Abstract
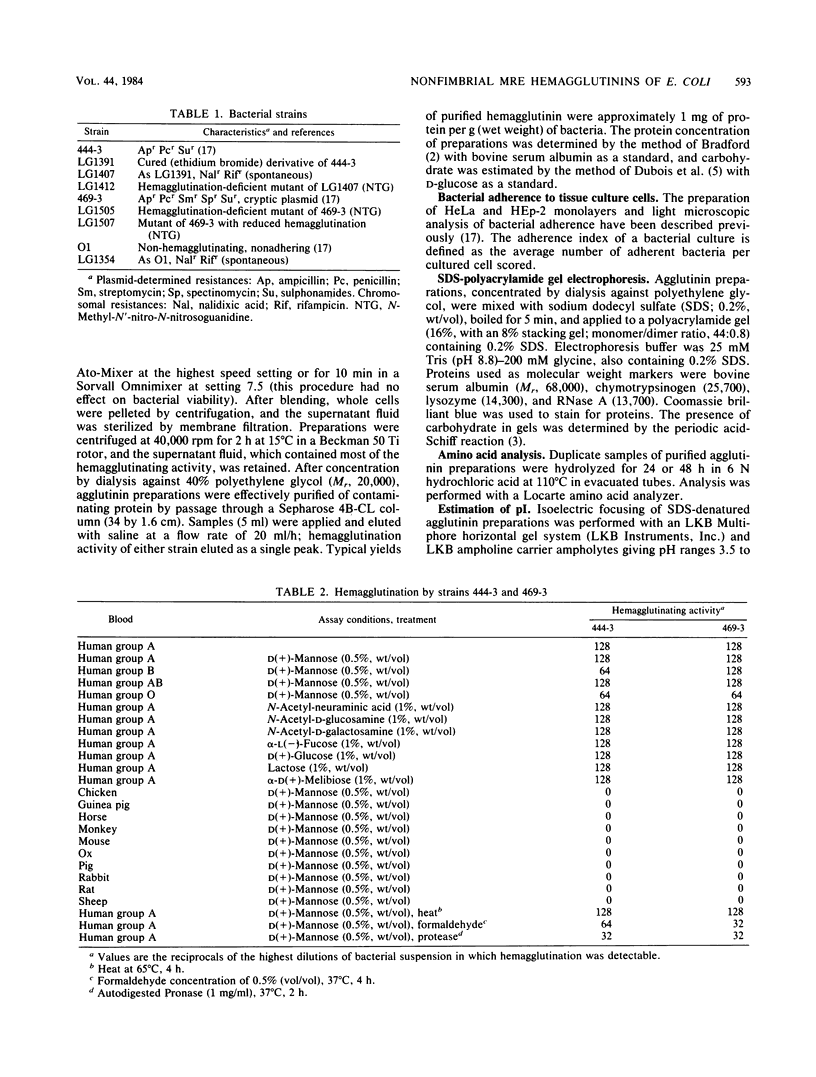
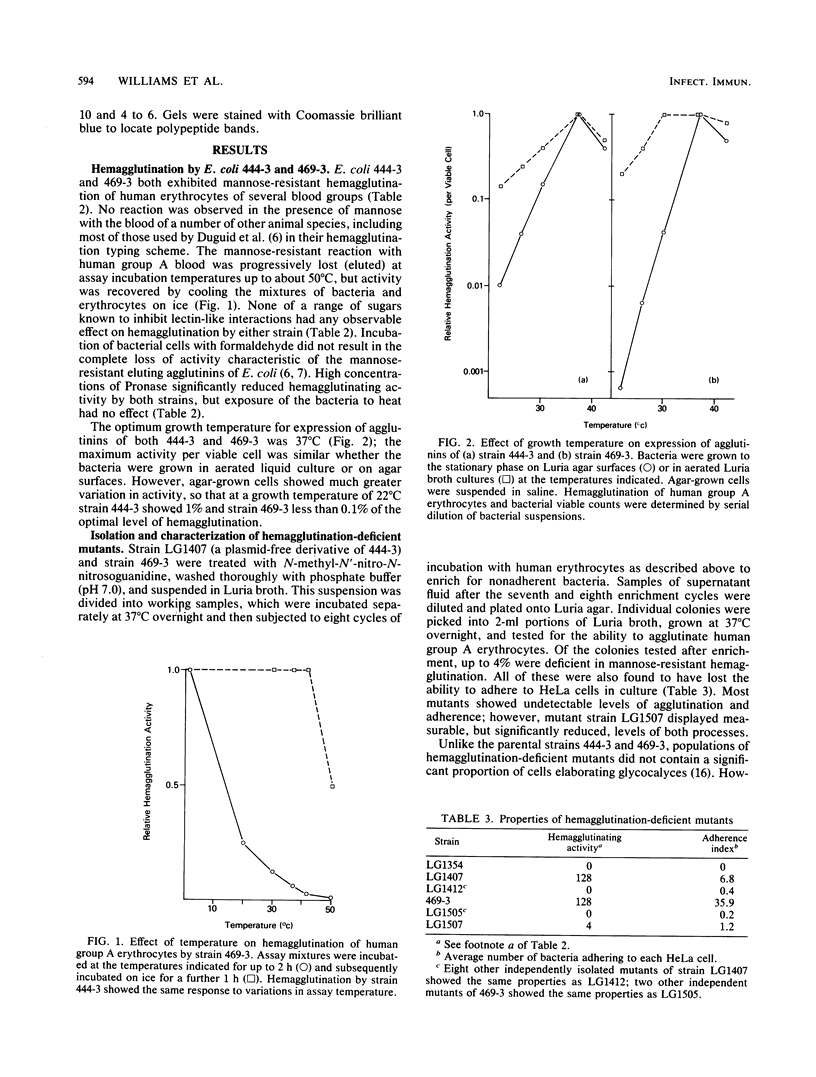
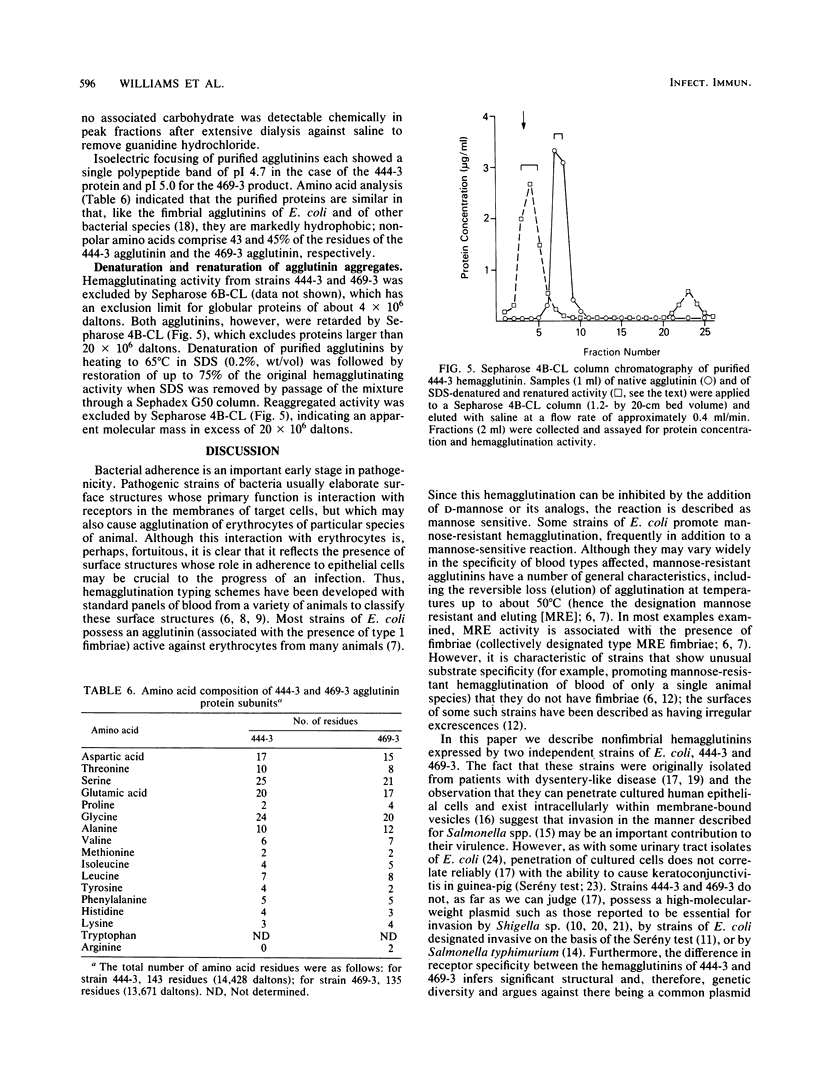
Escherichia coli strains 444-3 and 469-3, isolated from patients with severe infantile enteritis, are able to adhere to and penetrate human epithelial cells in culture. In addition to type 1 fimbriae and glycocalyces , both strains elaborate mannose-resistant nonfimbrial protein hemagglutinins specific for human erythrocytes. Purified agglutinins are aggregates (greater than 4 X 10(6) daltons) of a single protein subunit of apparent Mr 14,000 (469-3) to 14,500 (444-3). The optimal temperature for expression of the agglutinins is 37 degrees C. Bacteria grown at 22 degrees C, which show 1% or less of maximal activity, and mutants deficient in the ability to agglutinate human erythrocytes do not synthesize detectable levels of these surface proteins and, moreover, do not adhere to cultured epithelial cells. Coupled with the observation that purified agglutinins competitively inhibit bacterial adherence to cultured cells, these data indicate that the nonfimbrial surface proteins expressed by strains 444-3 and 469-3 are essential for adherence both to erythrocytes and to cultured epithelial cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouanchaud D. H., Scavizzi M. R., Chabbert Y. A. Elimination by ethidium bromide of antibiotic resistance in enterobacteria and staphylococci. J Gen Microbiol. 1968 Dec;54(3):417–425. doi: 10.1099/00221287-54-3-417. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- CLARKE J. T. SIMPLIFIED "DISC" (POLYACRYLAMIDE GEL) ELECTROPHORESIS. Ann N Y Acad Sci. 1964 Dec 28;121:428–436. doi: 10.1111/j.1749-6632.1964.tb14214.x. [DOI] [PubMed] [Google Scholar]
- Czirók E., Madár J., Budai J., Stverteczky Z., Kertész A., Dombi I., Gyengési L. The role in sporadic enteritis of facultatively enteropathogenic Escherichia coli serogroups. Acta Microbiol Acad Sci Hung. 1976;23(4):359–369. [PubMed] [Google Scholar]
