A number of biologically active prenylflavonoid natural products have been isolated from the mulberry tree and related plants.1 For example, kuwanon G (1)2 and multicaulisin (2)3 are Diels-Alder cycloadducts between prenylflavonoid dienes and 2′-hydroxychalcones (Figure 1). Related Diels-Alder cycloadducts4 include (−)-panduratin A (3)5 and nicolaioidesin C (4).6 In order to access these natural products, we wished to develop methodology to construct the cyclohexenyl chalcone nucleus employing electron rich 2′-hydroxychalcone dienophiles.7 In this Communication, we report examples of such [4+2] cycloadditions in a process likely involving electron transfer.8
Figure 1.
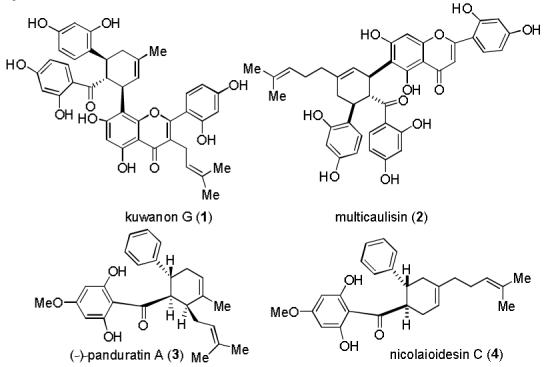
Select Diels-Alder Natural Products Derived from 2′-Hydroxychalcones
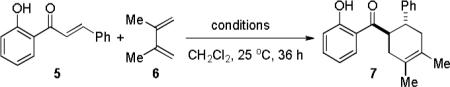
Our studies began with model reactions of trans-2′-hydroxy chalcone 5 and 2,3-dimethylbutadiene 6 (Table 1). Due to our inability to effect cycloaddition using Lewis acid-promoted (“LUMO” lowering) conditions,9 we considered alternative modes of catalysis. Based on a recent report involving Diels-Alder dimerization of piperine,10 we evaluated Co(I) catalysis11 for cycloaddition. Initial studies revealed that cycloadduct 7 was observed as a single trans- diastereomer using CoI2/1,10-phenanthroline (8)/ZnI2/Bu4NBH4 (10/10/30/10 mol%) (entry 1),12 which is in contrast to the 1,4-hydrovinylation of dienes and terminal alkenes employing a similar Co(I) catalyst system reported by Hilt and coworkers.11c Further investigation revealed that the amount of ZnI2 had a significant effect on the catalytic process (entries 1-3). Near quantitative conversion and isolated yield of 7 were obtained with CoI2/8/ZnI2/Bu4NBH4 (10/10/60/10 mol%) as catalyst (entry 3). Lower conversion was obtained in the absence of ligand 8 (entry 4). Remarkably, cycloaddition in the absence of cobalt proceeded in slightly lower yield employing ZnI2 and a catalytic amount of Bu4NBH4 (entry 5), either of which did not mediate the reaction alone (entries 6, 7). Moreover, no desired cycloadduct was observed with Zn(BH4)2 as catalyst.
Table 1.
Optimization of the Diels-Alder Cycloaddition of 5 and 6a
 | ||
|---|---|---|
| entry | CoI2:8:ZnI2:Bu4NBH4 | conv. (%)b |
| 1 | 10:10:30:10 mol% | 23 |
| 2 | 10:10: 0:10 mol% | <2d |
| 3 | 10:10:60:10 mol% | 96(95c) |
| 4 | 10: 0:60:10 mol% | 74 |
| 5 | 0: 0:60:10 mol% | 85(82c) |
| 6 | 0: 0:60: 0 mol% | <2d |
| 7 | 0: 0: 0:10 mol% | <2d |
See Supporting Information for experimental details.
Based on 1H NMR integration (average of two experiments).
Isolated yield.
Not observed.
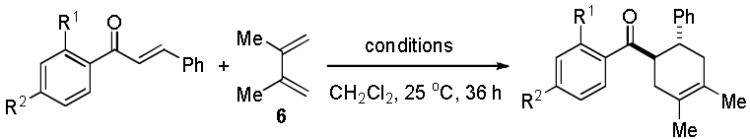
Further studies were undertaken to probe modifications of the chalcone dienophile (Table 2). Removal or methylation of the 2′- hydroxyl group (entries 1, 2) led to production of cycloadducts in lower overall yield in comparison to 7. Reactions conducted without cobalt generally afforded lower isolated yields. Surprisingly, 4′-hydroxychalcone 13 did not undergo cycloaddition (entry 3), implying that chelation of 5 to ZnI213 may be necessary for cycloaddition. Additionally, a counterion effect for the Zn(II) source was observed (I > Br > Cl) with ZnF2, Zn(OAc)2, and Zn(OTf)2 proving to be unreactive.12
Table 2.
