Abstract
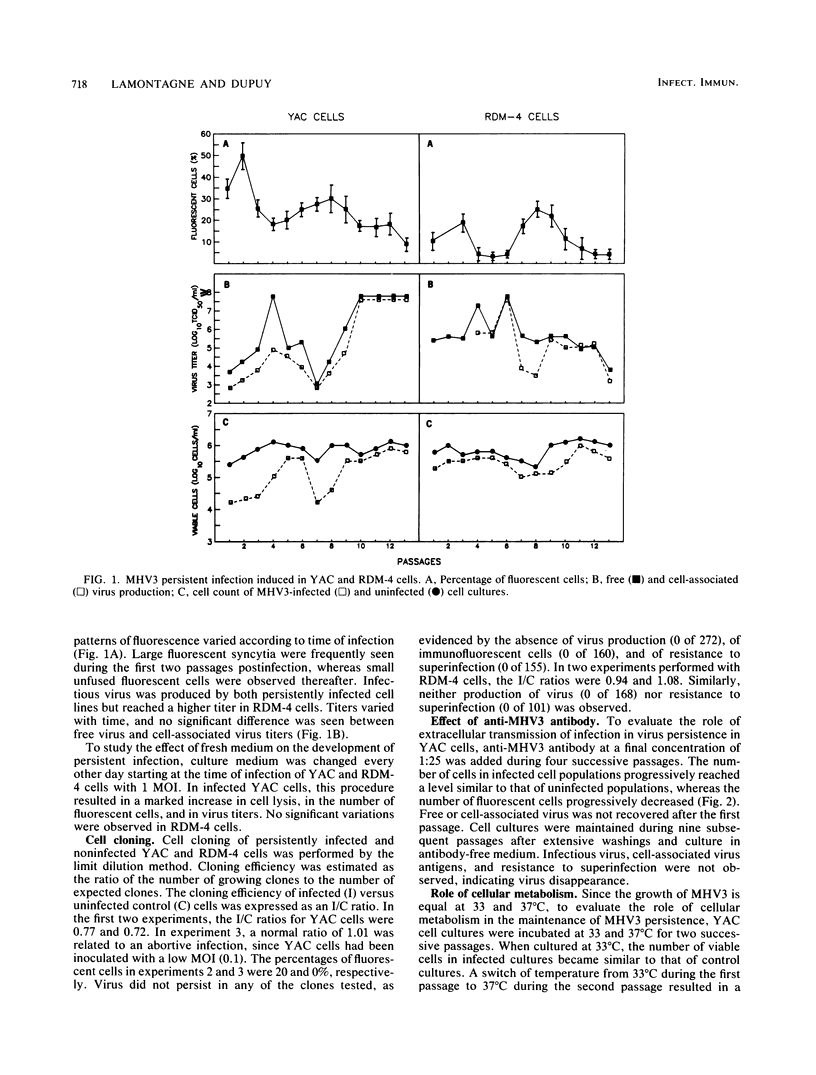
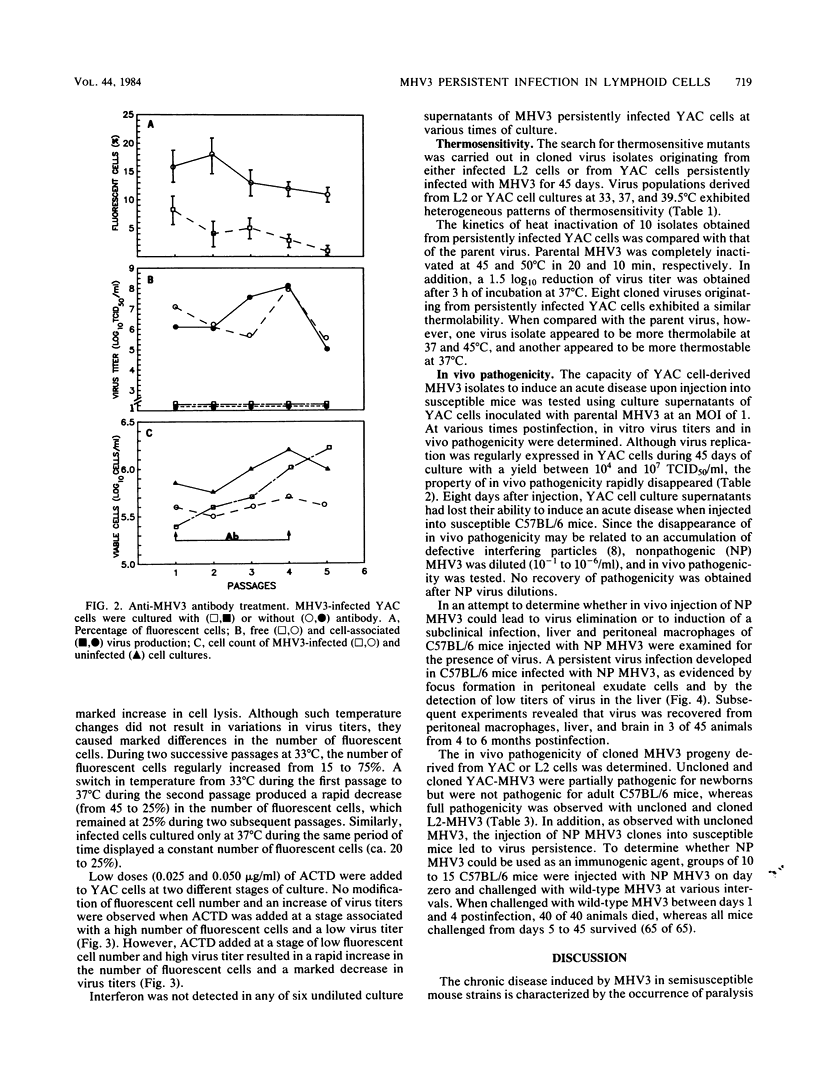
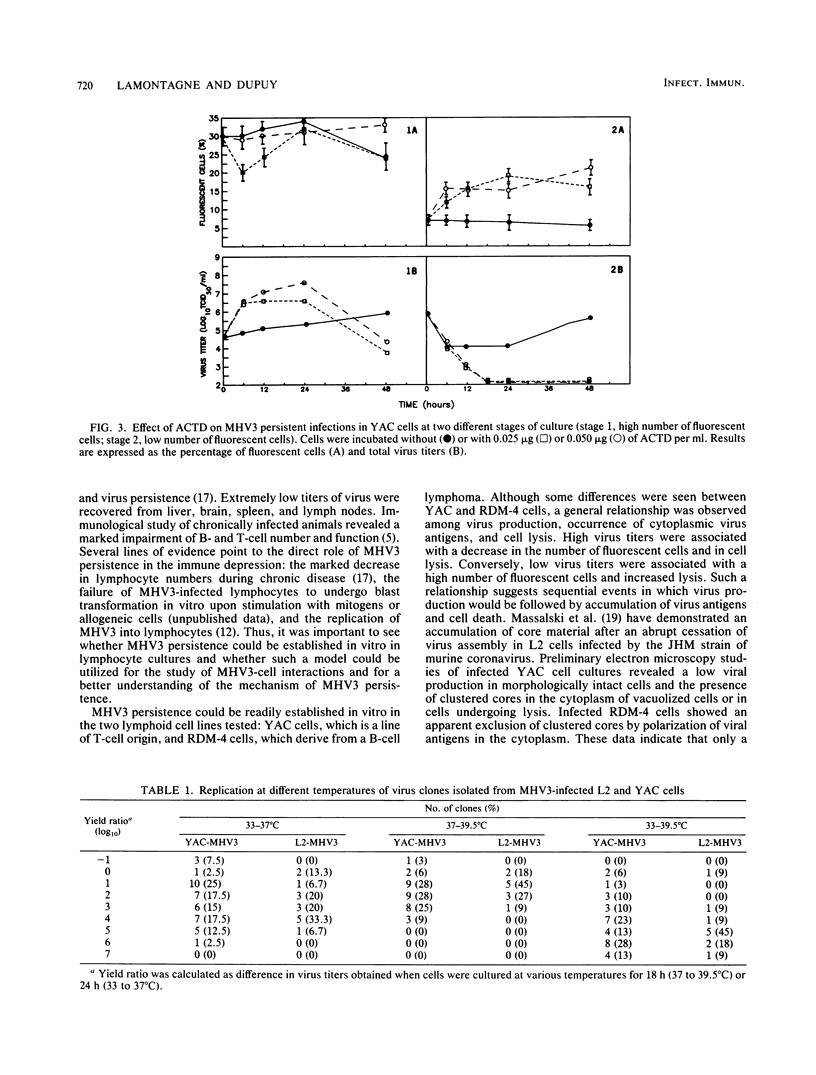
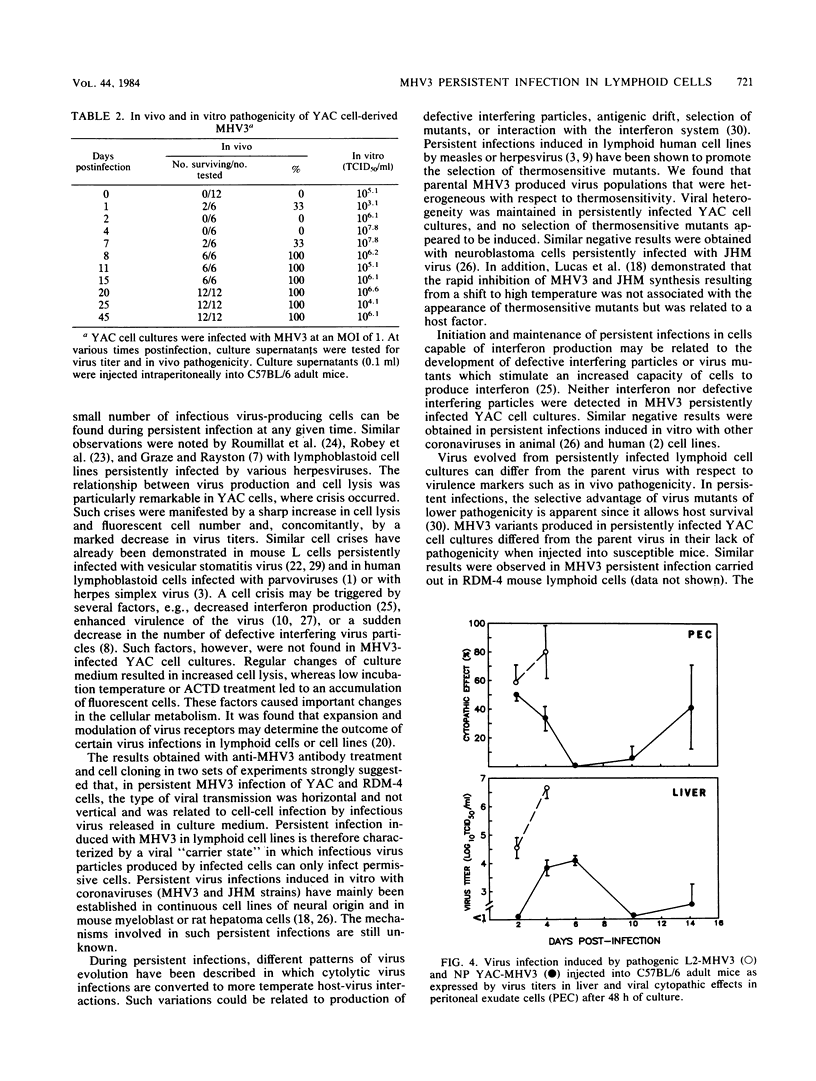
The sensitivity of mice to mouse hepatitis virus 3 (MHV3) varies according to strain, age, and immune status of the animals. In semisusceptible strains, mice surviving the acute phase of infection develop a chronic disease characterized by the occurrence of paralysis, virus persistence, and immunodeficiency. Persistent MHV3 infections established in vitro in YAC and RDM -4 mouse lymphoid cell lines were characterized by virus production, presence of cytoplasmic viral antigens, and cell lysis. The occurrence of cell "crisis" in YAC cells was manifested by a sharp increase in cell lysis and in the number of fluorescent cells and, concomitantly, by a marked decrease in virus titers. A relationship was observed among the percentage of fluorescent cells, cell lysis, and virus yield and was modulated by renewal of culture media, change in temperature, or inhibition of cellular RNA synthesis. Cell cloning and antibody treatment experiments indicated that viral transmission was performed by viral infection of newly permissive cells produced by the division of uninfected cells in the culture and not by transmission of viral information by infected dividing cells. The biological and biochemical properties of MHV3 variants derived from persistently infected YAC lymphoid cells were characterized. Thermosensitivity and thermolability of cloned viruses originating from persistently infected YAC cells, as well as parent virus suspensions, were studied. A similar heterogeneity was observed when YAC-derived cloned substrains (YAC-MHV3) were compared with parent-derived cloned viruses, indicating that no selection of temperature-sensitive mutants was induced in persistently