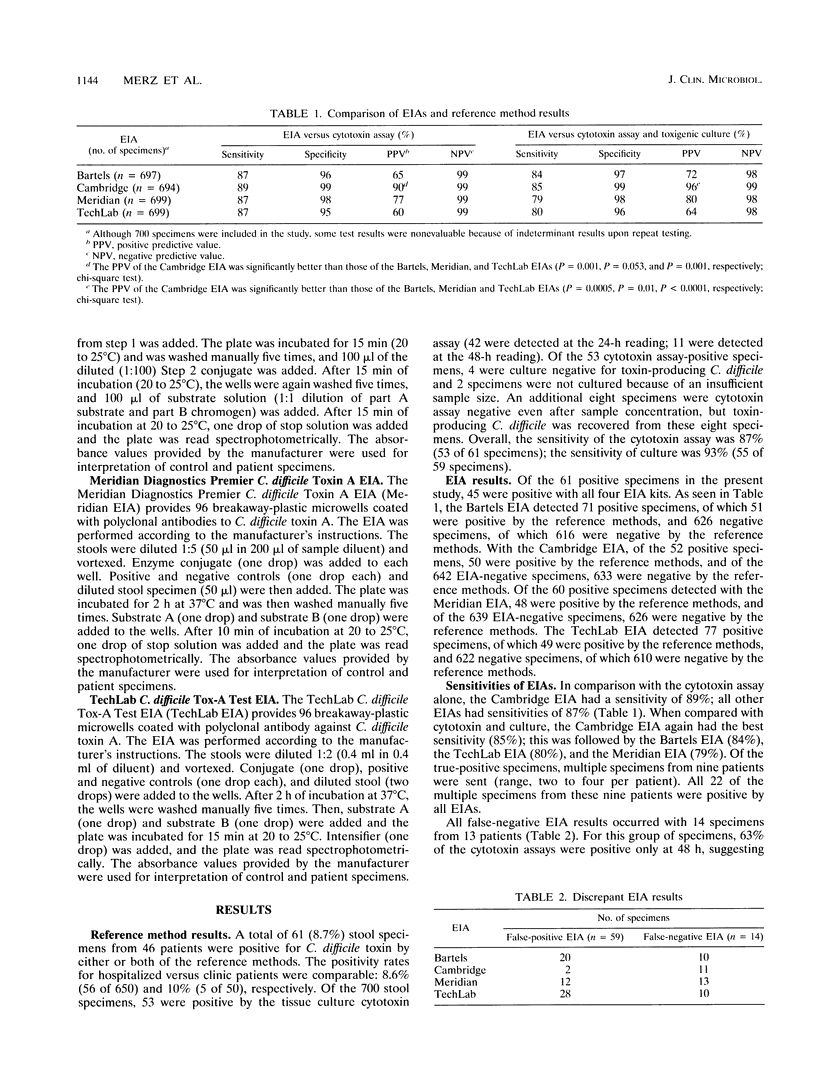
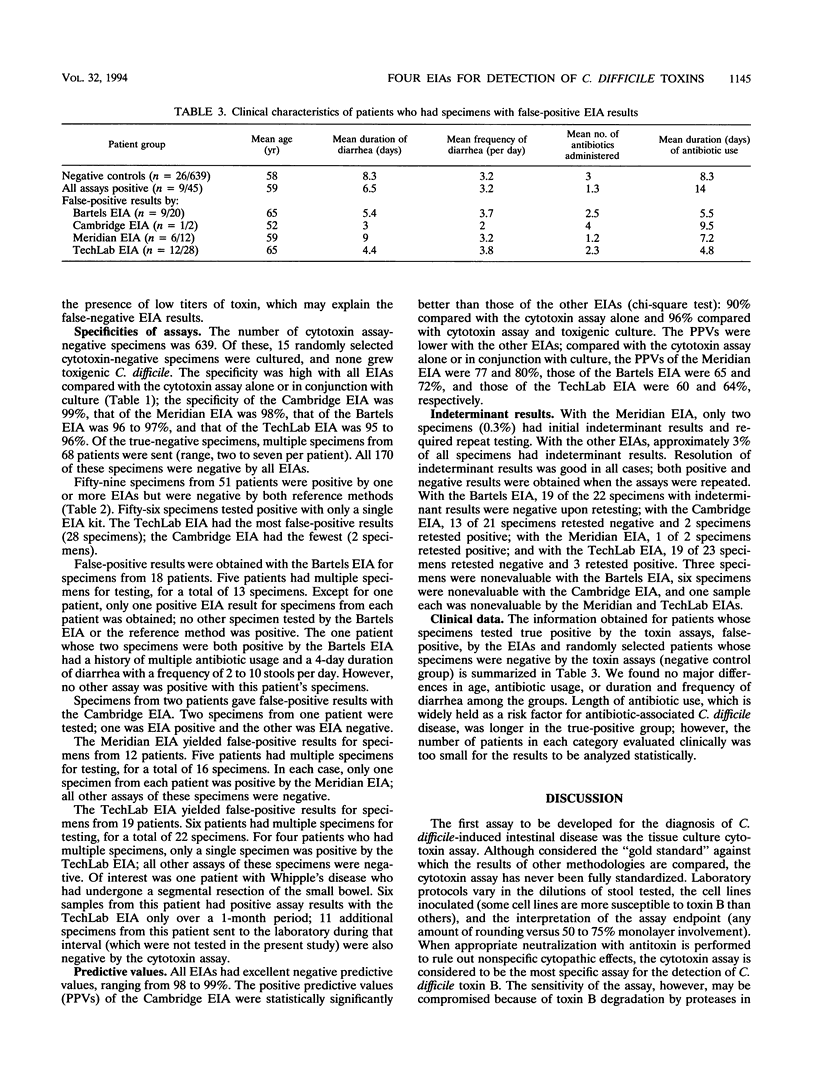
Abstract
Rapid (2.5- to 3.5-h) enzyme immunoassays (EIAs) for the detection of Clostridium difficile toxins have been developed. We report the results of simultaneous testing of 700 fresh stool specimens by the tissue culture cytotoxin assay and four EIAs (Bartels Prima System C. difficile Toxin A EIA, Cambridge Biotech Cytoclone A+B EIA, Meridian Diagnostics Premier C. difficile Toxin A EIA, and TechLab C. difficile Tox-A Test EIA). In cases of disagreement, culturing for toxigenic C. difficile was performed. A total of 61 (8.7%) specimens from 46 patients were positive for C. difficile toxin. The sensitivity of the cytotoxin assay was 87%, and that of culture was 93%. In comparison with the cytotoxin assay results, the sensitivity and specificity of the EIAs were as follows: Bartels, 87 and 96%; Cambridge, 89 and 99%; Meridian, 87 and 98%; and TechLab, 87 and 95%, respectively. In comparison with the cytotoxin assay plus toxigenic culture results, the sensitivity and specificity of the EIAs were as follows: Bartels, 84 and 97%; Cambridge, 85 and 99%; Meridian, 79 and 98%; and TechLab, 80 and 96%, respectively. The EIAs varied in positive predictive values (PPVs). A high PPV was seen with the Cambridge EIA (96%); lower PPVs were seen with the TechLab (64%), Bartels (72%), and Meridian (80%) EIAs because of high false-positive rates. The negative predictive values (98 to 99%) were excellent with all EIAs. Results were indeterminant with 0.3% of the samples by the Meridian EIA and 3% by all the other EIAs. Although the EIAs were less sensitive than the cytotoxin assay, they provide same-day results and may be useful in laboratories without tissue culture facilities.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbut F., Kajzer C., Planas N., Petit J. C. Comparison of three enzyme immunoassays, a cytotoxicity assay, and toxigenic culture for diagnosis of Clostridium difficile-associated diarrhea. J Clin Microbiol. 1993 Apr;31(4):963–967. doi: 10.1128/jcm.31.4.963-967.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borriello S. P., Vale T., Brazier J. S., Hyde S., Chippeck E. Evaluation of a commercial enzyme immunoassay kit for the detection of Clostridium difficile toxin A. Eur J Clin Microbiol Infect Dis. 1992 Apr;11(4):360–363. doi: 10.1007/BF01962079. [DOI] [PubMed] [Google Scholar]
