Abstract
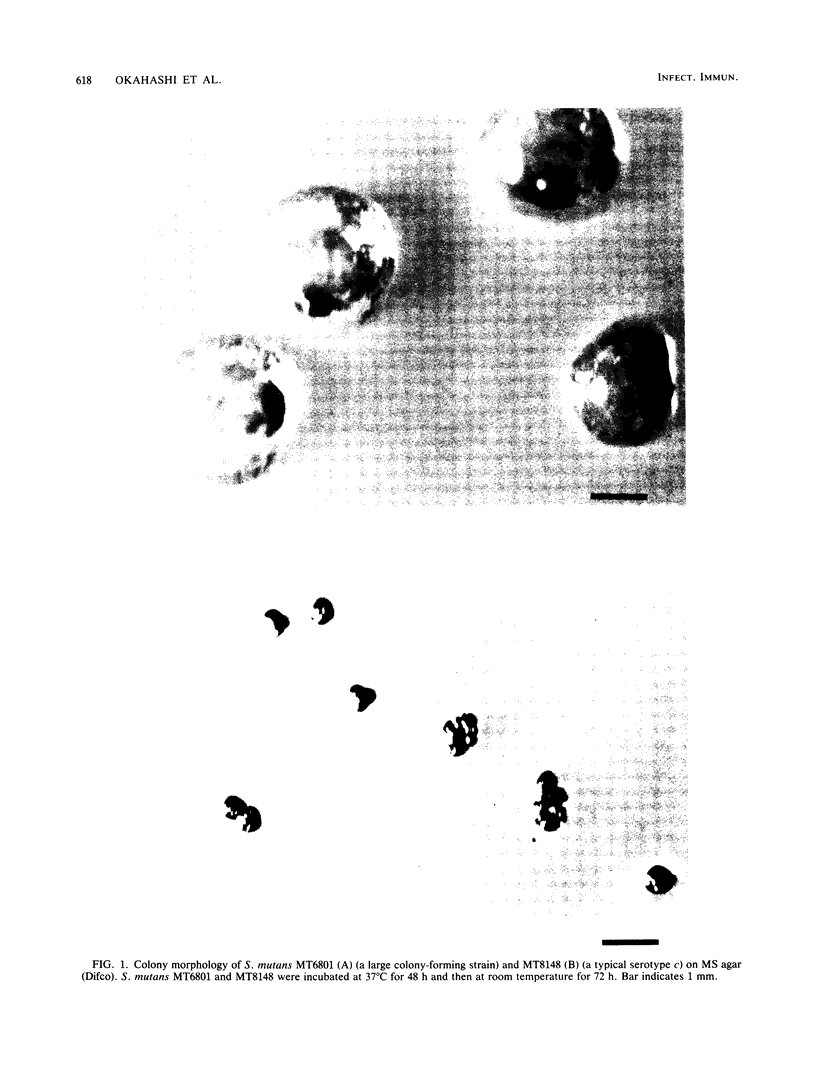
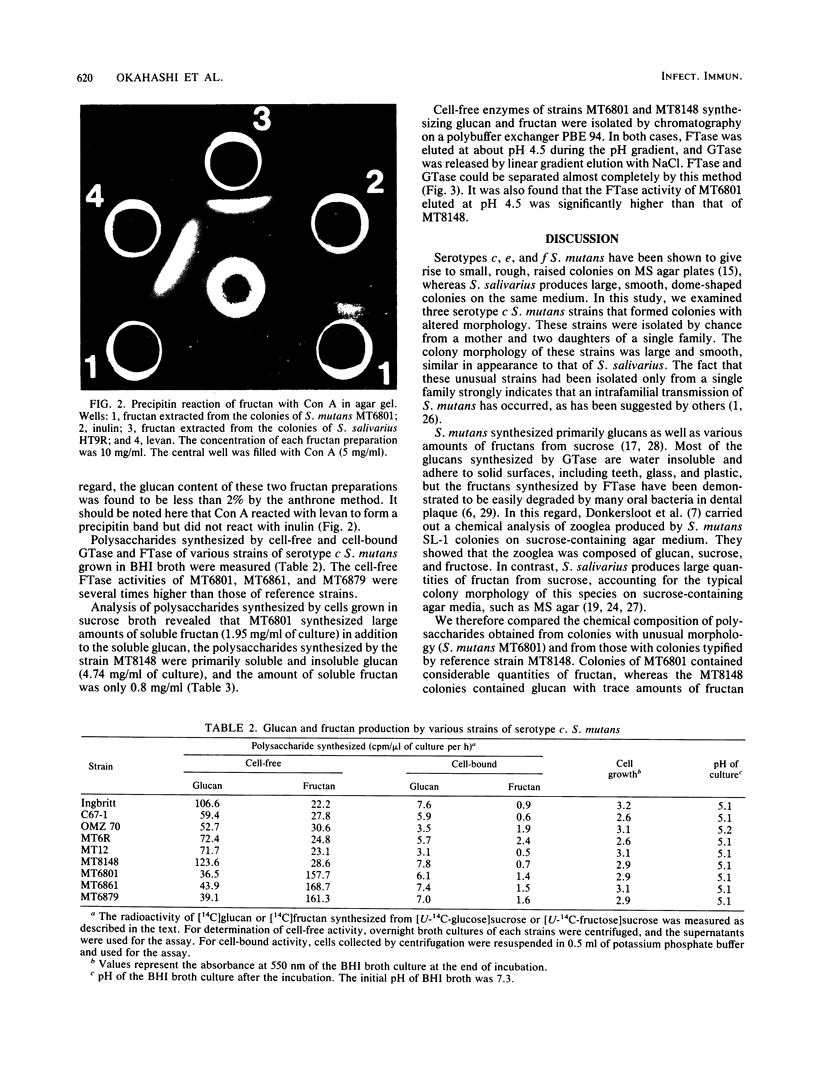
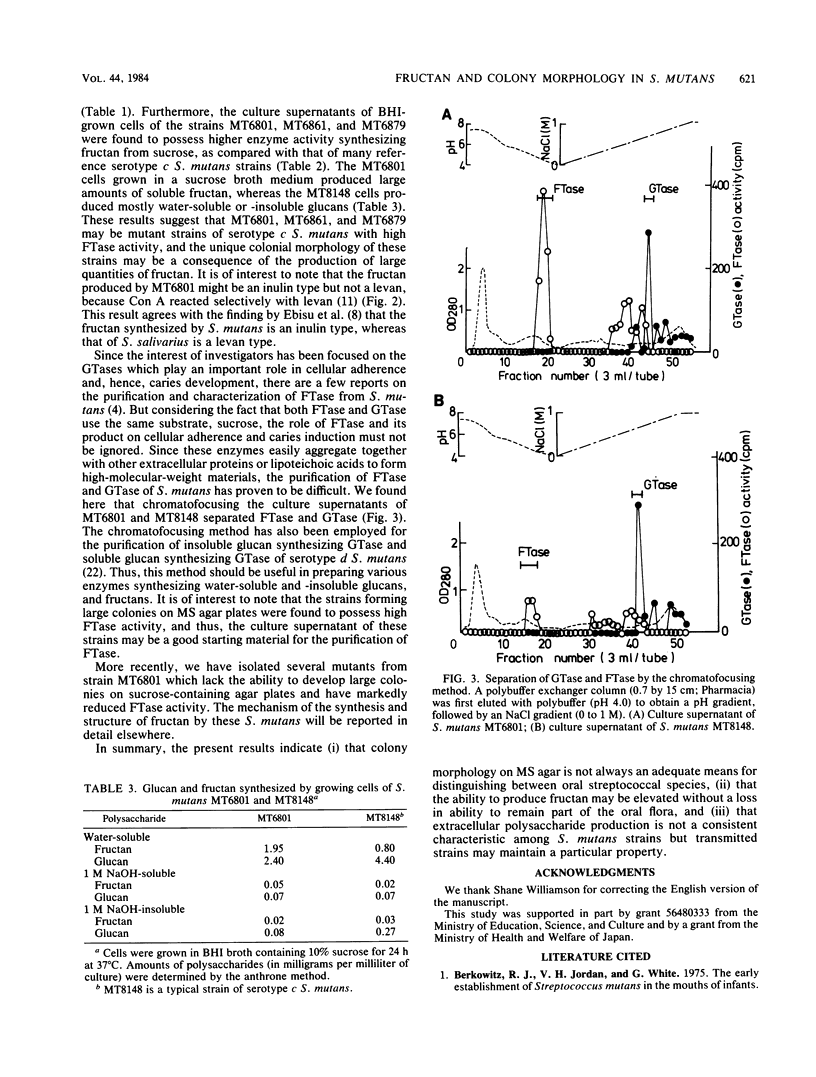
Streptococcus mutans MT6801 , MT6861 , and MT6879 , which form large mucoid colonies on mitis salivarius agar, were isolated from a mother and her two daughters. These isolates were identified as serotype c by immunodiffusion with serotype-specific antisera. The large colonies formed on sucrose-containing agar were found to contain water-soluble fructan . The cell-free fructosyltransferase ( FTase ) activity of the strains which formed large colonies was five to eight times higher than that of serotype c S. mutans which produced small, rough colonies typical of this serotype. Furthermore, greater quantities of fructan were synthesized from sucrose by growing cells of MT6801 when compared with MT8148 , a typical serotype c S. mutans. Glucosyltransferase and FTase could be isolated by chromatofocusing from culture supernatants of MT6801 and MT8148 . The FTase activity of both strains was eluted at pH 4.5, and glucosyltransferase was released by elution with an NaCl linear gradient. The eluted FTase activity of MT6801 was significantly higher than that of MT8148 . Strains MT6861 and MT6879 were also found to possess a similar property in terms of FTase activity. These results suggest that formation of large mucoid colonies by these strains is a consequence of high FTase activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bratthall D. Demonstration of Streptococcus mutans strains in some selected areas of the world. Odontol Revy. 1972;23(4):401–410. [PubMed] [Google Scholar]
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Carlsson J. A levansucrase from Streptococcus mutans. Caries Res. 1970;4(2):97–113. doi: 10.1159/000259632. [DOI] [PubMed] [Google Scholar]