infected YAC cells. However, the capacity of MHV3 to induce a lethal acute disease when injected into susceptible mice was lost very rapidly. The absence of pathogenicity was related to the induction of a subclinical infection which elicited defense mechanisms. These data suggest, therefore, that MHV3 replication in lymphoid cell lines leads to induction or selection of variants which maintain pathogenicity in vitro but display reduced pathogenic effects in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass L. R., Hetrick F. M. Persistent infection of a human lymphocyte cell line (Molt-4) with the kilham rat virus. J Infect Dis. 1978 Feb;137(2):210–212. doi: 10.1093/infdis/137.2.210. [DOI] [PubMed] [Google Scholar]
- Chaloner Larsson G., Johnson-Lussenburg C. M. Establishment and maintenance of a persistent infection of L132 cells by human coronavirus strain 229E. Arch Virol. 1981;69(2):117–129. doi: 10.1007/BF01315155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings P. J., Lakomy R. J., Rinaldo C. R., Jr Characterization of herpes simplex virus persistence in a human T lymphoblastoid cell line. Infect Immun. 1981 Dec;34(3):817–827. doi: 10.1128/iai.34.3.817-827.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cytolytic thymus-derived lymphocytes specific for allogeneic stimulator cells crossreact with chemically modified syngeneic cells. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1229–1233. doi: 10.1073/pnas.74.3.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl H., Degré M. A micro assay for mouse and human interferon. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(6):863–870. doi: 10.1111/j.0365-5563.1973.tb00012.x. [DOI] [PubMed] [Google Scholar]
- Dupuy J. M., Levey-Leblond E., Le Prevost C. Immunopathology of mouse hepatitis virus type 3mii. effect of immunosuppression in resistant mice. J Immunol. 1975 Jan;114(1 Pt 1):226–230. [PubMed] [Google Scholar]