Chalcone modifications
 | ||||
|---|---|---|---|---|
| entry | 2′-hydroxychalcone | product | conditiona | yield(%)b |
| 1 | 9: R1, R2 = H | 10 | A B |
38 28 |
| 2 | 11: R1 = OMe, R2 = H | 12 | A B |
55 50 |
| 3 | 13: R1 = H, R2 = OH | 14 | A B |
<2c <2c |
Condition A: 10/10/60/10 mol% CoI2/8/ZnI2/Bu4NBH4; condition B: 60/10 mol% ZnI2/Bu4NBH4, see Supporting Information.
Isolated yield.
Not observed.
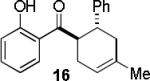
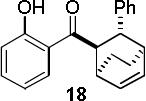
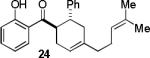
Encouraged by the success of the model reaction, we next evaluated a range of dienes and 2′-hydroxychalcones. [4+2] cycloadditions of select dienes and 5 were conducted in satisfactory isolated yield using CoI2/8/ZnI2/Bu4NBH4 (10/10/60/10 mol%) at 40 °C (Table 3). Reactions without cobalt showed decreased reactivity (entries 1, 2). Notably, single regioisomers were observed for unsymmetrical dienes (entries 1, 4-6). Trisubstituted diene 25, poorly reactive in conventional [4+2] cycloadditions,14 afforded cycloadduct 26 in moderate yield (entry 6). A number of highly electron-rich 2′-hydroxychalcones were also investigated (Table 4). For these dienophiles, a 20/40/120/20 mol% CoI2/8/ZnI2/Bu4NBH4 catalyst loading was found to be optimal. Lower yields were obtained with additional alkoxy substitution of the chalcone (entries 1, 3, and 5). The corresponding acetylated 2′-hydroxychalcones maintained high reactivity likely due to their less electron rich character (entries 2, 4, and 6).
Table 3.
Diels-Alder Reactions of 5 and Dienes
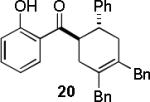
| entry | diene | product | conditiona | yield(%)b |
|---|---|---|---|---|
| 1 |  |
 |
A B |
97c 67c |
| 2 | 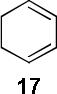 |
 |
A B |
97d 65d |
| 3 | 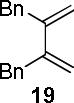 |
 |
A | 99 |
| 4 | 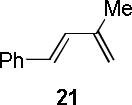 |
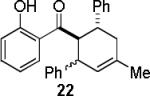 |
A | 97c,e |
| 5 | 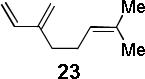 |
 |
A | 96c |
| 6 | 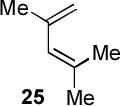 |
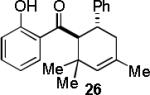 |
A | 55c |
Condition A: 10/10/60/10 mol% CoI2/8/ZnI2/Bu4NBH4, 40°C; condition B: 60/10 mol% ZnI2/Bu4NBH4, 40°C, see Supporting Information.
Isolated yields.
Single regioisomer.
Single endo isomer.
1.5:1 exo/endo ratio.
Table 4.
Diels-Alder Reactions of Electron-rich 2′-Hydroxychalcones
 | ||||
|---|---|---|---|---|
| entry | 2′-hydroxychalcone | product | conditiona | Yield(%)b |
| 1 | 27: R1=OMe, R2,R3,R4=H | 28 | A B |
68 36 |
| 2 | 29: R1=OAc, R2,R3,R4=H | 30 | A B |
84 55 |
| 3 | 31: R1,R2=OMe, R3,R4=H | 32 | A | 33 |
| 4 | 33: R1,R2=OAc, R3,R4=H | 34 | A | 72 |
| 5 | 35: R1,R3,R4=OMe, R2=H | 36 | A | 18 |
| 6 | 37: R1,R3,R4=OAc, R2=H | 38 | A | 61 |
Condition A: 20/40/120/20 mol% CoI2/8/ZnI2/Bu4NBH4; condition B: 120/20 mol% ZnI2/Bu4NBH4, see Supporting Information.
Isolated yields.