- Corrigan A. J., Robyt J. F. Nature of the fructan of Streptococcus mutans OMZ 176. Infect Immun. 1979 Oct;26(1):387–389. doi: 10.1128/iai.26.1.387-389.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaCosta T., Gibbons R. J. Hydrolysis of levan by human plaque streptococci. Arch Oral Biol. 1968 Jun;13(6):609–617. doi: 10.1016/0003-9969(68)90139-8. [DOI] [PubMed] [Google Scholar]
- Donkersloot J. A., de Leon H. A., Chassy B. M., Krichevsky M. I. Analysis of the exudate produced by Streptococcus mutans SL-1 colonies of sucrose-containing agar media. Appl Environ Microbiol. 1976 Sep;32(3):448–450. doi: 10.1128/aem.32.3.448-450.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisu S., Kato K., Kotani S., Misaki A. Structural differences in fructans elaborated by streptococcus mutans and Strep. salivarius. J Biochem. 1975 Nov;78(5):879–887. doi: 10.1093/oxfordjournals.jbchem.a130993. [DOI] [PubMed] [Google Scholar]
- Edwardsson S. Characteristics of caries-inducing human streptococci resembling Streptococcus mutans. Arch Oral Biol. 1968 Jun;13(6):637–646. doi: 10.1016/0003-9969(68)90142-8. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., So L. L. Protein-carbonhydrate interaction. 3. Agar gel-diffusion studies on the interaction of Concanavalin A, a lectin isolated from jack bean, with polysaccharides. Arch Biochem Biophys. 1965 Aug;111(2):407–414. doi: 10.1016/0003-9861(65)90203-1. [DOI] [PubMed] [Google Scholar]
- Hamada S., Masuda N., Kotani S. Demonstration of serotype d and g specificities of Streptococcus mutans by immunodiffusion. Arch Oral Biol. 1978;23(6):495–499. doi: 10.1016/0003-9969(78)90083-3. [DOI] [PubMed] [Google Scholar]
