Abstract
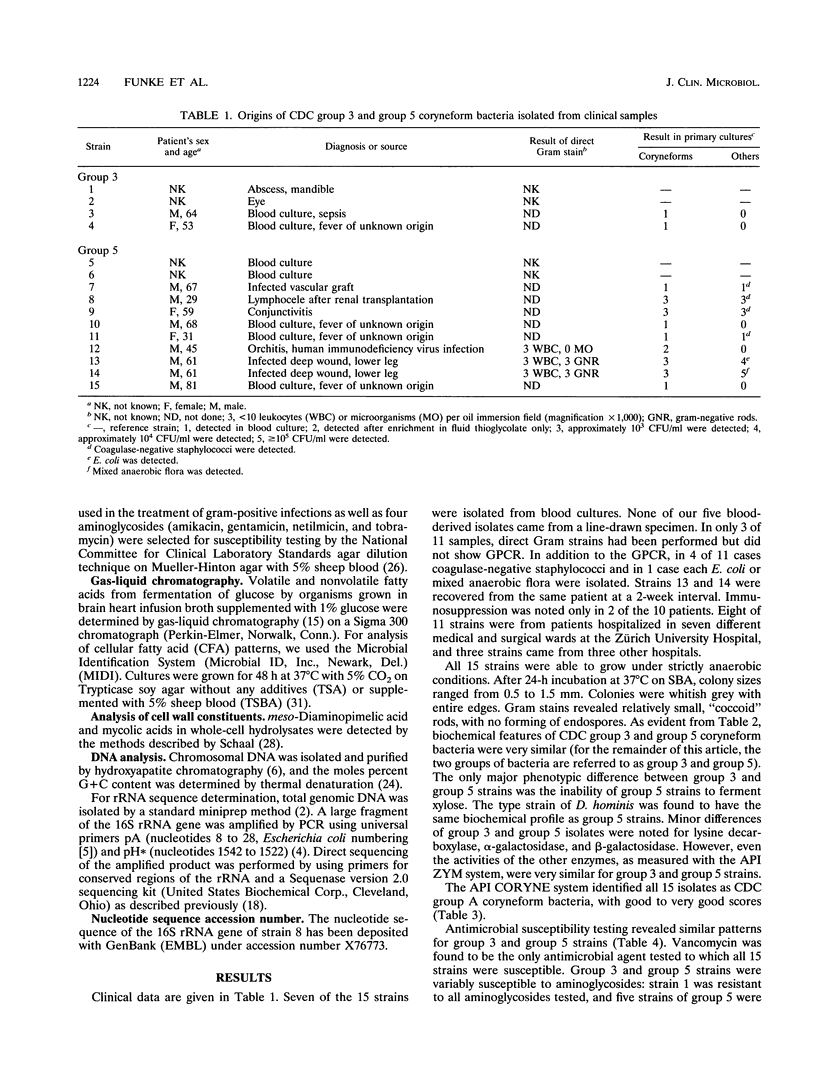
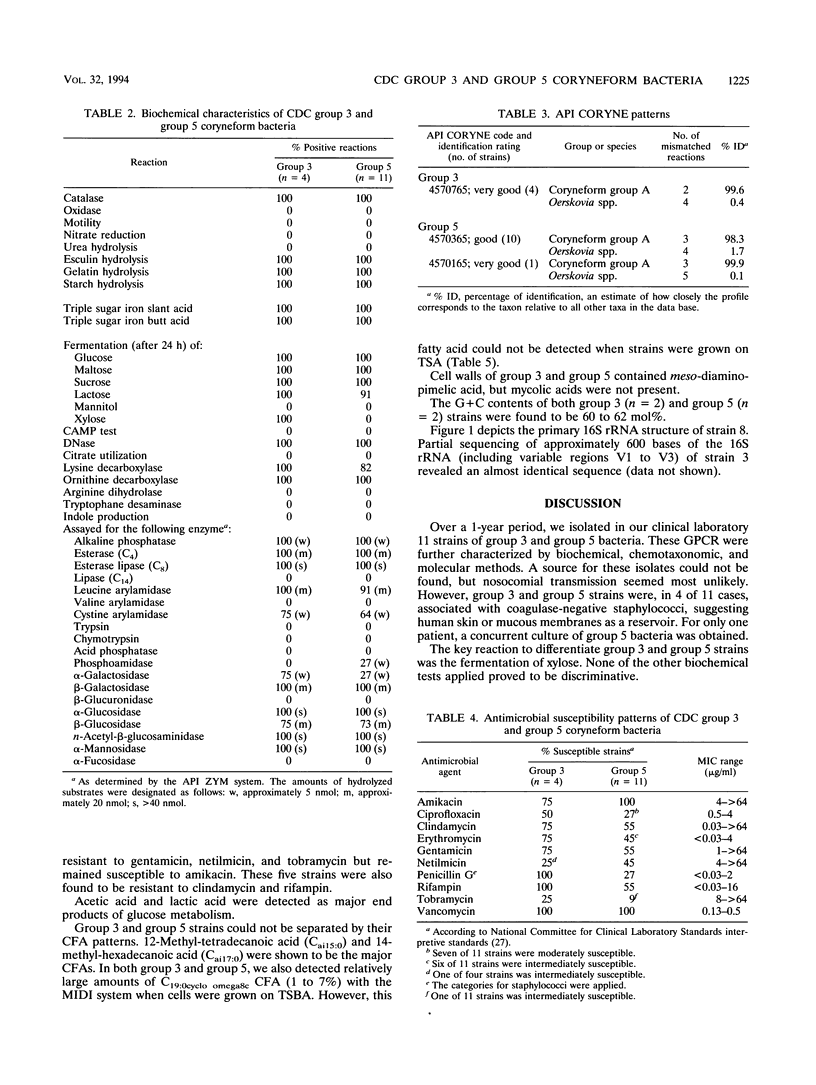
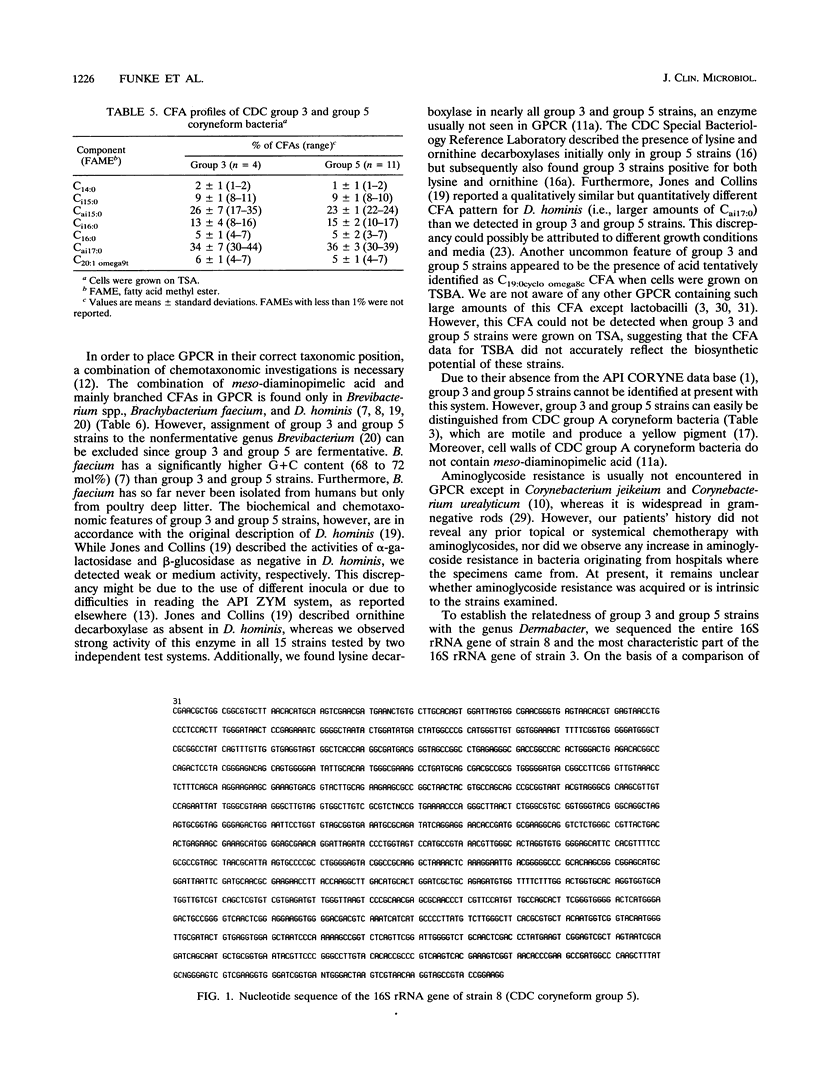
Over a 1-year period, 11 isolates (including 5 from blood cultures) of the recently described CDC group 3 and group 5 coryneform bacteria were derived from clinical specimens and compared with reference strains. Biochemical characteristics indicated a very close relationship between CDC group 3 and group 5 coryneform bacteria. The ability of CDC group 3 and the inability of CDC group 5 coryneform bacteria to ferment xylose were the only reactions that were different for the two taxa. Chemotaxonomic features of the two groups included the presence of meso-diaminopimelic acid, a lack of mycolic acids, and the presence of predominantly branched cellular fatty acids, a combination found among gram-positive rods only in Brevibacterium spp., Brachybacterium faecium, and Dermabacter hominis. 16S rRNA gene sequence analysis revealed that CDC group 3 and group 5 coryneform bacteria are members of the genus Dermabacter, which to date has been isolated exclusively from human skin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard K. A., Bellefeuille M., Ewan E. P. Cellular fatty acid composition as an adjunct to the identification of asporogenous, aerobic gram-positive rods. J Clin Microbiol. 