Abstract
The epithelial Na+ channel (ENaC) is a major regulator of salt and water reabsorption in a number of epithelial tissues. Abnormalities in ENaC function have been directly linked to several human disease states including Liddle's syndrome, psuedohypoaldosteronism, and cystic fibrosis and may be implicated in states as diverse as salt-sensitive hypertension, nephrosis, and pulmonary edema. ENaC activity in epithelial cells is highly regulated both by open probability and number of channels. Open probability is regulated by a number of factors, including proteolytic processing, while ENaC number is regulated by cellular trafficking. This review discusses current understanding of apical membrane delivery, cell surface stability, endocytosis, retrieval, and recycling of ENaC and the molecular partners that have so far been shown to participate in these processes. We review known sites and mechanisms of hormonal regulation of trafficking by aldosterone, vasopressin, and insulin. While many details of the regulation of ENaC trafficking remain to be elucidated, knowledge of these mechanisms may provide further insights into ENaC activity in normal and disease states.
Keywords: clathrin-mediated endocytosis, deubiquitination, vesicle recycling
the epithelial na+ channel (ENaC) is the rate-limiting step for Na+ reabsorption in absorptive epithelia which can develop steep lumen-to-blood concentration gradients (65). The channel is located in the apical membrane of epithelial tissues throughout the body, including colon, sweat glands, salivary duct, airway, and cortical collecting duct (CCD) of the kidney (65, 134). Final regulation of Na+ excretion by the kidney occurs in the cortical collecting tubules and collecting ducts where regulatory mechanisms influencing Na+ absorption impact extracellular fluid volume and thus blood pressure. Alterations in channel structure and function have been shown to be important in several human diseases, including the hypertension seen in patients with Liddle's syndrome (69, 163), and the salt wasting seen in some variants of psuedohypoaldosteronism (32). In addition, excessive Na+ absorption occurs when the apical membrane CFTR density is reduced by genetic mutations that cause cystic fibrosis, a process that appears to be linked to disease pathogenesis (19, 111). The effects manifested in genetic diseases point to potentially significant roles for Na+ channel dysregulation that may result in conditions such as pulmonary edema (116, 138), chronic obstructive pulmonary disease (111), acute respiratory distress syndrome (182) and nephrosis (92), as well as hypertension (9, 178, 181). The salt-sensitive hypertension of Liddle's syndrome is now well understood as being caused by an ENaC mutation which alters its trafficking and cell surface expression, the mechanism of which was elucidated through basic studies of ENaC function (1, 57, 68, 69, 105, 152, 163). Liddle's syndrome represents a small minority of salt-sensitive hypertensives, and in most cases the abnormalities leading to enhanced renal Na+ retention in other forms of hypertension remain unclear (149, 151). Many aspects of ENaC trafficking and regulation also remain unresolved. One trafficking defect has already been shown to lead to clinical disease (105), so it seems reasonable that a fuller understanding of the molecular mechanisms involved in ENaC trafficking and its regulation might identify other sites where abnormalities in ENaC activity would lead to hypertension or other disease states (140).
Surface residency of ENaC is under strict control and can be regulated by the action of hormones and initiation of their specific second messenger cascades. One of the major actions of all the hormones involved in ENaC regulation is an alteration in the channel density at the apical membrane of polarized epithelial cells. This is brought about either by the translocation of additional channels to the membrane surface or by increasing the residence of ENaC at the cell surface to increase sodium transport. This homeostatic control ensures the fine-tuning of sodium homeostasis, and along with chloride movement provides to osmotic driving force for water transport. A more complete discussion of hormonal regulation of ENaC follows the descriptions of the mechanisms involved in ENaC trafficking in the following sections.