- Cooperstock M., Riegle L., Woodruff C. W., Onderdonk A. Influence of age, sex, and diet on asymptomatic colonization of infants with Clostridium difficile. J Clin Microbiol. 1983 May;17(5):830–833. doi: 10.1128/jcm.17.5.830-833.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Girolami P. C., Hanff P. A., Eichelberger K., Longhi L., Teresa H., Pratt J., Cheng A., Letourneau J. M., Thorne G. M. Multicenter evaluation of a new enzyme immunoassay for detection of Clostridium difficile enterotoxin A. J Clin Microbiol. 1992 May;30(5):1085–1088. doi: 10.1128/jcm.30.5.1085-1088.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmée M., Mackey T., Hamitou A. Evaluation of a new commercial Clostridium difficile toxin A enzyme immunoassay using diarrhoeal stools. Eur J Clin Microbiol Infect Dis. 1992 Mar;11(3):246–249. doi: 10.1007/BF02098089. [DOI] [PubMed] [Google Scholar]
- Doern G. V., Coughlin R. T., Wu L. Laboratory diagnosis of Clostridium difficile-associated gastrointestinal disease: comparison of a monoclonal antibody enzyme immunoassay for toxins A and B with a monoclonal antibody enzyme immunoassay for toxin A only and two cytotoxicity assays. J Clin Microbiol. 1992 Aug;30(8):2042–2046. doi: 10.1128/jcm.30.8.2042-2046.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglow R., Pothoulakis C., Itzkowitz S., Israel E. J., O'Keane C. J., Gong D., Gao N., Xu Y. L., Walker W. A., LaMont J. T. Diminished Clostridium difficile toxin A sensitivity in newborn rabbit ileum is associated with decreased toxin A receptor. J Clin Invest. 1992 Sep;90(3):822–829. doi: 10.1172/JCI115957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. T., Champagne S. G., Sherlock C. H., Noble M. A., Freeman H. J., Smith J. A. Commercial latex agglutination test for detection of Clostridium difficile-associated diarrhea. J Clin Microbiol. 1987 Jul;25(7):1244–1247. doi: 10.1128/jcm.25.7.1244-1247.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoop F. C., Owens M., Crocker I. C. Clostridium difficile: clinical disease and diagnosis. Clin Microbiol Rev. 1993 Jul;6(3):251–265. doi: 10.1128/cmr.6.