Abstract
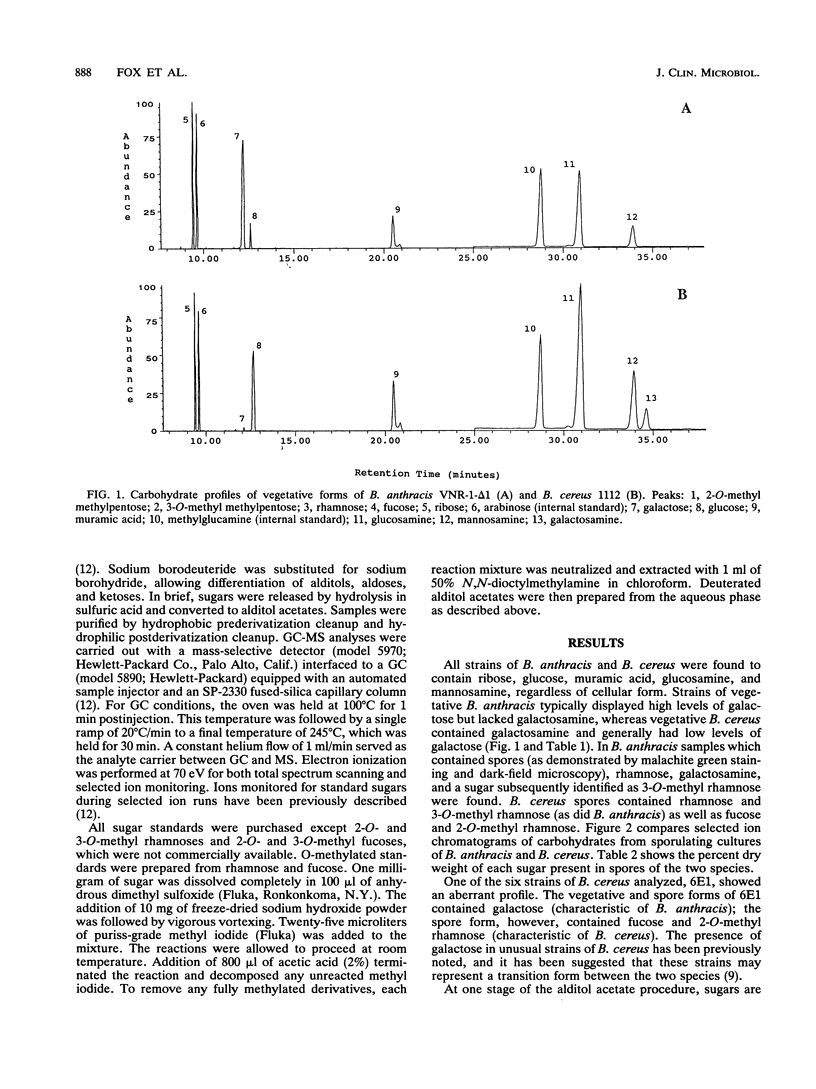
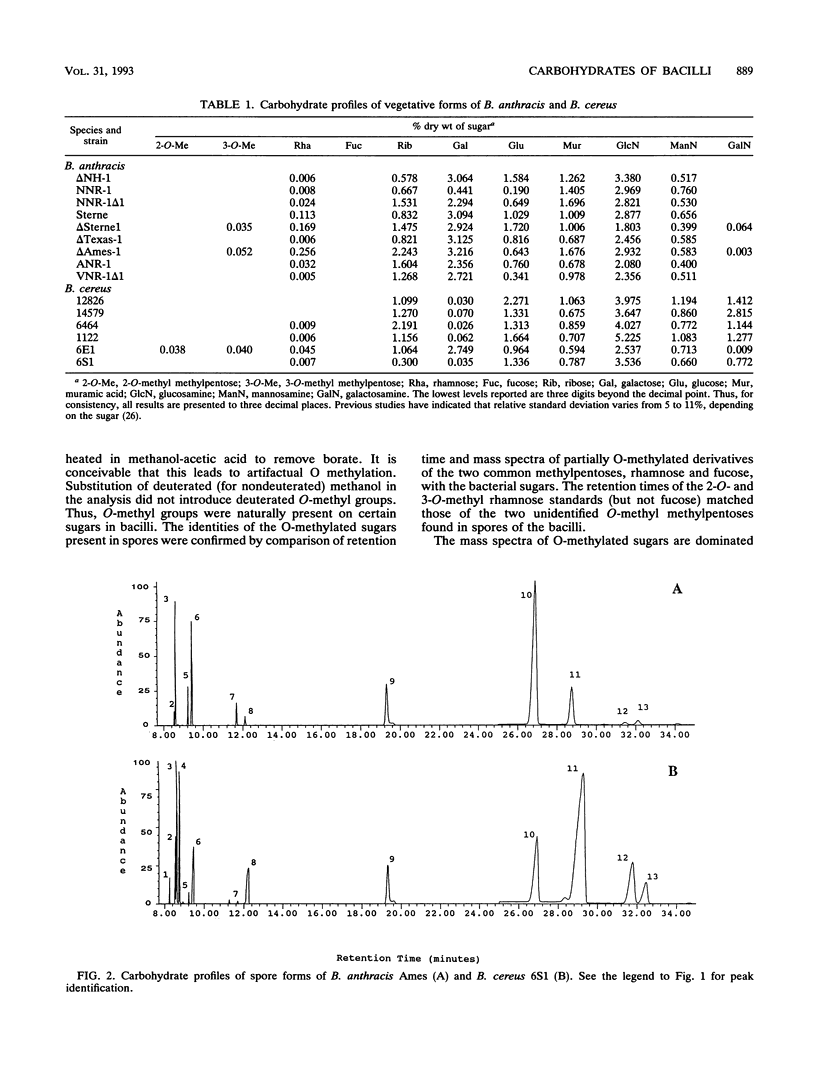
Bacillus anthracis and Bacillus cereus are closely related pathogenic organisms that are difficult to differentiate phenotypically or genotypically. It is well known that vegetative and spore forms of bacilli are quite distinct both morphologically and chemically, but spore-specific chemical markers allowing these species to be distinguished have not been previously described. By using gas chromatography-mass spectrometry, vegetative cells and spores of the two species were shown to exhibit distinct carbohydrate profiles. Profiles of vegetative B. anthracis typically contained high levels of galactose but did not contain galactosamine, whereas B. cereus contained galactosamine and generally low levels of galactose. Spore cultures exhibited unique carbohydrate profiles compared with those of vegetative cultures. B. anthracis spore profiles contained rhamnose alone, whereas B. cereus spore profiles contained rhamnose and fucose. Additionally, two spore-specific O-methylated methylpentoses were discovered. Both B. anthracis and B. cereus spores contained 3-O-methyl rhamnose, whereas B. cereus spores also contained 2-O-methyl rhamnose. Carbohydrate profiling is demonstrated to be a powerful tool for differentiating the two closely related species. Differentiation does not depend on whether organisms are in the vegetative or spore stage of growth.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ash C., Collins M. D. Comparative analysis of 23S ribosomal RNA gene sequences of Bacillus anthracis and emetic Bacillus cereus determined by PCR-direct sequencing. FEMS Microbiol Lett. 1992 Jul 1;73(1-2):75–80. doi: 10.1016/0378-1097(92)90586-d. [DOI] [PubMed] [Google Scholar]
- Ash C., Farrow J. A., Dorsch M., Stackebrandt E., Collins M. D. Comparative analysis of Bacillus anthracis, Bacillus cereus, and related species on the basis of reverse transcriptase sequencing of 16S rRNA. Int J Syst Bacteriol. 1991 Jul;41(3):343–346. doi: 10.1099/00207713-41-3-343. [DOI] [PubMed] [Google Scholar]
