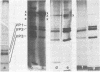
Abstract
Growth and neurovirulence of a number of neurotropic viruses show pronounced differences after passage in cell culture compared with continued in vivo passage in the central nervous system. The DA strain of Theiler's murine encephalomyelitis virus provides a model for studying these issues since DA virus grown in mouse brain produces acute neuronal disease in weanling mice, but tissue culture-passed DA virus does not. In addition, DA virus grown in mouse brain has a greater 50% mouse lethal dose/50% tissue culture infective dose ratio than tissue culture-passed DA virus. Comparison of these viruses required the analysis of virus purified directly from infected mouse brain, without tissue culture passage. Capsid proteins from DA virus grown in mouse brain were resolved on sodium dodecyl sulfate-polyacrylamide gels and shown to have the same profile as tissue culture-passed DA virus. Viral RNAs were the same size, with no evidence of defective interfering particle production. Two-dimensional gels of in vitro-labeled RNase T1-digested RNA showed that virus variants were more apparent during acute in vivo passage. These genomic differences may be critical in determining the biological behavior of the virus.
Full text
PDF

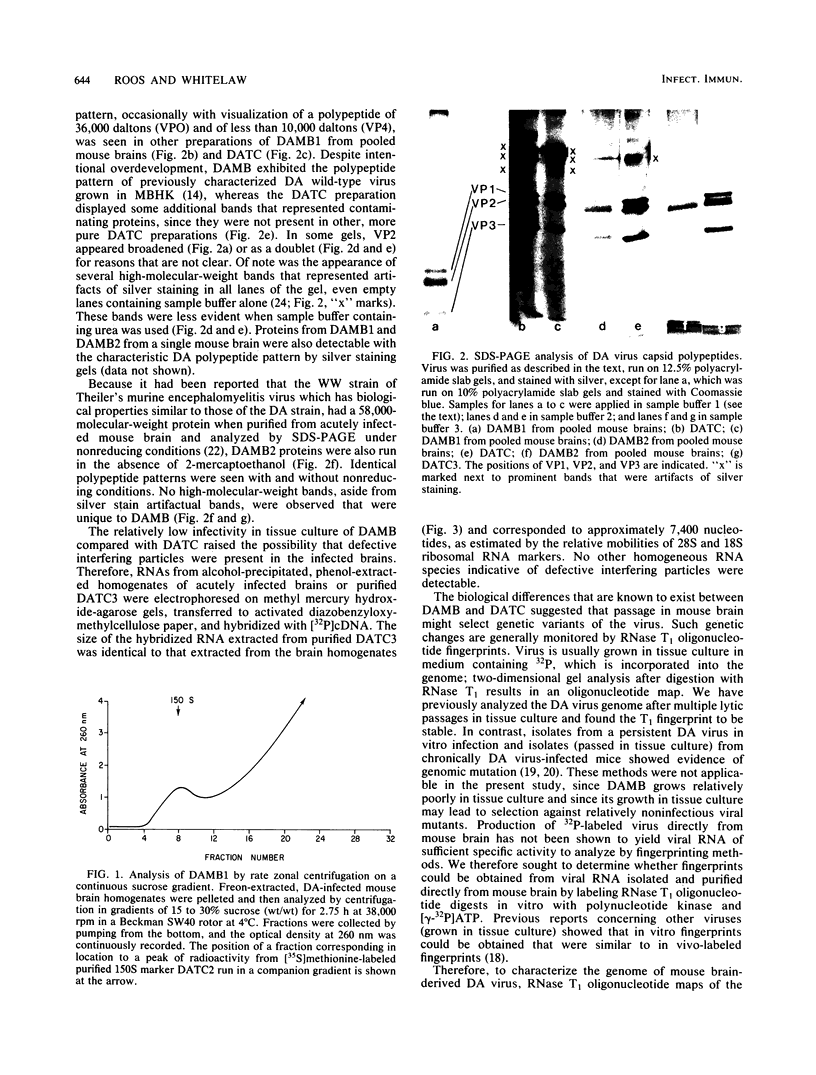

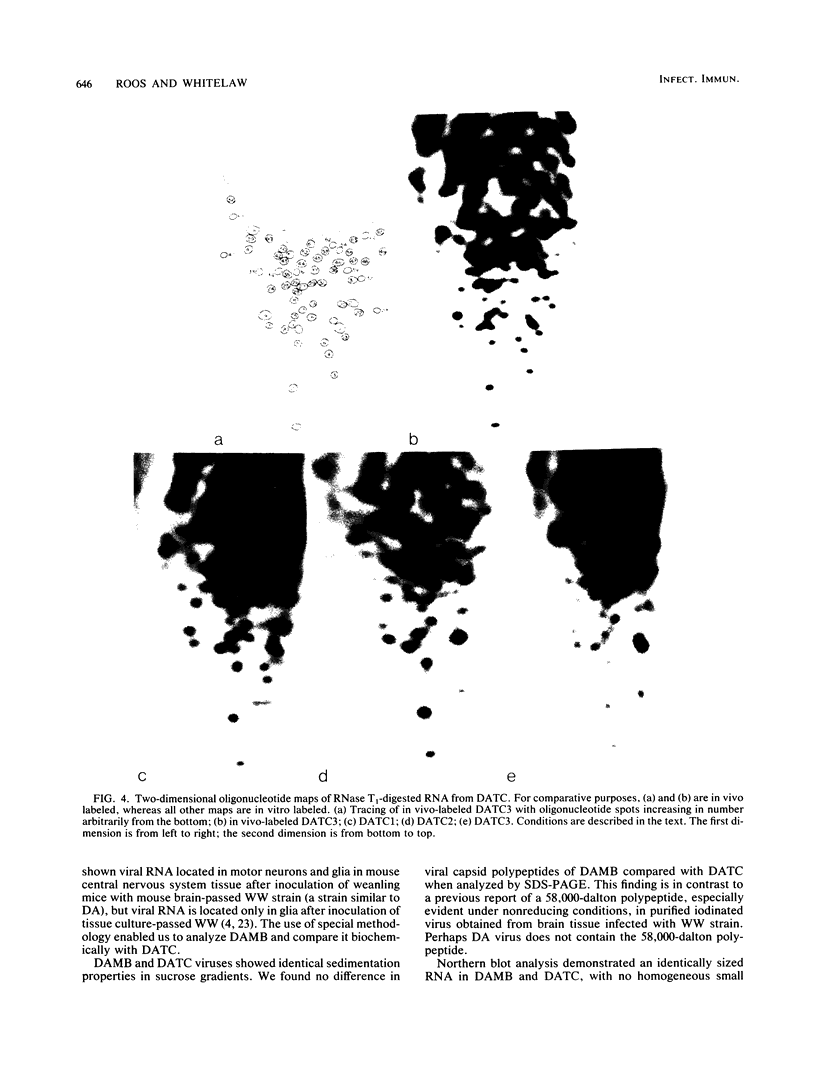



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. P., Klessig D. F. Synthesis of human adenovirus early RNA species is similar in productive and abortive infections of monkey and human cells. J Virol. 1982 May;42(2):748–754. doi: 10.1128/jvi.42.2.748-754.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batt-Humphries S., Simonsen C., Ehrenfeld E. Full-length viral RNA synthesized in vitro by vesicular stomatitis virus-infected HeLa cell extracts. Virology. 1979 Jul 15;96(1):88–99. doi: 10.1016/0042-6822(79)90175-2. [DOI] [PubMed] [Google Scholar]