1991 Jan;29(1):83–89. doi: 10.1128/jcm.29.1.83-89.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böddinghaus B., Wolters J., Heikens W., Böttger E. C. Phylogenetic analysis and identification of different serovars of Mycobacterium intracellulare at the molecular level. FEMS Microbiol Lett. 1990 Jul;58(2):197–203. doi: 10.1111/j.1574-6968.1990.tb13978.x. [DOI] [PubMed] [Google Scholar]
- Cashion P., Holder-Franklin M. A., McCully J., Franklin M. A rapid method for the base ratio determination of bacterial DNA. Anal Biochem. 1977 Aug;81(2):461–466. doi: 10.1016/0003-2697(77)90720-5. [DOI] [PubMed] [Google Scholar]
- Coyle M. B., Lipsky B. A. Coryneform bacteria in infectious diseases: clinical and laboratory aspects. Clin Microbiol Rev. 1990 Jul;3(3):227–246. doi: 10.1128/cmr.3.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Briel D., Langs J. C., Rougeron G., Chabot P., Le Faou A. Multiresistant corynebacteria in bacteriuria: a comparative study of the role of Corynebacterium group D2 and Corynebacterium jeikeium. J Hosp Infect. 1991 Jan;17(1):35–43. doi: 10.1016/0195-6701(91)90075-j. [DOI] [PubMed] [Google Scholar]