The utility of acetylated 2′-hydroxychalcones in [4+2] cycloadditions was further established by the total synthesis of nicolaioidesin C (4)6 (Scheme 1). Acetylated chalcone 39 was prepared in four steps12 (74% overall yield) from commercially available 2′,6′-dihydroxy-4′-methoxyacetophenone. Diels-Alder cycloaddition of 39 and myrcene 23, followed by saponification, afforded 4 as a single regioisomer in 52% yield. A 15% yield of 4 was observed in the corresponding reaction conducted without cobalt.12
Scheme 1.
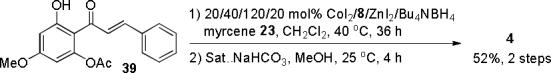
Synthesis of nicolaioidesin C
Our finding that cycloadditions are observed with Bu4NBH4/ZnI215 in conjunction with literature reports documenting electron transfer from Bu4NBH4 to acceptor substrates16 suggests that radical anions17 may be involved in the catalysis. As shown in Scheme 2, coordination of ZnI2 to 2′-hydroxychalcone 5 may afford complex 40. Preliminary cyclic voltammetry studies12 indicate that 5 in the presence of ZnI2 (CH2Cl2) shows two new irreversible reduction peaks (Ep,c −0.59 V, 0.36 V vs. SCE, respectively) compared to 5 alone (Ep,c −1.25 V vs. SCE). The apparent shift in the halfwave reduction potentials to less negative values is expected to parallel the promotion of electron transfer, and may be attributed to carbonyl activation by ZnI2. In the presence of electron donors such as Co(I)11a or borohydride, 40 may undergo metal ion-promoted single electron transfer18 to generate a chalcone radical anion 41.19 Regioselective addition of 41 to isoprene20 should afford a stabilized, allylic radical 42a which may undergo ring-closing cyclization to produce ketyl intermediate 43. Subsequent single electron transfer between 43 and another complex 40 may afford cycloadduct 16 and radical anion 41, thereby restarting the catalytic cycle.
Scheme 2.
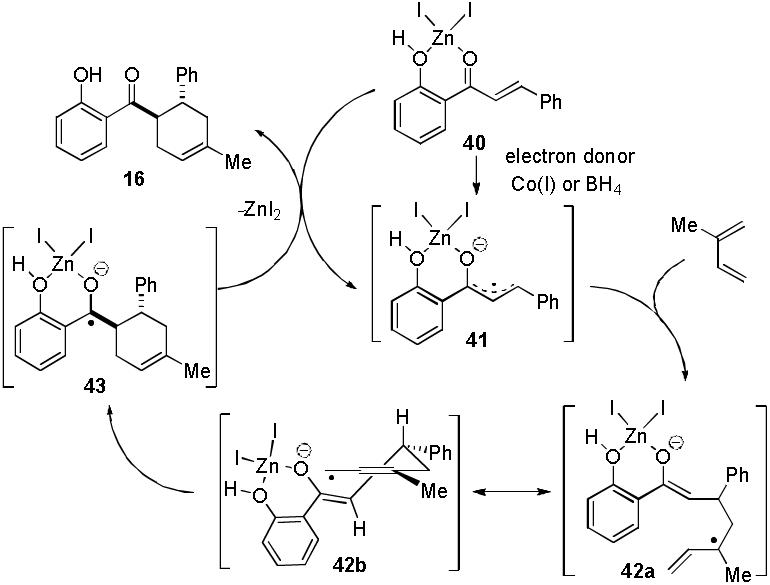
Generalized Mechanism for [4+2] Cycloadditions
In summary, we have developed [4+2] cycloadditions of highly electron rich 2′-hydroxychalcones and dienes using catalyst systems composed of electron donor (Co(I) or BH4−) and a Lewis acid (ZnI2). Mechanistic studies and further applications towards the syntheses of other natural product targets are currently in progress and will be reported in due course.
Supplementary Material
Acknowledgment
Financial support from the NIH (GM-073855), Merck, and Wyeth is gratefully acknowledged. We thank Dr. Aaron Beeler and Ms. Susan Cunningham (CMLD-BU) for HPLC assistance, and Dr. Branko Mitasev (Boston University) and Dr. Bruce Branchaud (Invitrogen) for helpful discussions.