- Black D. N., Brown F. Effect of actinomycin D and guanidine on the formation of a ribonucleic acid polymerase induced by foot-and mouth-disease virus and on the replication of virus and viral ribonucleic acid. Biochem J. 1969 Apr;112(3):317–323. doi: 10.1042/bj1120317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahic M., Stroop W. G., Baringer J. R. Theiler's virus persists in glial cells during demyelinating disease. Cell. 1981 Oct;26(1 Pt 1):123–128. doi: 10.1016/0092-8674(81)90040-4. [DOI] [PubMed] [Google Scholar]
- Dasgupta A., Zabel P., Baltimore D. Dependence of the activity of the poliovirus replicase on the host cell protein. Cell. 1980 Feb;19(2):423–429. doi: 10.1016/0092-8674(80)90516-4. [DOI] [PubMed] [Google Scholar]
- Dmitrieva T. M., Shcheglova M. V., Agol V. I. Inhibition of activity of encephalomyocarditis virus-induced RNA polymerase by antibodies against cellular components. Virology. 1979 Jan 30;92(2):271–277. doi: 10.1016/0042-6822(79)90131-4. [DOI] [PubMed] [Google Scholar]
- Etchison D., Ehrenfeld E. Comparison of replication complexes synthesizing poliovirus RNA. Virology. 1981 May;111(1):33–46. doi: 10.1016/0042-6822(81)90651-6. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Choppin P. W. Measles-virus proteins in the brain tissue of patients with subacute sclerosing panencephalitis: absence of the M protein. N Engl J Med. 1981 May 7;304(19):1152–1155. doi: 10.1056/NEJM198105073041906. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrich J. R., Arnason B. G., Hochberg F. H. Demyelinative myelopathy in mice induced by the DA virus. J Neurol Sci. 1976 Oct;29(2-4):149–160. doi: 10.1016/0022-510x(76)90167-2. [DOI] [PubMed] [Google Scholar]
- Lipton H. L., Dal Canto M. C. The TO strains of Theiler's viruses cause "slow virus-like" infections in mice. Ann Neurol. 1979 Jul;6(1):25–28. doi: 10.1002/ana.410060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L., Friedmann A. Purification of Theiler's murine encephalomyelitis virus and analysis of the structural virion polypeptides: correlation of the polypeptide profile with virulence. J Virol. 1980 Mar;33(3):1165–1172. doi: 10.1128/jvi.33.3.1165-1172.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L. Persistent Theiler's murine encephalomyelitis virus infection in mice depends on plaque size. J Gen Virol. 1980 Jan;46(1):169–177. doi: 10.1099/0022-1317-46-1-169. