licks occur at time 0 and are marked as a single red line on the raster plot. Previous and following dry licks are visible as red triangles at ± 0.15 s. (B) Correct predictions of nicotine and quinine stimuli (white and black bars respectively) across all ensembles were compared with chance (red bars). Because 1 or 2 concentrations of each tastant were presented in different recording sessions, a weighted chance level was calculated for each stimulus from the number of presentations at each chance level (50 or 25%–see methods). Nicotine (3 mM) was predicted at chance levels. All other stimuli were predicted above chance levels.
The firing patterns of GC neural ensembles have been shown to predict tastant identity and concentration on a single lick basis, particularly when firing rate and spike timing are combined (27). Using this methodology, we determined whether ensembles of GC neurons could discriminate between nicotine and quinine. A generalized linear model (GLM), based on neural ensemble activity recorded during licking, was used to predict, for each ensemble, stimulus identity for the deliveries withheld from the GLM. Data pooled from all ensembles showed that, with the exception of 3 mM nicotine, all bitter stimuli were correctly predicted above the respective weighted chance level (Fig. 5B). This indicates that even small ensembles of GC neurons contain sufficient information to discriminate between nicotine and quinine. Furthermore, we hypothesized that the information contained in each ensemble would increase with its size (i.e., number of neurons) (31). Accordingly, a measure of ensemble efficacy (proportion of tested stimuli that were predicted above chance by each ensemble) was shown to correlate positively with ensemble size (Spearman ρ = 0.6, P < 0.04; Fig. S7).
Although the GLM could correctly identify most stimuli, it remained unclear if the GC treated different concentrations of a single tastant as unique stimuli or if, alternatively, the ensemble firing patterns for all concentrations of one tastant could be more similar to each other than those observed with any concentration of the other. To clarify this issue, GLM predictions were reclassified, considering correct tastant predictions irrespective of any concentration errors. Across all ensembles, nicotine and quinine were correctly classified in 62.9% and 54% of trials respectively, in both cases above chance (50% given that only tastant identity, nicotine vs. quinine, was considered; Fig. S8). Thus, GC activity contains sufficient information to discriminate the identity of both tastants and stimuli (i.e., different concentrations of either tastant).
nAChR-Dependent Sensory Pathways Are Required for Discrimination Between Nicotine and Quinine.
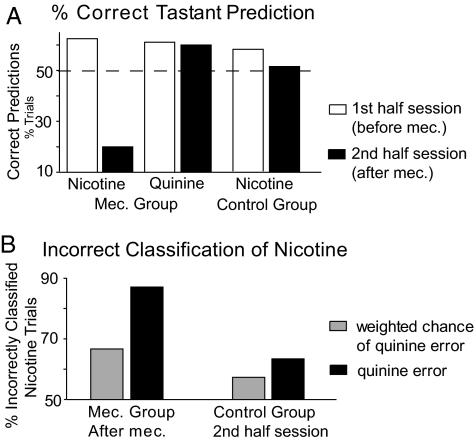
We also tested whether nAChR-dependent taste pathways contributed to the neural discrimination of nicotine from quinine in the GC. The effect of nAChR-antagonism on ensemble discrimination was verified in two recording sessions, where additional trials were run with mecamylamine (0.3 mM) in water and in all nicotine solutions. Although nicotine and quinine were correctly classified above chance at baseline (62.5 and 61.1% respectively), mecamylamine reduced the identification of nicotine to only 20% of trials, whereas quinine was still predicted above chance (60%) (Fig. 6A). This reduction was not due to time because, in 4 control ensembles, where tastants were unadulterated, prediction of nicotine was above chance across the whole recording session (Fig. 6A). To verify if the effect of mecamylamine was exerted by modifying the representation of nicotine selectively toward that of quinine, GLMs were reconstructed with the inclusion of water trials and the distribution of nicotine errors between water and quinine was calculated for the second half of the sessions. Although, in control ensembles, the proportion of incorrectly predicted nicotine trials predicted as quinine was only 6.1% above the weighted chance of a quinine error, in ensembles where mecamylamine was used, nicotine errors were classified as quinine 20.5% above chance (Fig. 6B).
