Abstract
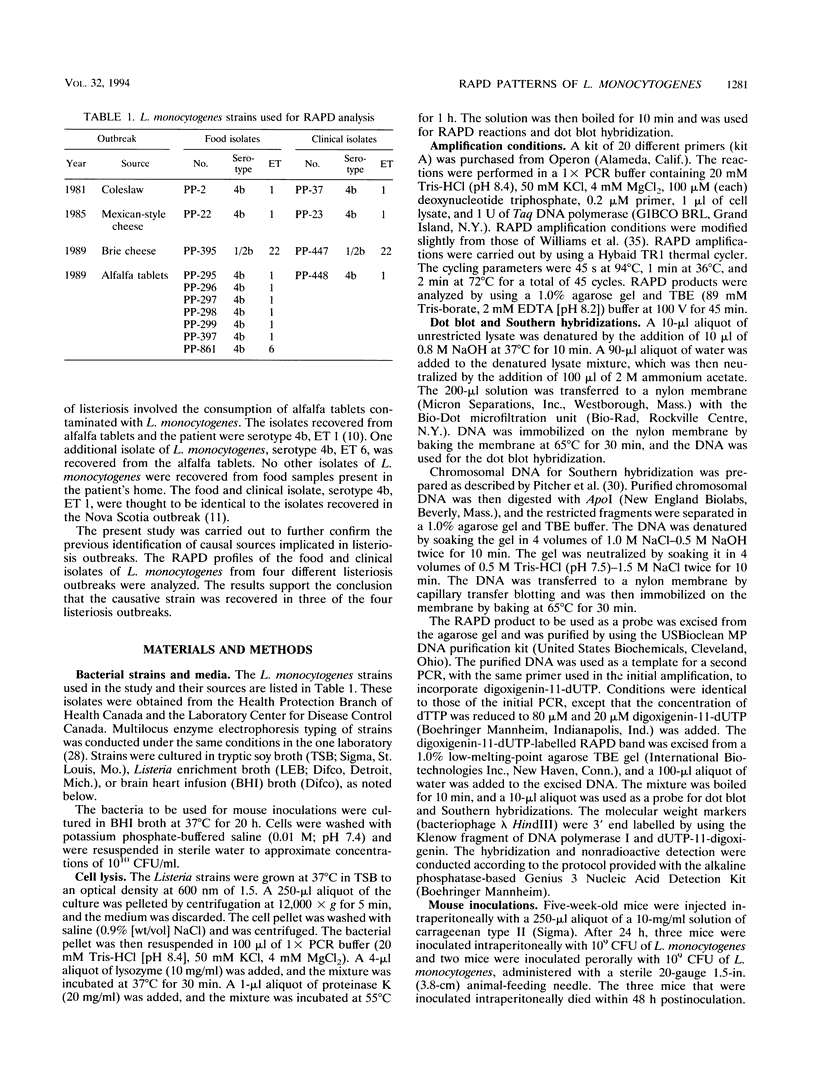
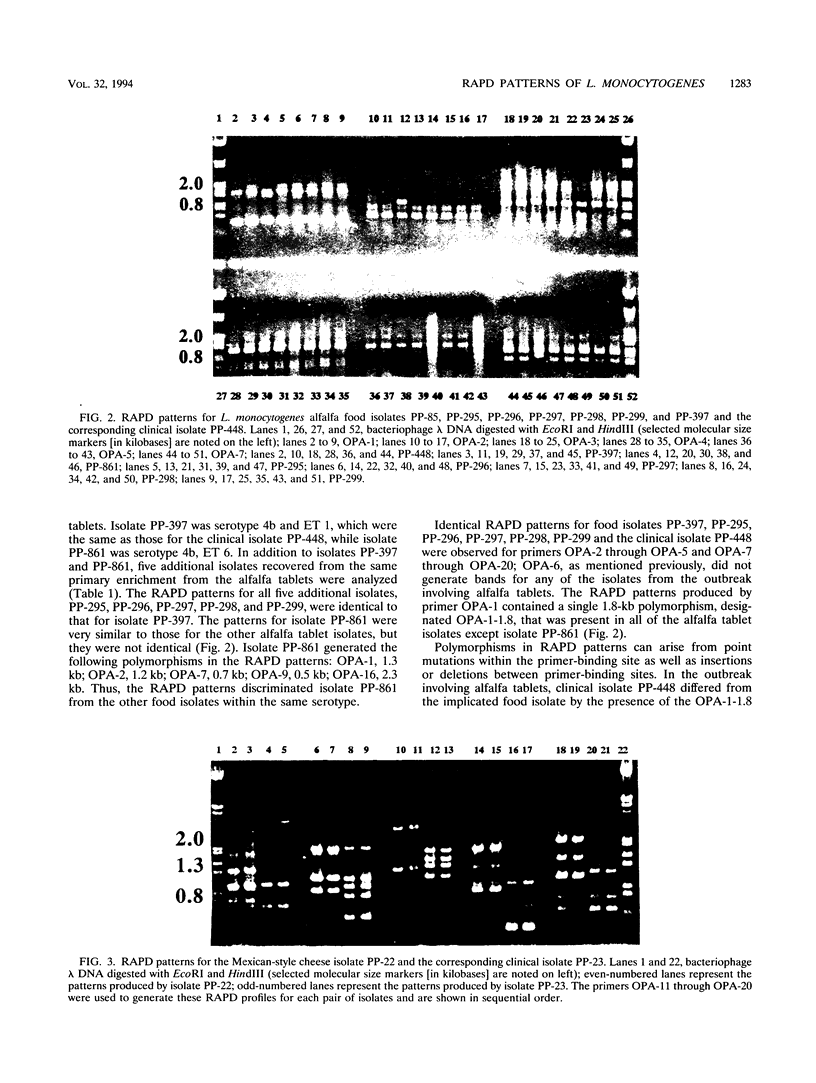
Food and clinical isolates of Listeria monocytogenes recovered from four different outbreaks of listeriosis were analyzed by their PCR-based randomly amplified polymorphic DNA (RAPD) patterns to verify their causal relationships. The generation of DNA fingerprints by PCR-based RAPD analysis is a fast and sensitive method for the epidemiological tracking and identification of bacteria implicated in food poisoning outbreaks. The L. monocytogenes strains used in the study were obtained from the following four outbreaks: California, 1985, Mexican-style cheese; Canadian Maritime Provinces, 1981, coleslaw; Canada, 1989, brie cheese; and Canada, 1989, alfalfa tablets. RAPD profiles were generated by using random 10-mer primers for at least one food and one clinical isolate recovered from each outbreak. Identical profiles for 20 different primers were observed for each pair of food and clinical isolates from two of the four outbreaks. Isolates from the outbreak involving alfalfa tablets exhibited identical patterns for 19 primers; however, primer OPA-1 produced one additional 1.8-kb fragment, designated OPA-1-1.8, that was found in the food isolate but not in the corresponding clinical isolate. Hybridization analysis revealed that the absence of the OPA-1-1.8 polymorphic fragment in the clinical isolate was due to a deletion of at least 1.8 kb. Loss of the OPA-1-1.8 polymorphic fragment could not be induced by infective passage of the L. monocytogenes isolate from the alfalfa tablet through a mouse or by growth of this isolate under selective conditions. This suggests that the isolate recovered from the food was not identical to the isolate recovered from the patient. The ability to produce unique RAPD patterns allows for the discrimination between isolates even if they are of the same serotype and multilocus enzyme electrophoretic type.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Audurier A., Taylor A. G., Carbonnelle B., McLauchlin J. A phage typing system for Listeria monocytogenes and its use in epidemiological studies. Clin Invest Med. 1984;7(4):229–232. [PubMed] [Google Scholar]