- Dupuy J. M., Rodrigue D. Heterogeneity in evolutive patterns of inbred mice infected with a cloned substrain of mouse hepatitis virus type 3. Intervirology. 1981;16(2):114–117. doi: 10.1159/000149255. [DOI] [PubMed] [Google Scholar]
- Graze P. R., Royston I. Infection of human and rhesus lymphoblastoid cells with Herpesvirus macaca. Arch Virol. 1975;49(2-3):165–174. doi: 10.1007/BF01317535. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P., Welsh R. M., Oldstone M. B., Kohne D., Lazzarini R., Scolnick E. Long-term persistent vesicular stomatitis virus and rabies virus infection of cells in vitro. J Gen Virol. 1976 Nov;33(2):193–211. doi: 10.1099/0022-1317-33-2-193. [DOI] [PubMed] [Google Scholar]
- Ju G., Udem S., Rager-Zisman B., Bloom B. R. Isolation of a heterogeneous population of temperature-sensitive mutants of measles virus from persistently infected human lymphoblastoid cell lines. J Exp Med. 1978 Jun 1;147(6):1637–1652. doi: 10.1084/jem.147.6.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai A., Matsumoto S. Interfering and noninterfering defective particles generated by a rabies small plaque variant virus. Virology. 1977 Jan;76(1):60–71. doi: 10.1016/0042-6822(77)90282-3. [DOI] [PubMed] [Google Scholar]
- Krzystyniak K., Dupuy J. M. Early interaction between mouse hepatitis virus 3 and cells. J Gen Virol. 1981 Nov;57(Pt 1):53–61. doi: 10.1099/0022-1317-57-1-53. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Brayton P. R., Armen R. C., Patton C. D., Pugh C., Stohlman S. A. Mouse hepatitis virus A59: mRNA structure and genetic localization of the sequence divergence from hepatotropic strain MHV-3. J Virol. 1981 Sep;39(3):823–834. doi: 10.1128/jvi.39.3.823-834.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prevost C., Levy-Leblond E., Virelizier J. L., Dupuy J. M. Immunopathology of mouse hepatitis virus type 3 infection. Role of humoral and cell-mediated immunity in resistance mechanisms. J Immunol. 1975 Jan;114(1 Pt 1):221–225. [PubMed] [Google Scholar]