Fig. 6.
Effects of mecamylamine on GC responses to nicotine. (A) In two GC neural ensemble recording sessions, mecamylamine was added to nicotine solutions at midsession. Although nicotine and quinine were initially (white bars) predicted ≈10% above chance, mecamylamine (black bars) reduced the identification of nicotine to 30% below chance (dashed line at the 50% chance level). In control ensembles, correct prediction of nicotine dropped by 6.7% from the first to the second half of the session (white and black bar respectively) but was, nevertheless, still above chance. (B) The distribution of nicotine errors between quinine or water prediction was calculated for trials with mecamylamine and the corresponding dataset in control ensembles. Weighted chance levels for a quinine error were calculated because the number of concentrations of quinine that were presented was not constant (1 or 2).
Discussion
We showed that nicotine activates the taste system via two parallel receptor-transduction pathways: one that is TRPM5-dependent and common to other bitter tastants and another that is more specific for nicotine and essential for its sensory representation. Specifically, although quinine was not aversive for Trpm5-KO mice, nicotine was equally aversive for WT and KO mice and, even though CT responses to nicotine were reduced in KO mice, they were not abolished. Furthermore, CT responses to nicotine were inhibited by nAChR-antagonism with mecamylamine, both in WT and KO mice and in rats, whereas responses to quinine and both a sweet and a salty tastant were unaffected. These effects of mecamylamine were also found to be behaviorally relevant. For these reasons, we conclude that nicotine engages TRPM5-dependent responses common to other bitter tastants and also more specific TRPM5-independent taste pathways that depend on nAChRs. In accordance with these findings we found that, in rats, behavioral and GC neural ensemble responses were discriminatory between nicotine and quinine. Importantly, lingual nAChR antagonism made these responses more similar, demonstrating that nAChR-dependent responses to nicotine are important for this tastant to be discriminated from quinine.
Previously, nAChRs had been proposed as taste receptors for nicotine (22, 23). Here, we demonstrate that nAChRs are, in fact, expressed in taste buds and the CT and, furthermore, that nAChR-dependent taste responses are relatively specific for nicotine, TRPM5-independent and reversible (Figs. 3 and 4). Thus, nicotine may elicit taste responses by activating nAChRs expressed on TRPM5-negative TRCs and/or CT nerve terminals. The physiological function of nAChRs in taste buds remains, nevertheless, undetermined. Acetylcholine has been proposed to modulate TRCs via muscarinic AChRs (32) but could also act through nAChRs. In fact, the effects of cigarette smoking on food consumption and taste preferences (33) could result, among other factors (23), from peripheral taste modulation due to chronic exposure to nicotine, acting on nAChRs in taste buds.
CT recordings performed in this study also provided the first definitive demonstration that nicotine activates a taste transduction pathway that is common to other bitter tastants (Fig. 2B). This finding was further supported by the fact that mecamylamine, by inhibiting TRPM5-independent responses to nicotine while leaving TRPM5-dependent responses intact (Figs. 3 and 4), made the central sensory representation of nicotine more similar to that of quinine (Fig. 6). Because quinine is described by humans as purely bitter (22), and, at concentrations <10 mM, is not detected by Trpm5-KO mice (13), we propose that this common, TRPM5-dependent pathway, is the peripheral substrate for the “bitterness” of both tastants (6).
Another important finding was the demonstration of behavioral and GC neural discrimination between quinine and nicotine, resulting from nAChR-dependent orosensory input (Fig. 6 and Fig. S6D). Previous studies gave inconsistent results as to the possibility of gustatory neurons to discriminate between bitter tastants, including nicotine and quinine (16–18). However, perceptual intensity had not been considered in the choice of stimuli concentrations, and neural responses to tastants were analyzed across long periods (up to 10 s) considering only the rate component of the response. Here, we tested intensity-matched concentrations of nicotine and quinine (Fig. S6 A and B) and analyzed both the firing rate and temporal firing pattern of responses occurring in the first 150 ms after tastant exposure (Fig. 5A), a time-window shown to include most of the GC chemosensory activity in freely licking rats (27, 30). Presumably, these fast GC responses result from the phasic activity of taste nerves that, as was demonstrated for the CT, is disrupted when mecamylamine is added to nicotine (Figs. 3 and 4).