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl S. J., Tang Y. J., Navarro L., Gumerlock P. H., Silva J., Jr Diagnosis and monitoring of Clostridium difficile infections with the polymerase chain reaction. Clin Infect Dis. 1993 Jun;16 (Suppl 4):S234–S238. doi: 10.1093/clinids/16.supplement_4.s234. [DOI] [PubMed] [Google Scholar]
- Lima A. A., Lyerly D. M., Wilkins T. D., Innes D. J., Guerrant R. L. Effects of Clostridium difficile toxins A and B in rabbit small and large intestine in vivo and on cultured cells in vitro. Infect Immun. 1988 Mar;56(3):582–588. doi: 10.1128/iai.56.3.582-588.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Krivan H. C., Wilkins T. D. Clostridium difficile: its disease and toxins. Clin Microbiol Rev. 1988 Jan;1(1):1–18. doi: 10.1128/cmr.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Lockwood D. E., Richardson S. H., Wilkins T. D. Biological activities of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1147–1150. doi: 10.1128/iai.35.3.1147-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peach S. L., Borriello S. P., Gaya H., Barclay F. E., Welch A. R. Asymptomatic carriage of Clostridium difficile in patients with cystic fibrosis. J Clin Pathol. 1986 Sep;39(9):1013–1018. doi: 10.1136/jcp.39.9.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe R. D., Song W. Purification of a functional receptor for Clostridium difficile toxin A from intestinal brush border membranes of infant hamsters. Clin Infect Dis. 1993 Jun;16 (Suppl 4):S219–S227. doi: 10.1093/clinids/16.supplement_4.s219. [DOI] [PubMed] [Google Scholar]
- Shanholtzer C. J., Willard K. E., Holter J. J., Olson M. M., Gerding D. N., Peterson L. R. Comparison of the VIDAS Clostridium difficile toxin A immunoassay with C. difficile culture and cytotoxin and latex tests. J Clin Microbiol. 1992 Jul;30(7):1837–1840. doi: 10.1128/jcm.30.7.1837-1840.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N. S., Thorne G. M., Bartlett J. G. Comparison of two toxins produced by Clostridium difficile. Infect Immun. 1981 Dec;34(3):1036–1043. doi: 10.1128/iai.34.3.1036-1043.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