- Duguid J. P., Clegg S., Wilson M. I. The fimbrial and non-fimbrial haemagglutinins of Escherichia coli. J Med Microbiol. 1979 May;12(2):213–227. doi: 10.1099/00222615-12-2-213. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., DuPont H. L. Hemagglutination patterns of enterotoxigenic and enteropathogenic Escherichia coli determined with human, bovine, chicken, and guinea pig erythrocytes in the presence and absence of mannose. Infect Immun. 1979 Feb;23(2):336–346. doi: 10.1128/iai.23.2.336-346.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Young L. S., Pitt J. Hemagglutination typing of Escherichia coli: definition of seven hemagglutination types. J Clin Microbiol. 1980 Aug;12(2):235–242. doi: 10.1128/jcm.12.2.235-242.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Sansonetti P. J., Schad P. A., Austin S., Formal S. B. Characterization of virulence plasmids and plasmid-associated outer membrane proteins in Shigella flexneri, Shigella sonnei, and Escherichia coli. Infect Immun. 1983 Apr;40(1):340–350. doi: 10.1128/iai.40.1.340-350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. R., Wachsmuth I. K., Davis B. R., Cohen M. L. High-molecular-weight plasmid correlates with Escherichia coli enteroinvasiveness. Infect Immun. 1982 Sep;37(3):1295–1298. doi: 10.1128/iai.37.3.1295-1298.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip S. M., Crichton P. B., Old D. C., Duguid J. P. Mannose-resistant and eluting haemagglutinins and fimbriae in Escherichia coli. J Med Microbiol. 1981 May;14(2):223–226. doi: 10.1099/00222615-14-2-223. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Rabert D. K., Svinarich D. M., Whitfield H. J. Association of adhesive, invasive, and virulent phenotypes of Salmonella typhimurium with autonomous 60-megadalton plasmids. Infect Immun. 1982 Nov;38(2):476–486. doi: 10.1128/iai.38.2.476-486.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihlström E., Nilsson L. Endocytosis of Salmonella typhimurium 395 MS and MR10 by HeLa cells. Acta Pathol Microbiol Scand B. 1977 Oct;85B(5):322–328. doi: 10.1111/j.1699-0463.1977.tb01982.x. [DOI] [PubMed] [Google Scholar]