Critical to the relevance of these findings is the proposal that behavioral and central GC responses to low concentrations of nicotine delivered orally reflect the peripheral taste nerve activation. The consistency of the effects of mecamylamine on CT responses with those on the animal's behavior and GC neuronal responses support this proposal. However, in rats, behavioral (EC50 = 0.69 mM) (Fig. S6A) and whole nerve CT (phasic − EC50 = 2.74 mM) (Fig. S3C) responses to nicotine differed by a factor of 4. These differences can be rationalized by differences in the conditions of data collection (e.g., anesthetized vs. awake), and the fact that responses of more sensitive single CT and glossopharyngeal units could be masked in whole nerve recordings (5, 16).
Collectively, our findings contribute to the explanation of the oral sensations produced by tobacco products. Furthermore, we have demonstrated a previously unknown link between peripheral nAChR-dependent taste pathways and the sensory representation of nicotine in the GC. Although antagonists of nAChRs are currently used in the treatment of tobacco addiction because of their effects on CNS receptors (1), they also modify the sensory effects of cigarette smoke (25). Because brain lesions including the GC have also been shown to disrupt addiction to cigarette smoking, possibly because of an effect on sensory representations (12), the above mentioned link could have a previously underappreciated role in the oral sensory effects of tobacco products.
Methods
Full methods are in SI Methods and SI Appendix. All procedures were approved by the Duke University or Virginia Commonwealth University Institutional Animal Care and Use Committees.
Behavioral Measurements.
Two-bottle and brief access preference tests were conducted according to standard procedures (34). Two-alternative choice tests were used to measure tastant discrimination (29). Newborn KO mice were treated with capsaicin (35) and, as adults, were tested for nicotine preference in 2-bottle tests.
CT Recordings.
Recordings were performed in anesthetized animals and analyzed following protocols described in ref. 36. Data are given as mean ± SD. Equations for nicotine concentration-response and mecamylamine dose-inhibition functions are in SI Appendix. The effect of 0.3 mM mecamylamine in KO compared with WT was calculated as the asymptotic percentage of inhibition.
Reverse Transcriptase PCR for nAChR Subunits.
Fungiform and circumvallate taste buds were collected according to methods described in ref. 36. CT nerve tissue was collected in its exit from the tympanic bulla. RT-PCR was performed to screen for the presence of α-3, α-4, β-2, β-4 nAChR subunits (37), and α-gustducin and β-actin.
GC Neuronal Recordings.
Recordings were conducted and analyzed according to the protocol described in ref. 30. In 4 animals, electrodes were lowered to allow recording of a second session. After completion of the experiments, rat brains were processed to verify correct electrode placement (30).
Statistical Analysis.
Analyses made use of 2-way or 1-way ANOVAs (with Bonferroni or Newman–Keuls posthoc tests) and 2-sample or 1-sample t tests. Sequential Bonferroni correction for multiple comparisons was performed with the Holm's method (38) whenever multiple independent t tests were used in the same dataset. Unless otherwise specified, data are given as mean ± SEM.
Supplementary Material
Acknowledgments.
We thank Teresa Maia for outstanding support in mouse colony management, genotyping, and behavioral experiments; Pedro Nicolelis for assistance in behavioral experiments; Jim Meloy and Gary Lehew for technical support; Dr. Shih-Chieh Lin, Dr. Ian Davison, and Mônica Coelho for comments on the manuscript; and Dr. Jeff Seeman for encouraging us to explore the unique taste of nicotine. This work was supported by National Institutes of Health Grants DC001065 (to S.A.S.), DC005981 (to V.L.), and DC00122 (to J.A.D.); grants from Philip Morris USA, Inc. and Philip Morris International (to S.A.S.); and a doctoral fellowship from the Portuguese Foundation for Science and Technology and the Graduate Program in Areas of Basic and Applied Biology (to A.J.O.-M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810184106/DCSupplemental.