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Omata T., Toyoda H., Kuge S., Horie H., Kataoka Y., Genba Y., Nakano Y., Imura N. Complete nucleotide sequence of the attenuated poliovirus Sabin 1 strain genome. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5793–5797. doi: 10.1073/pnas.79.19.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottay B. K., Kew O. M., Hatch M. H., Heyward J. T., Obijeski J. F. Molecular variation of type 1 vaccine-related and wild polioviruses during replication in humans. Virology. 1981 Jan 30;108(2):405–423. doi: 10.1016/0042-6822(81)90448-7. [DOI] [PubMed] [Google Scholar]
- Pedersen F. S., Haseltine W. A. Analysis of the genome of an endogenous, ecotropic retrovirus of the AKR strain of mice: micromethod for detailed characterization of high-molecular-weight RNA. J Virol. 1980 Jan;33(1):349–365. doi: 10.1128/jvi.33.1.349-365.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos R. P., Richards O. C., Ehrenfeld E. Analysis of Theiler's virus isolates from persistently infected mouse nervous tissue. J Gen Virol. 1983 Mar;64(Pt 3):701–706. doi: 10.1099/0022-1317-64-3-701. [DOI] [PubMed] [Google Scholar]
- Roos R. P., Richards O. C., Green J., Ehrenfeld E. Characterization of a cell culture persistently infected with the DA strain of Theiler's murine encephalomyelitis virus. J Virol. 1982 Sep;43(3):1118–1122. doi: 10.1128/jvi.43.3.1118-1122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop W. G., Baringer J. R. Biochemistry of Theiler's murine encephalomyelitis virus isolated from acutely infected mouse brain: identification of a previously unreported polypeptide. Infect Immun. 1981 May;32(2):769–777. doi: 10.1128/iai.32.2.769-777.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop W. G., Brahic M., Baringer J. R. Detection of tissue culture-adapted Theiler's virus RNA in spinal cord white matter cells throughout infection. Infect Immun. 1982 Aug;37(2):763–770. doi: 10.1128/iai.37.2.763-770.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasheva B., Dessev G. Artifacts in sodium dodecyl sulfate-polyacrylamide gel electrophoresis due to 2-mercaptoethanol. Anal Biochem. 1983 Feb 15;129(1):98–102. doi: 10.1016/0003-2697(83)90057-x. [DOI] [PubMed] [Google Scholar]