- Knutton S., Williams P. H., Lloyd D. R., Candy D. C., McNeish A. S. Ultrastructural study of adherence to and penetration of cultured cells by two invasive Escherichia coli strains isolated from infants with enteritis. Infect Immun. 1984 Jun;44(3):599–608. doi: 10.1128/iai.44.3.599-608.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandadasa H. G., Sargent G. F., Brown M. G., McNeish A. S., Williams P. H. The role of plasmids in adherence of invasive Escherichia coli to mammalian cells. J Infect Dis. 1981 Feb;143(2):286–290. doi: 10.1093/infdis/143.2.286. [DOI] [PubMed] [Google Scholar]
- Rudoy R. C. Enteroinvasive and enterotoxigenic Escherichia coli. Occurrence in acute diarrhea of infants and children. Am J Dis Child. 1975 Jun;129(6):668–672. doi: 10.1001/archpedi.1975.02120430008004. [DOI] [PubMed] [Google Scholar]
- SERENY B. Experimental shigella keratoconjunctivitis; a preliminary report. Acta Microbiol Acad Sci Hung. 1955;2(3):293–296. [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Shigella sonnei plasmids: evidence that a large plasmid is necessary for virulence. Infect Immun. 1981 Oct;34(1):75–83. doi: 10.1128/iai.34.1.75-83.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varian S. A., Cooke E. M. Adhesive properties of Escherichia coli from urinary-tract infections. J Med Microbiol. 1980 Feb;13(1):111–119. doi: 10.1099/00222615-13-1-111. [DOI] [PubMed] [Google Scholar]
- Williams P. H., Sedgwick M. I., Evans N., Turner P. J., George R. H., McNeish A. S. Adherence of an enteropathogenic strain of Escherichia coli to human intestinal mucosa is mediated by a colicinogenic conjugative plasmid. Infect Immun. 1978 Nov;22(2):393–402. doi: 10.1128/iai.22.2.393-402.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