- BOLTON E. T., McCARTHY B. J. A general method for the isolation of RNA complementary to DNA. Proc Natl Acad Sci U S A. 1962 Aug;48:1390–1397. doi: 10.1073/pnas.48.8.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloga A. O., Harlander S. K. Comparison of methods for discrimination between strains of Listeria monocytogenes from epidemiological surveys. Appl Environ Microbiol. 1991 Aug;57(8):2324–2331. doi: 10.1128/aem.57.8.2324-2331.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerlin P., Rocourt J., Piffaretti J. C. Taxonomy of the genus Listeria by using multilocus enzyme electrophoresis. Int J Syst Bacteriol. 1991 Jan;41(1):59–64. doi: 10.1099/00207713-41-1-59. [DOI] [PubMed] [Google Scholar]
- Brousseau R., Saint-Onge A., Préfontaine G., Masson L., Cabana J. Arbitrary primer polymerase chain reaction, a powerful method to identify Bacillus thuringiensis serovars and strains. Appl Environ Microbiol. 1993 Jan;59(1):114–119. doi: 10.1128/aem.59.1.114-119.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajka J., Bsat N., Piani M., Russ W., Sultana K., Wiedmann M., Whitaker R., Batt C. A. Differentiation of Listeria monocytogenes and Listeria innocua by 16S rRNA genes and intraspecies discrimination of Listeria monocytogenes strains by random amplified polymorphic DNA polymorphisms. Appl Environ Microbiol. 1993 Jan;59(1):304–308. doi: 10.1128/aem.59.1.304-308.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber J. M., Carter A. O., Varughese P. V., Ashton F. E., Ewan E. P. Listeriosis traced to the consumption of alfalfa tablets and soft cheese. N Engl J Med. 1990 Feb 1;322(5):338–338. doi: 10.1056/nejm199002013220519. [DOI] [PubMed] [Google Scholar]
- Farber J. M., Peterkin P. I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991 Sep;55(3):476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete A., Bantle J. A., Halling S. M., Stich R. W. Amplification fragment length polymorphism in Brucella strains by use of polymerase chain reaction with arbitrary primers. J Bacteriol. 1992 Dec;174(23):7778–7783. doi: 10.1128/jb.174.23.7778-7783.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming D. W., Cochi S. L., MacDonald K. L., Brondum J., Hayes P. S., Plikaytis B. D., Holmes M. B., Audurier A., Broome C. V., Reingold A. L. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N Engl J Med. 1985 Feb 14;312(7):404–407. doi: 10.1056/NEJM198502143120704. [DOI] [PubMed] [Google Scholar]
- Gellin B. G., Broome C. V. Listeriosis. JAMA. 1989 Mar 3;261(9):1313–1320. [PubMed] [Google Scholar]