- Funke G., Lucchini G. M., Pfyffer G. E., Marchiani M., von Graevenitz A. Characteristics of CDC group 1 and group 1-like coryneform bacteria isolated from clinical specimens. J Clin Microbiol. 1993 Nov;31(11):2907–2912. doi: 10.1128/jcm.31.11.2907-2912.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson R. A., Thompson D. E., Collins M. D. Genetic interrelationships of saccharolytic Clostridium botulinum types B, E and F and related clostridia as revealed by small-subunit rRNA gene sequences. FEMS Microbiol Lett. 1993 Mar 15;108(1):103–110. doi: 10.1111/j.1574-6968.1993.tb06081.x. [DOI] [PubMed] [Google Scholar]
- Lechevalier M. P. Lipids in bacterial taxonomy - a taxonomist's view. CRC Crit Rev Microbiol. 1977;5(2):109–210. doi: 10.3109/10408417709102311. [DOI] [PubMed] [Google Scholar]
- Shaw K. J., Rather P. N., Hare R. S., Miller G. H. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev. 1993 Mar;57(1):138–163. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerkamp J. H. Fatty acid composition of Bifidobacterium and Lactobacillus strains. J Bacteriol. 1971 Nov;108(2):861–867. doi: 10.1128/jb.108.2.861-867.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Graevenitz A., Osterhout G., Dick J. Grouping of some clinically relevant gram-positive rods by automated fatty acid analysis. Diagnostic implications. APMIS. 1991 Feb;99(2):147–154. doi: 10.1111/j.1699-0463.1991.tb05132.x. [DOI] [PubMed] [Google Scholar]