The contribution of ENaC to Na+ transport both in vitro and in vivo is commonly determined by its sensitivity to the potassium-sparing diuretic amiloride (65). ENaC activity is regulated by the number of channels (N) in the apical membrane, by the single-channel conductance (i), and by the open probability of the channel (PO). Although amiloride-sensitive channels with differing single-channel conductance properties have been reported (85, 192), there are no examples of significant regulatory or genetic alterations in ENaC function due to changes in i. Thus it appears that most regulation of channel activity occurs through alterations in either N or PO. Although some examples of physiologic regulation of PO are known (30, 65), most long-term regulation of ENaC activity occurs via regulation of N (150), the number of functional channels present in the apical membrane. Apical membrane channel number is determined by the balance between delivery and retrieval of the channel at the apical membrane, but can also be regulated by increase in synthesis and delivery of channel subunits, by impaired retrieval, or by insertion of preformed channels from subapical locations. The relation of functional channels to those detectable by biochemical methods has been impacted by our emerging understanding of the role of proteolytic activation of ENaC. To understand the various regulatory influences acting on ENaC, proteolysis raises the additional possibility that the number of functional Na+ channels may be increased by the activation of near-silent channels present in the apical membrane. Moreover, the Po of apical ENaC may be increased if residency time in the apical membrane is increased. This review will focus on the mechanisms that govern apical membrane ENaC density via regulation of membrane trafficking. The review will begin by relating our understanding of the factors that influence the density and residency of ENaC in the apical membrane. Next, the events involved in ENaC internalization will be traced, including routing decisions regarding the fate of the endocytosed channel, and finally we will track the channel back to the apical membrane by relating our small but growing knowledge about the recycling pathways involved. Schematic diagrams outlining the major regulatory pathways discussed are presented (Figs. 1 and 2).
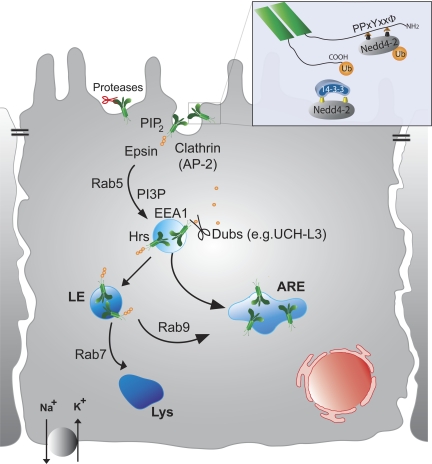
Fig. 1.
Schematic diagram outlining steps in epithelial sodium channel (ENaC) endocytosis and progress through early endosomes as it is recycled. Each of the steps in ENaC internalization is discussed in detail in the text. ENaC internalization is initiated by the binding of Nedd4-2 to the intracellular NH2 terminus (NH2) of each subunit and the addition of ubiquitin (Ub) moieties to the COOH tail (COOH) of the ENaC subunits (enlarged inset). Binding of 14-3-3 proteins to phosphorylated Nedd4-2 (yellow symbols on Nedd4-2) prevents Nedd4-2 interaction with the PY motifs on ENaC. Following ubiquitination, the channel is internalized by clathrin-dependant endocytosis through an interaction with epsin and passes through an early endosomal compartment (EEA1 and Hrs positive). If ENaC is deubiquitinated by deubiquitinating enzymes (Dubs), it will be transferred to the apical recycling endosome (ARE) for recycling to the apical membrane (see Fig. 2). If it remains ubiquitinated, it is likely trafficked via the late endosomes (LE) to the lysosomes (Lys) for degration. From studies on other transporters, it is likely that Rab proteins may be involved in rescuing ENaC from the LE back to the ARE or facilitating its movement to Lys for degradation.
Fig. 2.
Schematic diagram outlining the steps in ENaC recycling and delivery to the apical membrane. Following synthesis and assembly (yellow Golgi apparatus), channels are transported to the apical surface (yellow vesicle) and can be localized in lipid raft membrane domains (Rafts). It is also possible that ENaC enters the regulated recycling pathway directly (? and arrow), but no support for this idea has yet emerged. Those channels retrieved from the apical surface (see Fig. 1) and delivered to the ARE are trafficked back to the apical membrane through the involvement of Rab proteins (most likely Rab11, 27). Delivery of ENaC to the apical surface is regulated by RhoA and sorting nexin 3 (SN3) and vesicle fusion is mediated by SNARE proteins (SNAREs). Details of the mechanisms of ENaC delivery are provided in the text.
ENaC at the Surface (Apical Surface Residency)
A factor that permits membrane trafficking events to significantly impact ENaC-mediated Na+ entry is the relatively short half-life of the channel on the apical surface. While there is a wide range of both reported apical membrane ENaC half-lives and estimates of channel lifetimes, all are relatively brief in relation to those of other membrane proteins, for example, aquaporin-2 (AQP2) at ∼50 h (24). The calculated half-lives are derived from a number of model systems and estimates have varied from as short as ∼15 min to over 3 h. What could account for these differences? Estimation of half-life may be complicated by numerous factors. For example, recycling of ENaC results in net retention of channels at or returning to the apical membrane and an increase in estimated channel half-life, since channels have the ability to reach the apical surface multiple times before being diverted to degradation. Added to this is the idea that there may be a large intracellular storage pool with the ability to deliver ENaC to the apical surface as needed (107). This would make channel half-life estimations from functional data and current rundown experiments challenging. Second, a biochemical pool of nearly inactive channels found concurrently with active ENaC in the apical membranes of cells and Na+-transporting tissues has been described. Activation of the silent channels has been associated with proteolytic cleavage, structural alterations in the channel architecture, and shifts in apparent protein sizes observed by Western blotting (2, 29, 30, 38, 78–80, 96, 187, 190, 191). Proteolysis adds further difficulties in comparing biochemical results from earlier studies where this phenomenon was not considered. Third, different cell types or model systems may differ in their capacity for maintaining significant intracellular ENaC pools or in their ability to traffic and return ENaC to the apical membrane.