- Howard P. J., Harsono K. D., Luchansky J. B. Differentiation of Listeria monocytogenes, Listeria innocua, Listeria ivanovii, and Listeria seeligeri by pulsed-field gel electrophoresis. Appl Environ Microbiol. 1992 Feb;58(2):709–712. doi: 10.1128/aem.58.2.709-712.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C., Noyer-Weidner M., Trautner T. A., Weiner M., Zahler S. A. M.H2I, a multispecific 5C-DNA methyltransferase encoded by Bacillus amyloliquefaciens phage H2. Gene. 1991 Apr;100:213–218. doi: 10.1016/0378-1119(91)90369-m. [DOI] [PubMed] [Google Scholar]
- Loessner M. J., Busse M. Bacteriophage typing of Listeria species. Appl Environ Microbiol. 1990 Jun;56(6):1912–1918. doi: 10.1128/aem.56.6.1912-1918.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurier S. I., Audurier A., Marquet-Van der Mee N., Notermans S., Wernars K. A comparative study of randomly amplified polymorphic DNA analysis and conventional phage typing for epidemiological studies of Listeria monocytogenes isolates. Res Microbiol. 1992 Jun;143(5):507–512. doi: 10.1016/0923-2508(92)90097-8. [DOI] [PubMed] [Google Scholar]
- Mazurier S. I., Wernars K. Typing of Listeria strains by random amplification of polymorphic DNA. Res Microbiol. 1992 Jun;143(5):499–505. doi: 10.1016/0923-2508(92)90096-7. [DOI] [PubMed] [Google Scholar]
- McLauchlin J., Audurier A., Taylor A. G. The evaluation of a phage-typing system for Listeria monocytogenes for use in epidemiological studies. J Med Microbiol. 1986 Dec;22(4):357–365. doi: 10.1099/00222615-22-4-357. [DOI] [PubMed] [Google Scholar]
- Meier J. T., Simon M. I., Barbour A. G. Antigenic variation is associated with DNA rearrangements in a relapsing fever Borrelia. Cell. 1985 Jun;41(2):403–409. doi: 10.1016/s0092-8674(85)80013-1. [DOI] [PubMed] [Google Scholar]
- Monod M., Denoya C., Dubnau D. Sequence and properties of pIM13, a macrolide-lincosamide-streptogramin B resistance plasmid from Bacillus subtilis. J Bacteriol. 1986 Jul;167(1):138–147. doi: 10.1128/jb.167.1.138-147.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munthali M., Ford-Lloyd B. V., Newbury H. J. The random amplification of polymorphic DNA for fingerprinting plants. PCR Methods Appl. 1992 May;1(4):274–276. doi: 10.1101/gr.1.4.274. [DOI] [PubMed] [Google Scholar]
- Muralidharan K., Wakeland E. K. Concentration of primer and template qualitatively affects products in random-amplified polymorphic DNA PCR. Biotechniques. 1993 Mar;14(3):362–364. [PubMed] [Google Scholar]