- Lucas A., Coulter M., Anderson R., Dales S., Flintoff W. In vivo and in vitro models of demyelinating diseases. II. Persistence and host-regulated thermosensitivity in cells of neural derivation infected with mouse hepatitis and measles viruses. Virology. 1978 Jul 15;88(2):325–337. doi: 10.1016/0042-6822(78)90289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massalski A., Coulter-Mackie M., Knobler R. L., Buchmeier M. J., Dales S. In vivo and in vitro models of demyelinating diseases. V. Comparison of the assembly of mouse hepatitis virus, strain JHM, in two murine cell lines. Intervirology. 1982;18(3):135–146. doi: 10.1159/000149316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima T., McClintock P. R., Billups L. C., Notkins A. L. Expression and modulation of virus receptors on lymphoid and myeloid cells: relationship to infectivity. Virology. 1982 Jan 30;116(2):605–618. doi: 10.1016/0042-6822(82)90152-0. [DOI] [PubMed] [Google Scholar]
- Prévost C. L., Virelizier J. L., Dupuy J. M. Immunopathology of mouse hepatitis virus type 3 infection. III. Clinical and virologic observation of a persistent viral infection. J Immunol. 1975 Sep;115(3):640–643. [PubMed] [Google Scholar]
- Ramseur J. M., Friedman R. M. Prolonged infection of L cells with vesicular stomatitis virus. Defective interfering forms and temperature-sensitive mutants as factors in the infection. Virology. 1978 Mar;85(1):253–261. doi: 10.1016/0042-6822(78)90429-4. [DOI] [PubMed] [Google Scholar]