A new paradigm for considering the relationship of the biochemically detectable pool of subunits and the functional pool of channels is therefore required. Indeed, the last several years have seen evidence presented that the biochemical pool of channels in cell membranes significantly exceeds the number of active channels (17, 57). This phenomenon is exemplified by the ability of trypsin to augment amiloride-sensitive Na+ transport via proteolytic activation of inactive channels resident in the plasma membrane (78–80, 187, 190, 191). The turnover and half-life of cleaved and uncleaved channels will need to be examined to determine whether there is a difference in the rates of degradation or whether they are processed differently from uncleaved channels. Two recent publications have begun to address these issues and demonstrate that conditions that reduce channel retrieval from the apical membrane, increasing its residency time, result in increased appearance of cleaved active channels (91, 203). These results point to a possible role of trafficking in regulating the abundance of active vs. inactive channels. Possible difference in the trafficking itinerary of cleaved channels will also need to be addressed. It is possible that some of the discrepancies in channel half-life estimates from earlier studies may be accounted for as a difference in biochemical identity of ENaC investigated.
Despite the potential pitfalls, some consensus for estimates for ENaC surface residency obtained from surface labeling and pulse-chase studies has recently emerged. Apical membrane half-life estimates of ∼20–30 min have been made from a number of studies employing ENaC expression in several polarized cell systems or for endogenously expressed ENaC (A6, MDCK, FRT) (4, 70, 91, 107). In several studies investigating ENaC half-life, an initial rapid loss of ENaC from the apical surface was followed by stabilization in subunit lifetimes. This biphasic response would suggest a recycling mechanism which maintains a longer lived ENaC population in preference to ENaC degradation. In a study investigating the role of deubiquitinating enzymes (DUBs) in ENaC regulation, we reported that the inhibition of a specific DUB resulted in ENaC-dependant short-circuit current rundown with a t½ of ∼60 min without cAMP stimulation and ∼25 min under cAMP-stimulated conditions (26). These estimates parallel the biochemical data and agree with the results of pulse-chase studies in a scenario where ENaC is retrieved from the apical surface and directly degraded. However, when ENaC deubiquitination was not inhibited, the functional half-life as estimated from current rundown was longer, consistent with a longer lived channel population at the apical domain. These findings agree with biochemical estimates of channel lifetimes made in studies using cells endogenously expressing ENaC (A6) (195). Overall, the results from studies investigating ENaC deubiquitination are consistent with the idea of channel recycling, and this process may contribute to differences in ENaC half-life estimated in different systems.
ENaC Cleavage and Trafficking
Several channel-activating proteases (CAPs) have been described which cleave the extracellular loops of ENaC and increase Na+ transport (2, 3, 29, 30, 78–80, 94, 160, 187, 190, 191). In the CCD, inhibition of these serine proteases results in significantly reduced ENaC currents. Patch-clamp studies demonstrated that CAP action dramatically increases channel open probability (PO) changing ENaC from a near electrically silent to a constitutively active channel (22, 30). The serine protease furin has been shown to specifically cleave the α- and γ-ENaC subunits at extracellular sites close to the first transmembrane spanning domain (78–80). A number of other proteases have also been shown to activate ENaC, and much has still to be elucidated about CAP regulation itself. The populations of proteases, and protease cascades including protease inhibitors, in different tissues which are physiologically important in processing ENaC need to be determined. However, CAPs provide another mode of ENaC regulation, distinct from changes in channel number, with the ability to modulate PO.