- Ou J. T., Baron L. S., Rubin F. A., Kopecko D. J. Specific insertion and deletion of insertion sequence 1-like DNA element causes the reversible expression of the virulence capsular antigen Vi of Citrobacter freundii in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4402–4405. doi: 10.1073/pnas.85.12.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piffaretti J. C., Kressebuch H., Aeschbacher M., Bille J., Bannerman E., Musser J. M., Selander R. K., Rocourt J. Genetic characterization of clones of the bacterium Listeria monocytogenes causing epidemic disease. Proc Natl Acad Sci U S A. 1989 May;86(10):3818–3822. doi: 10.1073/pnas.86.10.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Díaz J. C., Vicente M. F., Baquero F. Plasmids in Listeria. Plasmid. 1982 Sep;8(2):112–118. doi: 10.1016/0147-619x(82)90049-x. [DOI] [PubMed] [Google Scholar]
- Salvi R. J., Ahroon W., Saunders S. S., Arnold S. A. Evoked potentials: computer-automated threshold-tracking procedure using an objective detection criterion. Ear Hear. 1987 Jun;8(3):151–156. [PubMed] [Google Scholar]
- Schlech W. F., 3rd, Lavigne P. M., Bortolussi R. A., Allen A. C., Haldane E. V., Wort A. J., Hightower A. W., Johnson S. E., King S. H., Nicholls E. S. Epidemic listeriosis--evidence for transmission by food. N Engl J Med. 1983 Jan 27;308(4):203–206. doi: 10.1056/NEJM198301273080407. [DOI] [PubMed] [Google Scholar]
- Schwartz B., Ciesielski C. A., Broome C. V., Gaventa S., Brown G. R., Gellin B. G., Hightower A. W., Mascola L. Association of sporadic listeriosis with consumption of uncooked hot dogs and undercooked chicken. Lancet. 1988 Oct 1;2(8614):779–782. doi: 10.1016/s0140-6736(88)92425-7. [DOI] [PubMed] [Google Scholar]
- Schwartz B., Hexter D., Broome C. V., Hightower A. W., Hirschhorn R. B., Porter J. D., Hayes P. S., Bibb W. F., Lorber B., Faris D. G. Investigation of an outbreak of listeriosis: new hypotheses for the etiology of epidemic Listeria monocytogenes infections. J Infect Dis. 1989 Apr;159(4):680–685. doi: 10.1093/infdis/159.4.680. [DOI] [PubMed] [Google Scholar]
- Wesley I. V., Ashton F. Restriction enzyme analysis of Listeria monocytogenes strains associated with food-borne epidemics. Appl Environ Microbiol. 1991 Apr;57(4):969–975. doi: 10.1128/aem.57.4.969-975.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Kubelik A. R., Livak K. J., Rafalski J. A., Tingey S. V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990 Nov 25;18(22):6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]