- Robey W. G., Graham B. J., Harris C. L., Madden M. J., Pearson G. R., Vande Woude G. F. Persistent herpes simplex virus infections established in two Burkitt lymphoma derived cell lines. J Gen Virol. 1976 Jul;32(1):51–62. doi: 10.1099/0022-1317-32-1-51. [DOI] [PubMed] [Google Scholar]
- Roumillat L. F., Feorino P. M., Lukert P. D. Persistent infection of a human lymphoblastoid cell line with equine herpesvirus 1. Infect Immun. 1979 May;24(2):539–544. doi: 10.1128/iai.24.2.539-544.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekellick M. J., Marcus P. I. Persistent infection. II. Interferon-inducing temperature-sensitive mutants as mediators of cell sparing: possible role in persistent infection by vesicular stomatitis virus. Virology. 1979 May;95(1):36–47. doi: 10.1016/0042-6822(79)90399-4. [DOI] [PubMed] [Google Scholar]
- Stohlman S. A., Weiner L. P. Stability of neurotropic mouse hepatitis virus (JHM strain) during chronic infection of neuroblastoma cells. Arch Virol. 1978;57(1):53–61. doi: 10.1007/BF01315637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacore H., Youngner J. S. Cells persistently infected with newcastle disease virus: I. Properties of mutants isolated from persistently infected L cells. J Virol. 1969 Sep;4(3):244–251. doi: 10.1128/jvi.4.3.244-251.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogeeswaran G., Gronberg A., Hansson M., Dalianis T., Kiessling R., Welsh R. M. Correlation of glycosphingolipids and sialic acid in YAC-1 lymphoma variants with their sensitivity to natural killer-cell-mediated lysis. Int J Cancer. 1981 Oct 15;28(4):517–526. doi: 10.1002/ijc.2910280419. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Dubovi E. J., Quagliana D. O., Kelly M., Preble O. T. Role of temperature-sensitive mutants in persistent infections initiated with vesicular stomatitis virus. J Virol. 1976 Jul;19(1):90–101. doi: 10.1128/jvi.19.1.90-101.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