Exogenous expression of ENaC engineered with specific tags has allowed biochemical studies of ENaC half-lives to be undertaken for cleaved and uncleaved ENaC populations and such studies may ultimately address the discrepancies in biochemical half-lives from earlier work (91, 96). However, it will still be important to obtain suitable antibodies which are able to distinguish these various forms in cells and tissues which express ENaC endogenously to determine whether the regulation defined in the model cells is similar to that achieved in vivo. A recent publication sought to clarify the regulation of cleaved ENaC and estimated the surface half-life of cleaved ENaC to be ∼20 min (91). These data would suggest that cleaved ENaC is regulated in a similar manner to the full-length species reported in other studies and that cleavage does not significantly impact ENaC regulation by trafficking. However, these findings would need to be directly compared with studies investigating cleaved vs. uncleaved channels in the same system and verified in systems that endogenously express ENaC.
ENaC Internalization and Regulation by Ubiquitination
It is widely accepted that the brief residency of ENaC at the cell surface is related to its ability to be modified and negatively regulated by the addition of ubiquitin (107, 165, 199, 203, 204). This pathway has been detailed in a number of elegant studies and has recently been reviewed (59, 109). The E3 ubiquitin ligase Nedd4-2 binds to consensus PPxY motifs in COOH terminus ENaC subunits and mediates ubiquitination of lysine residues (see Fig. 1, inset) on the NH2 terminus of α- and γ-ENaC subunits in Madin-Darby canine kidney (MDCK) cells (153). Ubiquitination of the β subunit has now also been reported in a number of endogenously expressing and overexpressing cell lines (110, 203). Ubiquitination of membrane proteins can serve as a signal for retrieval of these proteins into the endosomal/lysosomal pathway where they are either degraded or sorted for recycling (145) (see Fig. 1). Rotin and colleagues (176) originally proposed that excess ENaC subunits synthesized in endoplasmic reticulum (ER), but not destined to reach plasma membrane, are degraded through a process of polyubiquitination and proteosomal degradation, while assembled αβγ-ENaC that reaches the plasma membrane is monoubiquitinated and targeted to endosomal/lysosomal degradation pathways. They demonstrated that mutation of lysines in NH2 terminal α- and γ-ENaC results in prolonged channel half-life due to apical retention of the channels (177). Several recent studies showed that ENaC subunits are either polyubiquitinated (26) or multimonoubiquitinated (199) at the cell surface in both endogenously expressing cell lines and overexpression systems (203). It is clear from studies of overexpression of Nedd4-2 in oocytes or other cellular expression systems as well as mutagenesis studies within the PPxY motif that ubiquitination is the major pathway contributing to retention of ENaC at the cell surface under basal transport conditions (175). The gain-of-function mutations in ENaC that lead to enhanced Na+ reabsorption and hypertension in Liddle's syndrome are clearly caused by impaired Nedd4-2 binding to ENaC and inhibition of ubiquitin-mediated channel retrieval (69, 163).
The interaction between Nedd4-2 and ENaC has also been identified as a site of physiologic regulation of ENaC. The aldosterone-induced protein SGK1 has been shown to phosphorylate Nedd4-2 inducing its interaction with specific, aldosterone-induced, 14-3-3 protein isoforms (14, 83, 104). The phosphorylation of Nedd4-2 and its interaction with 14-3-3 proteins result in a decrease in affinity for Nedd4-2 binding to ENaC and disrupt channel ubiquitination (see Figs. 1 and 3). This leads to increased retention of channels at the apical membrane of aldosterone-responsive cells. Interestingly, the opposite effect has been ascribed to ERK which phosphorylates sites on ENaC and apparently leads to an increase in affinity for Nedd4-2 binding (54). The ERK cascade may be constitutively active in native ENaC expressing tissues or may be activated through the EGF signaling pathway or by progesterone (see Fig. 3). The aldosterone-induced protein GILZ appears to disrupt the activation of ERK, blocking this phosphorylation mechanism and leading to a decrease in affinity of the channel for Nedd4-2 (13, 169, 170). In this manner, aldosterone can lead to reduced Nedd4-2 affinity for the channel by its direct phosphorylation of Nedd4-2, or by blocking ERK-mediated phosphorylation. Thus aldosterone does not act by decreasing Nedd4-2 expression, but by manipulating its posttranslational regulation, and thereby, its ability to interact with ENaC. These effects of aldosterone are complementary and lead to decreased channel ubiquitination, and increased apical membrane channel expression and function.
Fig. 3.