References
- 1.Hatsukami DK, Stead LF, Gupta PC. Tobacco addiction. Lancet. 2008;371:2027–2038. doi: 10.1016/S0140-6736(08)60871-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pritchard WS, Robinson JH, Guy TD, Davis RA, Stiles MF. Assessing the sensory role of nicotine in cigarette smoking. Psychopharmacology (Berlin) 1996;127:55–62. doi: 10.1007/BF02805975. [DOI] [PubMed] [Google Scholar]
- 3.Rose JE, Behm FM, Levin ED. Role of nicotine dose and sensory cues in the regulation of smoke intake. Pharmacol Biochem Behav. 1993;44:891–900. doi: 10.1016/0091-3057(93)90021-k. [DOI] [PubMed] [Google Scholar]
- 4.Thuerauf N, et al. The influence of mecamylamine on trigeminal and olfactory chemoreception of nicotine. Neuropsychopharmacology. 2006;31:450–461. doi: 10.1038/sj.npp.1300842. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki K, Sato M. Neural responses and aversion to bitter stimuli in rats. Chemical Senses. 1981;6:119–128. [Google Scholar]
- 6.Pfaffmann C. The sense of taste. In: Field J, Magoun HW, Hall VE, editors. Handbook of Physiology: Neurophysiology. Vol 1. Washington, DC: American Physiological Society; 1959. pp. 507–533. [Google Scholar]
- 7.Carstens E, Kuenzler N, Handwerker HO. Activation of neurons in rat trigeminal subnucleus caudalis by different irritant chemicals applied to oral or ocular mucosa. J Neurophysiol. 1998;80:465–492. doi: 10.1152/jn.1998.80.2.465. [DOI] [PubMed] [Google Scholar]
- 8.Feyerabend C, Higenbottam T, Russell MA. Nicotine concentrations in urine and saliva of smokers and non-smokers. Br Med J Clin Res Ed. 1982;284:1002–1004. doi: 10.1136/bmj.284.6321.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim UK, et al. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299:1221–1225. doi: 10.1126/science.1080190. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan AR, Glanville EV, Fischer R. Taste thresholds for bitterness and cigarette smoking. Nature. 1964;202:1366. doi: 10.1038/2021366a0. [DOI] [PubMed] [Google Scholar]
- 11.Snedecor SM, Pomerleau CS, Mehringer AM, Ninowski R, Pomerleau OF. Differences in smoking-related variables based on phenylthiocarbamide “taster” status. Addict Behav. 2006;31:2309–2312. doi: 10.1016/j.addbeh.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, et al. Coding of sweet, bitter, and umami tastes: Different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 14.Ming D, Ruiz-Avila L, Margolskee RF. Characterization and solubilization of bitter-responsive receptors that couple to gustducin. Proc Natl Acad Sci USA. 1998;95:8933–8938. doi: 10.1073/pnas.95.15.8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He W, et al. Umami taste responses are mediated by alpha-transducin and alpha-gustducin. J Neurosci. 2004;24:7674–7680. doi: 10.1523/JNEUROSCI.2441-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahl M, Erickson RP, Simon SA. Neural responses to bitter compounds in rats. Brain Res. 1997;756:22–34. doi: 10.1016/s0006-8993(97)00131-5. [DOI] [PubMed] [Google Scholar]
- 17.Scott TR, Giza BK, Yan J. Gustatory neural coding in the cortex of the alert cynomolgus macaque: The quality of bitterness. J Neurophysiol. 1999;81:60–71. doi: 10.1152/jn.1999.81.1.60. [DOI] [PubMed] [Google Scholar]
- 18.Lemon CH, Smith DV. Neural representation of bitter taste in the nucleus of the solitary tract. J Neurophysiol. 2005;94:3719–3729. doi: 10.1152/jn.00700.2005. [DOI] [PubMed] [Google Scholar]
- 19.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez CA, et al. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- 21.Lin W, Ogura T, Margolskee RF, Finger TE, Restrepo D. TRPM5-expressing solitary chemosensory cells respond to odorous irritants. J Neurophysiol. 2008;99:1451–1460. doi: 10.1152/jn.01195.2007. [DOI] [PubMed] [Google Scholar]