Footnotes
Supporting Information Available: Experimental procedures and characterization data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Nomura T. Pure Appl. Chem. 1999;71:1115. [Google Scholar]
- 2.Nomura T, Fukai T. Chem. Pharm. Bull. 1980;28:2548. [Google Scholar]
- 3.Ferrari F, Delle Monache F, Suarez AI, Compagnone RS. Fitoterapia. 2000;71:213. doi: 10.1016/s0367-326x(99)00156-2. [DOI] [PubMed] [Google Scholar]
- 4.Stocking EM, Williams RM. Angew. Chem. Int. Ed. 2003;42:3078. doi: 10.1002/anie.200200534. [DOI] [PubMed] [Google Scholar]
- 5.Tuntiwachwuttikul P, Pancharoen O, Reutrakul V, Byrne LT. Aust. J. Chem. 1984;37:449. [Google Scholar]
- 6.Gu J-Q, Park EJ, Vigo JS, Graham JG, Fong HHS, Pezzuto JM, Kinghorn AD. J. Nat. Prod. 2002;65:1616. doi: 10.1021/np020249p. [DOI] [PubMed] [Google Scholar]
- 7.Brito CM, Pinto DCGA, Silva AMS, Silva AMG, Tome AC, Cavaleiro JAS. Eur. J. Org. Chem. 2006:2558. [Google Scholar]; For Diels-Alder cycloaddition of 2′-hydroxychalcone, see:
- 8.Bauld NL. Tetrahedron. 1989;45:5307. [Google Scholar]; Radical cation cycloadditions:
- 9.a Otto S, Engberts JBFNJ. Am. Chem. Soc. 1999;121:6798. [Google Scholar]; b Barroso S, Blay G, Pedro JR. Org. Lett. 2007;9:1983. doi: 10.1021/ol0705752. [DOI] [PubMed] [Google Scholar]
- 10.Wei K, Li W, Koike K, Nikaido T. Org. Lett. 2005;7:2833. doi: 10.1021/ol050689i. [DOI] [PubMed] [Google Scholar]
- 11.a Baik T-G, Wang L-C, Luiz A-L, Krische MJ. J. Am. Chem. Soc. 2002;124:9448. doi: 10.1021/ja020223k. [DOI] [PubMed] [Google Scholar]; b Chang H-T, Jayanth TT, Cheng C-H. J. Am. Chem. Soc. 2007;129:4166. doi: 10.1021/ja0710196. [DOI] [PubMed] [Google Scholar]; c Hilt G, Lüers S, Schmidt F. Synthesis. 2004:634. [Google Scholar]; d Hilt G, Janikowski J, Hess W. Angew. Chem. Int. Ed. 2006;45:5204. doi: 10.1002/anie.200601974. [DOI] [PubMed] [Google Scholar]; e Lautens M, Tam W, Lautens JC, Edwards LG, Crudden CM, Smith AC. J. Am. Chem. Soc. 1995;117:6863. [Google Scholar]; f Ma B, Snyder JK. Organometallics. 2002;21:4688. [Google Scholar]; g Achard M, Mosrin M, Tenaglia A, Buono G. J. Org. Chem. 2006;71:2907. doi: 10.1021/jo052630v. [DOI] [PubMed] [Google Scholar]; Select examples of Co(I)-catalyzed cycloadditions:
- 12.See Supporting Information for complete experimental details.
- 13.Swamy SJ, Lingaiah P. Indian J. Chem., Sect A. 1978;16:723. [Google Scholar]
- 14.Roush WR, Barda DA. J. Am. Chem. Soc. 1997;119:7402. [Google Scholar]
- 15.Lau CK, Dufresne C, Bélanger PC, Piétré S, Scheigetz J. J. Org. Chem. 1986;51:3038. and references cited therein. [Google Scholar]
- 16.a Lucarini M, Pedulli GF, Alberti A, Paradisi C, Roffia S. J. Chem. Soc., Perkin Trans. 2. 1993:2083. [Google Scholar]; b Lucarini M, Pedulli GF. J. Organomet. Chem. 1995;494:123. [Google Scholar]
- 17.a Borhani DW, Greene FD. J. Org. Chem. 1986;51:1563. [Google Scholar]; b Roh Y, Jang H-Y, Lynch V, Bauld NL, Krische MJ. Org. Lett. 2002;4:611. doi: 10.1021/ol0172065. [DOI] [PubMed] [Google Scholar]
- 18.Fukuzumi S, Okamoto T. J. Am. Chem. Soc. 1993;115:11600. [Google Scholar]
- 19.Quintana-Espinoza P, Yáñez C, Escobar CA, Sicker D, Araya-Maturana R, Squella JA. Electroanalysis. 2006;18:521. [Google Scholar]
- 20.Hilt G, Bolze P, Harms K. Chem. Eur. J. 2007;13:4312. doi: 10.1002/chem.200601747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