Schematic diagram indicating points of hormonal regulation (in red) in the ENaC trafficking pathways previously depicted in Figs. 1 and 2. The actions of aldosterone (Aldo) and vasopressin are highlighted. Binding of Aldo to the mineralocorticoid receptor results in the long-term synthesis of additional ENaC, and in the regulation of Nedd4-2 (as depicted in Fig. 1) by the serum and glucocorticoid kinase (SGK). SGK phosphorylation (P) prevents Nedd4-2 binding and ENaC ubiquitination (Fig. 1, inset). Direct regulation of ENaC by phosphorylation through ERK is prevented by the action of GilZ, another Aldo-induced protein. Both vasopressin and insulin induce the translocation of ENaC from an intracellular vesicle storage population to the apical membrane. For vasopressin, this is facilitated by SN3.
In addition to these modulatory effects, the G protein receptor-coupled kinase GRK2 has also been reported to phosphorylate the COOH terminus of β-ENaC, and this renders the channel insensitive to inhibition by Nedd4-2 (46, 154). This observation provides a possible explanation for the observed association of GRK2 overactivity with hypertension and could represent a genetic candidate for altering the relationship between ENaC trafficking and hypertension. In earlier work, the same group reported that Nedd4-2 could also be phosphorylated by GRK2 which would in turn lead to a decrease in Nedd4-2 interaction with ENaC and offers another point of regulation for ENaC surface density (46).
Aldosterone and SGK1- or GRK2-mediated phosphorylation mechanisms provide major pathways that control the ability Nedd4-2 to ubiquitinate ENaC, and thereby regulate ENaC cell surface density by manipulating its endocytic rate. Ubiquitination appears to be the predominant signal for ENaC internalization, and channel endocytosis is a critical point of physiological control by the hormones that regulate Na+ balance.
Mechanism of Internalization
Membrane proteins may be internalized by varying mechanisms, including clathrin-mediated endocytosis, clathrin-independent endocytosis, or caveolar internalization. Although it has recently been proposed that ubiquitinated cargo may be endocytosed by clathrin-independent processes (34, 164) and that EGF receptor may be internalized by both clathrin-dependent and caveolar pathways, the current evidence regarding ENaC is that internalization is entirely via clathrin-mediated pathways and is not associated with caveolas (107, 193, 199). Early studies of ENaC in oocytes demonstrated that coexpression of a dominant-negative dynamin as well as mutation of the COOH-terminal Yxxφ motif by mutating the tyrosine in β-ENaC were both associated with increased activity of ENaC, consistent with regulation of surface expression by clathrin-mediated endocytosis (162). This sequence represents a classic tyrosine-based linear internalization motif, but it also overlaps with the PPxY segment representing the WW domain mediating Nedd4-2 binding (Fig. 1, inset). Since structural analysis of the Nedd 4:β ENaC interaction suggests that the second proline and the tyrosine are critical for interaction between the two proteins, it seems likely that the main effect of the tyrosine mutation in these early studies was to downregulate the interaction between the channel subunit and the ubiquitin ligase, rather than disrupting an AP-2-mediated internalization motif (162). Nevertheless, it has recently been suggested that the overlapping internalization motifs (the PPxY for Nedd4-2 binding and the Yxxφ for clathrin adaptor μ2 binding) could potentially function as independent sorting signals and that this switching function was dependent on upstream serine and threonine residues (173). This possibility has been challenged by observations that mutations disrupting the Yxxφ motif have little effect on endocytosis of ENaC, although these mutations did not involve either of the critical terminal amino acids of this motif (203). Whether the channel is endocytosed to some degree via linear internalization motifs, it seems clear that the major regulation of internalization under most conditions involves Nedd4-2-mediated ENaC ubiquitination (175).
Membrane proteins which are modified by attachment of one or more ubiquitin molecules appear to be internalized by signals unlike the classic internalization motifs and may require binding to accessory proteins which link them to clathrin adaptors (72, 198). Multimodular proteins of the epsin family have been implicated in performing such a function since they contain ubiquitin interaction motifs (UIM) as well as clathrin-binding domains (DPW) and phosphoinositide-interactive domains (ENTH) (198). Epsin orthologs in Drosophila and C. elegans target monoubiquitinated ligands of the Notch pathway for endocytosis, and subsequent deubiquitination and recycling of cargo are essential for Notch signaling (130, 183, 194). The EGF receptor is ubiquitinated by the ligase Cbl, is internalized via primarily clathrin-dependent mechanisms, and is then sorted for recycling or degradation by the action of serial deubiquitylating enzymes (119). A recent study has now shown that ENaC interacts with the clathrin adaptor μ2 component of the AP-2 complex, is enriched in clathrin-coated vesicles, and interacts with and is regulated by epsin (193). Interestingly, fully mature and cleaved isoforms of β- and γ-ENaC, respectively, appear to be particularly enriched in clathrin-coated vesicles, suggesting that they may be selectively retrieved by this mechanism (193). Overexpression of epsin downregulates ENaC expression in oocytes, HEK cells, and endogenously expressing mouse CCD cells (174, 193, 196).