- 22.Schiffman SS. Taste quality and neural coding: Implications from psychophysics and neurophysiology. Physiol Behav. 2000;69:147–159. doi: 10.1016/s0031-9384(00)00198-0. [DOI] [PubMed] [Google Scholar]
- 23.Simons CT, Boucher Y, Carstens MI, Carstens E. Nicotine suppression of gustatory responses of neurons in the nucleus of the solitary tract. J Neurophysiol. 2006;96:1877–1886. doi: 10.1152/jn.00345.2006. [DOI] [PubMed] [Google Scholar]
- 24.Shytle RD, Penny E, Silver AA, Goldman J, Sanberg PR. Mecamylamine (Inversine): An old antihypertensive with new research directions. J Hum Hypertens. 2002;16:453–457. doi: 10.1038/sj.jhh.1001416. [DOI] [PubMed] [Google Scholar]
- 25.Rose JE, Sampson A, Levin ED, Henningfield JE. Mecamylamine increases nicotine preference and attenuates nicotine discrimination. Pharmacol Biochem Behav. 1989;32:933–938. doi: 10.1016/0091-3057(89)90061-0. [DOI] [PubMed] [Google Scholar]
- 26.Lundy RF, Jr., Norgren R. Gustatory System. In: Paxinos G, editor. The Rat Nervous System. 3rd Ed. San Diego, CA and London: Elsevier, Academic; 2004. pp. 891–921. [Google Scholar]
- 27.Stapleton JR, Lavine ML, Nicolelis MA, Simon SA. Ensembles of gustatory cortical neurons anticipate and discriminate between tastants in a single lick. Frontiers in Neuroscience. 2007;1:161–174. doi: 10.3389/neuro.01.1.1.012.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faulkner DC, Growcott JW. Effects of neonatal capsaicin administration on the nociceptive response of the rat to mechanical and chemical stimuli. J Pharm Pharmacol. 1980;32:656–657. doi: 10.1111/j.2042-7158.1980.tb13027.x. [DOI] [PubMed] [Google Scholar]
- 29.Spector AC, Guagliardo NA, St John SJ. Amiloride disrupts NaCl versus KCl discrimination performance: Implications for salt taste coding in rats. J Neurosci. 1996;16:8115–8122. doi: 10.1523/JNEUROSCI.16-24-08115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stapleton JR, Lavine ML, Wolpert RL, Nicolelis MA, Simon SA. Rapid taste responses in the gustatory cortex during licking. J Neurosci. 2006;26:4126–4138. doi: 10.1523/JNEUROSCI.0092-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicolelis MA, et al. Simultaneous encoding of tactile information by three primate cortical areas. Nat Neurosci. 1998;1:621–630. doi: 10.1038/2855. [DOI] [PubMed] [Google Scholar]
- 32.Ogura T, et al. Immuno-localization of vesicular acetylcholine transporter in mouse taste cells and adjacent nerve fibers: Indication of acetylcholine release. Cell Tissue Res. 2007;330:17–28. doi: 10.1007/s00441-007-0470-y. [DOI] [PubMed] [Google Scholar]
- 33.Grunberg NE. The effects of nicotine and cigarette smoking on food consumption and taste preferences. Addict Behav. 1982;7:317–331. doi: 10.1016/0306-4603(82)90001-6. [DOI] [PubMed] [Google Scholar]
- 34.de Araujo IE, et al. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 35.Newson P, et al. Intrinsic sensory deprivation induced by neonatal capsaicin treatment induces changes in rat brain and behaviour of possible relevance to schizophrenia. Br J Pharmacol. 2005;146:408–418. doi: 10.1038/sj.bjp.0706349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyall V, et al. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J Physiol. 2004;558(Pt 1):147–159. doi: 10.1113/jphysiol.2004.065656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park KS, et al. An alpha3beta4 subunit combination acts as a major functional nicotinic acetylcholine receptor in male rat pelvic ganglion neurons. Pflugers Arch. 2006;452:775–783. doi: 10.1007/s00424-006-0086-1. [DOI] [PubMed] [Google Scholar]
- 38.Holm S. A simple sequential rejective multiple test procedure. Scand J Statistics. 1979;6:65–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.