- Hamada S., Masuda N., Kotani S. Isolation and serotyping of Streptococcus mutans from teeth and feces of children. J Clin Microbiol. 1980 Apr;11(4):314–318. doi: 10.1128/jcm.11.4.314-318.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Masuda N., Ooshima T., Sobue S., Kotani S. Epidemiological survey of Streptococcus mutans among Japanese children. Identification and serological typing of the isolated strains. Jpn J Microbiol. 1976 Feb;20(1):33–44. doi: 10.1111/j.1348-0421.1976.tb00905.x. [DOI] [PubMed] [Google Scholar]
- Hamada S., Masuda N., Shimamoto T. Some biological properties of Streptococcus mutans isolated from human mouths, with reference to the correlation with serotypes. Arch Oral Biol. 1979;24(8):627–631. doi: 10.1016/0003-9969(79)90025-6. [DOI] [PubMed] [Google Scholar]
- Hamada S., Mizuno J., Murayama Y., Ooshima Y., Masuda N. Effect of dextranase on the extracellular polysaccharide synthesis of Streptococcus mutans; chemical and scanning electron microscopy studies. Infect Immun. 1975 Dec;12(6):1415–1425. doi: 10.1128/iai.12.6.1415-1425.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelstrup J. Extracellular polysaccharides of smooth and rough variants of Streptococcus salivarius. Scand J Dent Res. 1981 Oct;89(5):374–383. doi: 10.1111/j.1600-0722.1981.tb01696.x. [DOI] [PubMed] [Google Scholar]
- Koga T., Hamada S., Murakawa S., Endo A. Effect of a glucosyltransferase inhibitor on glucan synthesis and cellular adherence of Streptococcus mutans. Infect Immun. 1982 Dec;38(3):882–886. doi: 10.1128/iai.38.3.882-886.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T., Inoue M. Inactivation of D-glucosyltransferases from oral Streptococcus mutans and Streptococcus sanguis by photochemical oxidation. Carbohydr Res. 1981 Jun 16;93(1):125–133. doi: 10.1016/s0008-6215(00)80757-0. [DOI] [PubMed] [Google Scholar]