The role of ENTH domains of accessory proteins such as epsin in clathrin-mediated endocytosis appears to be related to binding to PI(4,5)P2 and is a necessary prerequisite for endocytosis to proceed (86). PIP2-regulated recruitment of epsin to the plasma membrane initiates clathrin lattice formation and the budding of clathrin-coated vesicles (60, 61). Subsequently, dynamin is recruited to the neck of the forming vesicle by binding to PIP2 enabling fission of the vesicle from the plasma membrane. Clathrin lattices are next disassembled when synaptojanin dephosphorylates PIP2 converting it to PIP (123). It has been demonstrated that stimulation of PIP2 levels by PIP5-K results in enhanced rates of clathrin-mediated endocytosis (196) and overexpression of PIP5-Kα in both CCD cells and oocytes results in increase in PIP2 levels and decrease in surface ENaC expression consistent with increased retrieval (196). However, the role of PIP2 in ENaC regulation may be quite complex as PIP2 is known to have multiple effects on membrane channels (73). PIP2 has been demonstrated to bind directly to cationic sequences in the COOH termini of ENaC, to alter channel gating (108, 201), and to stimulate channel exocytosis (45, 121, 172). Of note, it is the PIP5-Kβ isoform which appears to stimulate exocytosis in overexpression systems, suggesting that variations in effects of PIP2 on channel activity may be isoform specific and related to the spatial and temporal subcellular localization of both channel and enzymes.
Membrane proteins destined for lysosomal degradation are sorted from those that are recycled to the plasma membrane in early endosomes (see Fig. 1) and ENaC has been convincingly shown to traffic through the endosomal pathway both in endogenously expressing CCD cells and in ENaC overexpressing MDCK cells (107, 193). The sorting of proteins in the endosomal pathway is a complex process that has been studied extensively in yeast through the isolation of vacuolar protein sorting (vps) mutants (145). Mammalian homologs of many vps proteins have been identified as making up the endosomal sorting complexes required for transport (ESCRT) and key proteins for sorting of ubiquitinated substrates (such as ENaC) are the hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs), the mammalian homolog of Vps27p and its binding partner, signal transducing adaptor molecule (STAM) (119). Hrs, like EEA1, contains a FYVE binding domain which serves to localize it to early endosomes by binding to PtdIns(3)P (PI3P) (86). EEA1 functions as a tethering molecule, binds to Rab proteins, and mediates endosomal fusion (67, 143, 145). Hrs, like epsin, has both a UIM and a clathrin-binding domain and it has been suggested that Hrs recruits clathrin to early endosomes and may mediate the sorting of ubiquitinated cargo into lysosomes for degradation (143, 144) or for sequential transfer to either regulatory or housekeeping deubiquitinating enzymes (DUBs) (119). By the action of DUBs, cargo which is deubiquitinated may be sorted for recycling (161, 197, 198). ENaC traffics through both EEA1, Hrs, and Rab11-positive endosomes and to avoid degradation by lysosomes, it must undergo deubiquitilaytion analogous to the EGF receptor. Two separate debuqiutylating enzymes, one isolated from EEA1-positive endosomes, have now been demonstrated to be involved in ENaC trafficking. The role of PI3P in the process of endosomal sorting has been examined using both PI3K inhibitors such as wortmannin, which impair trafficking through endosomal compartments by collapsing discreet sorting compartments, and PI4P phosphatases which appear to rescue it. PI3P, the product of class III PI3K, plays a major role in endosomal sorting (77, 81, 87, 90). Inhibitors of PI3K have been repeatedly shown to inhibit both basal and stimulated ENaC function (16, 146). Although this action has been attributed to PI3K activation of SGK1, the profound effect of these inhibitors on basal ENaC function suggests a possibly distinct site of action.
ENaC Deubiquitination
Once retrieved from the apical surface, ENaC traffics through early and sorting endosomal compartments. It is likely that the fate of ENaC is determined in these early endocytic compartments. ENaC that remains ubiquitinated is likely to be degraded in lysosomal compartments as inhibition of lysosomes results in an accumulation of ubiquitinated ENaC and the extension of the channel's half-life (199). To divert ENaC from a degradative fate in lysosomes, rapid deubiquitination of the endocytosed channel would be required (40, 120). DUBs are known to perform these functions (5, 36, 120, 128, 167) and in a recent study, evidence was presented for such a role by the ubiquitin COOH-terminal hydrolase (UCH-L3) in ENaC regulation (26). UCH-L3 is not induced by aldosterone and it appears to be ubiquitiously expressed. By specifically blocking the deubiquitinating action of UCH-L3, a loss in steady-state ENaC-mediated Na+ transport was observed. Knockdown of the DUB significantly reduced ENaC activity and little response to cAMP activation was detected following UCH-L3 knockdown or inhibition (26).