- BURDON K. L. Useful criteria for the identification of Bacillus anthracis and related species. J Bacteriol. 1956 Jan;71(1):25–42. doi: 10.1128/jb.71.1.25-42.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole H. B., Ezzell J. W., Jr, Keller K. F., Doyle R. J. Differentiation of Bacillus anthracis and other Bacillus species by lectins. J Clin Microbiol. 1984 Jan;19(1):48–53. doi: 10.1128/jcm.19.1.48-53.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekwunife F. S., Singh J., Taylor K. G., Doyle R. J. Isolation and purification of cell wall polysaccharide of Bacillus anthracis (delta Sterne). FEMS Microbiol Lett. 1991 Aug 15;66(3):257–262. doi: 10.1016/0378-1097(91)90270-k. [DOI] [PubMed] [Google Scholar]
- Ezzell J. W., Jr, Abshire T. G., Little S. F., Lidgerding B. C., Brown C. Identification of Bacillus anthracis by using monoclonal antibody to cell wall galactose-N-acetylglucosamine polysaccharide. J Clin Microbiol. 1990 Feb;28(2):223–231. doi: 10.1128/jcm.28.2.223-231.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A., Lau P. Y., Brown A., Morgan S. L., Zhu Z. T., Lema M., Walla M. D. Capillary gas chromatographic analysis of carbohydrates of Legionella pneumophila and other members of the family Legionellaceae. J Clin Microbiol. 1984 Mar;19(3):326–332. doi: 10.1128/jcm.19.3.326-332.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A., Rogers J. C., Fox K. F., Schnitzer G., Morgan S. L., Brown A., Aono R. Chemotaxonomic differentiation of legionellae by detection and characterization of aminodideoxyhexoses and other unique sugars using gas chromatography-mass spectrometry. J Clin Microbiol. 1990 Mar;28(3):546–552. doi: 10.1128/jcm.28.3.546-552.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison L. H., Ezzell J. W., Abshire T. G., Kidd S., Kaufmann A. F. Evaluation of serologic tests for diagnosis of anthrax after an outbreak of cutaneous anthrax in Paraguay. J Infect Dis. 1989 Oct;160(4):706–710. doi: 10.1093/infdis/160.4.706. [DOI] [PubMed] [Google Scholar]
- Ivins B. E., Ezzell J. W., Jr, Jemski J., Hedlund K. W., Ristroph J. D., Leppla S. H. Immunization studies with attenuated strains of Bacillus anthracis. Infect Immun. 1986 May;52(2):454–458. doi: 10.1128/iai.52.2.454-458.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda T. Fatty acids in the genus Bacillus. II. Similarity in the fatty acid compositions of Bacillus thuringiensis, Bacillus anthracis, and Bacillus cereus. J Bacteriol. 