- Niven C. F., Smiley K. L., Sherman J. M. The Hydrolysis of Arginine by Streptococci. J Bacteriol. 1942 Jun;43(6):651–660. doi: 10.1128/jb.43.6.651-660.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooshima T., Imanishi H., Hamada S. Changes in some biological properties of Streptococcus salivarius isolates from infected rats. Zentralbl Bakteriol A. 1980;247(4):431–439. [PubMed] [Google Scholar]
- Perch B., Kjems E., Ravn T. Biochemical and serological properties of Streptococcus mutans from various human and animal sources. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):357–370. doi: 10.1111/j.1699-0463.1974.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Rogers A. H. The source of infection in the intrafamilial transfer of Streptococcus mutans. Caries Res. 1981;15(1):26–31. doi: 10.1159/000260496. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Loken A. E., Schmitt M. K. Use of specifically labeled sucrose for comparison of extracellular glucan and fructan metabolism by oral streptococci. Infect Immun. 1972 Feb;5(2):263–266. doi: 10.1128/iai.5.2.263-266.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautner K., Felgenhauer B., Rieder H. Extracellular polysaccharide synthesized by the oral bacterium Streptococcus mutans of serotype a to e in vitro. Arch Oral Biol. 1981;26(12):1005–1013. doi: 10.1016/0003-9969(81)90111-4. [DOI] [PubMed] [Google Scholar]
- Van Handel E. Determination of fructose and fructose-yielding carbohydrates with cold anthrone. Anal Biochem. 1967 Apr;19(1):193–194. doi: 10.1016/0003-2697(67)90152-2. [DOI] [PubMed] [Google Scholar]
- van Houte J., Jansen H. M. Levan degradation by streptococci isolated from human dental plaque. Arch Oral Biol. 1968 Jul;13(7):827–830. doi: 10.1016/0003-9969(68)90102-7. [DOI] [PubMed] [Google Scholar]