These data highlighted the requirement for constitutive deubiquitination to maintain ENaC at the apical membrane. Of interest, while a loss in surface ENaC expression could be demonstrated biochemically, there was little decrease in whole cell levels of ENaC. This suggested that UCH-L3 did not regulate total cellular levels of ENaC, and therefore not likely involved in degradation at the level of the ER (26). As this DUB was biochemically detected in early endosomal and clathrin-coated vesicle compartments, it is likely that its action to deubiquitinate ENaC occurred near the apical surface as ENaC was internalized. As confirmation of this, surface biotinylation followed by immunoprecipitation of biotinylated ENaC using an anti-ubiquitin antibody demonstrated an increase in the levels of ENaC ubiquitination at the apical membrane of CCD cells following UCH-L3 inhibition.
The kinetics of UCH-L3 inhibition differed, depending on whether the inhibitor was applied under basal or stimulated conditions (26). The action of the DUB inhibitor was much more rapid in cells where ENaC recruitment to the plasma membrane was stimulated by cAMP/PKA. Accordingly, the actions of the UCH-L3 inhibitor indicate that ENaC is in transit between intracellular compartments and the apical membrane under both basal and stimulated conditions. With increased channel turnover induced by the agonist, the contribution of ENaC recycling is critical for the maintenance of apical channel density. Channel transit through intracellular pathways is maintained in the absence of acute stimulation under basal conditions since inhibition of DUB activity reduced ENaC currents in the absence of stimulation.
A second DUB, USP2-45, has also been demonstrated to deubiquitinate ENaC (53). In contrast to UCH-L3, USP2-45 is aldosterone induced and was isolated as an early aldosterone-induced gene product, whose intracellular level was induced by aldosterone (1.6-fold). Like UCH-L3, overexpression of USP2-45 increased ENaC activity and decreased levels of ENaC ubiquitination. However, it was not possible to determine the role of this DUB in ENaC recycling from the data presented in this study. Although the authors demonstrated an alteration in the level of ENaC ubiquitination with changes in USP2-45 expression, it is not clear where the cellular locus for this DUB's action on ENaC is and what role the aldosterone induction would have on overall ENaC regulation by this DUB. It remains a possibility that USP2-45 is involved in regulating ENaC in the ER, by rescue from ER-associated degradation to increase the number of channels in response to aldosterone stimulation.
While the role of USP2-45 deubiquitination of ENaC is unclear, a third DUB has been implicated in ENaC regulation by vasopressin through an intricate association with sorting nexin 3. Staub and colleagues (unpublished observations) identified Usp10 through a SAGE screen as a vasopressin-induced protein and linked its induction to ENaC regulation. Usp10 does not act to directly deubiquitinate ENaC, as no change in ENaC ubiquitination was observed with alterations in the levels of Usp10. Rather, by using a yeast two-hybrid screen, they demonstrated that Usp10 deubiquitinated sorting nexin 3, which then promoted the surface expression of ENaC. The mRNA levels of sorting nexin 3 were not altered by vasopressin, suggesting that Usp10 acted on synthesized endogenous nexin to deubiquitinate and increase protein abundance (O. Staub; unpublished results). This cascade of interactions illustrates the complex DUB associations which regulate ENaC. Not only does the deubiquitination of ENaC need to be taken into account, but there are a number of accessory and trafficking proteins which are likely regulated by DUBs and may alter ENaC surface residency. In addition, it is possible that ubiquitinating and deubiquitinating enzymes are themselves regulated by ubiquitination offering another level of regulation (5, 82).