1968 Jun;95(6):2210–2216. doi: 10.1128/jb.95.6.2210-2216.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T., Nozaki R., Aizawa K. Deoxyribonucleic acid relatedness between Bacillus anthracis, Bacillus cereus and Bacillus thuringiensis. Microbiol Immunol. 1978;22(10):639–641. doi: 10.1111/j.1348-0421.1978.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Lawrence D., Heitefuss S., Seifert H. S. Differentiation of Bacillus anthracis from Bacillus cereus by gas chromatographic whole-cell fatty acid analysis. J Clin Microbiol. 1991 Jul;29(7):1508–1512. doi: 10.1128/jcm.29.7.1508-1512.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan N. A., Carman J. A., Melling J., Berkeley R. C. Identification of Bacillus anthracis by API tests. J Med Microbiol. 1985 Aug;20(1):75–85. doi: 10.1099/00222615-20-1-75. [DOI] [PubMed] [Google Scholar]
- Matz L. L., Beaman T. C., Gerhardt P. Chemical composition of exosporium from spores of Bacillus cereus. J Bacteriol. 1970 Jan;101(1):196–201. doi: 10.1128/jb.101.1.196-201.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murazumi N., Araki Y., Ito E. Biosynthesis of the wall neutral polysaccharide in Bacillus cereus AHU 1356. Eur J Biochem. 1986 Nov 17;161(1):51–59. doi: 10.1111/j.1432-1033.1986.tb10123.x. [DOI] [PubMed] [Google Scholar]
- Talmont F., Fournet B. Isolation and characterization of methylated sugars from the tube of the hydrothermal vent tubiculous annelid worm Alvinella pompejana. FEBS Lett. 1991 Apr 9;281(1-2):55–58. doi: 10.1016/0014-5793(91)80357-9. [DOI] [PubMed] [Google Scholar]
- Warth A. D., Strominger J. L. Structure of the peptidoglycan from spores of Bacillus subtilis. Biochemistry. 1972 Apr 11;11(8):1389–1396. doi: 10.1021/bi00758a010. [DOI] [PubMed] [Google Scholar]
- Whiton R. S., Lau P., Morgan S. L., Gilbart J., Fox A. Modifications in the alditol acetate method for analysis of muramic acid and other neutral and amino sugars by capillary gas chromatography-mass spectrometry with selected ion monitoring. J Chromatogr. 1985 Oct 25;347(1):109–120. doi: 10.1016/s0021-9673(01)95474-3. [DOI] [PubMed] [Google Scholar]