ENaC Recycling: Rab GTPases
Following its deubiquitination, ENaC would be reorganized into sorting and recycling endosomes to traffic back to the apical surface (see Fig. 2). The itinerary of ENaC recycling can be determined by colocalization with markers of early endosomal and recycling compartments. Thanks to work investigating apical recycling in model cells, some prediction can be made as to the vesicular neighbors ENaC is likely to encounter as it recycles. Most notable of these are Rab GTPases which coordinate steps in the endocytic, recycling and exocytic trafficking pathways. In most trafficking systems, the initial steps in membrane fusion involve tethering of cargo containing vesicles at the target membrane. This process often involves a complex of accessory proteins that are organized on, or recruited to, trafficking vesicles by small GTPases, including Rab proteins (136, 157). Rabs are a ubiquitously expressed family of small (20–29 kDa) monomeric Ras-like GTPases and function as molecular switches, cycling between GTP-bound and GDP-bound states (33, 58, 142). Over 60 mammalian Rabs have been identified and they are known to be regulated by guanine nucleotide exchange factors (GEFs) which trigger the binding of GTP and GTPase-activating proteins to accelerate the hydrolysis of GTP to GDP. At this time, we have only sparse knowledge of the Rab GTPases that are involved in apical ENaC trafficking, but inferences can be made from other systems. The Rabs are distributed selectively at different steps along the protein secretory pathway, where they regulate trafficking between donor and acceptor membrane compartments. Rabs 4, 5, 18, and 21 have been associated with early endosomes and with early steps in endosomal internalization (11, 23, 148). Rab7 has been linked to late endosomes and its colocalization would suggest channels are destined for lysosomal degradation (6). Rab11 and Rab25 are found in recycling endosomes and Rab27 is involved in exocytic insertion (31, 35, 157). Rab proteins are known to play key roles in the trafficking of various channels and transporters.
For example, AQP2 is expressed in the same principal cell population as ENaC. AQP2 is also regulated by vasopressin and it is known to recycle to the apical surface in these cells following endocytosis (18, 21, 39, 84, 129, 186). An extensive proteomic screen demonstrated the association of AQP2-containing vesicles with a number of Rab family members (12, 20). For CFTR, Rab5 mediates its internalization from the plasma membrane and its delivery to early endosomes (66). As with ENaC, CFTR can be returned to the plasma membrane by transfer to recycling endosomes (Fig. 1). The recycling of CFTR back to the apical membrane is mediated by Rab11 (179, 180) and passes through a compartment that is regulated by the endocytic recycling regulator, Rme-1 (137). Alternately, CFTR may be delivered from early endosomes to late endosomes by a process facilitated by Rab7 (66), which leads the protein to lysosomes and degradation. However, Rab9 can rescue CFTR from degradation by facilitating its recovery from late endosomes and transfer the channel to the trans-Golgi network, to again enter the protein secretory pathway and find its way back to the apical membrane. These studies showed that alterations in Rab protein function can influence the density of CFTR channels at the apical membrane, by either interfering with Rab5 function and endocytosis, or by augmenting Rab11 function and recycling. These factors can be significant in controlling the apical density of mutant channels that have reduced apical residency, such as the common ΔF508 CFTR. The same behavior could be anticipated for ENaC.
ENaC has been localized in Rab11-positive compartments by immunofluorescent labeling, adding support to the idea that it traffics through this recycling compartment (107). Two studies documented the involvement of Rab4 (endocytosis) and 27a (exocytosis) with ENaC regulation in an immortalized human colonic cell line (155–158). Overexpression of either Rab protein resulted in an inhibition of ENaC current across H-29 cells, ostensibly by direct protein-protein interactions which reduced the ENaC surface density. Rab27 inhibited ENaC via a mechanism that involved the SNARE regulatory protein, munc 13–4 (155). Interactions of ENaC with SNAP-23 and synaptotagmin were also proposed in these studies, implying that there is a complex, and at present, poorly defined set of interactions with Rab proteins and other components of the exocytic machinery. It is likely that as more Rab family members are investigated, a number of specific GTPases will be associated with ENaC regulation by trafficking.
An interesting protein that links Rab27 to myosin V and the actin cytoskeleton in CCD (M1) cells was identified recently by Martel et al. (112) in a screen for aldosterone-induced proteins. Treatment of M1 cells expressing an inducible mineralocorticoid receptor with aldosterone led to the rapid (2 h) induction of melanophilin expression, a protein that functions in vesicular trafficking in malanocytes by linking Rab27a vesicles to the unconventional myosin isoform, Va. M1 cells expressed the myosin Vc isoform, which the authors proposed as a potential SGK1 substrate, since its COOH-terminal tail was recognized by a phospho-SGK/Akt antibody. Since the induction of increased melanophilin expression in M1 cells led to increased Na+ transport, these findings raise the interesting possibility that this aldosterone-induced protein couples motors to ENaC-containing vesicles via Rab27, to mediate steps on the exocytic pathway.
Intracellular ENaC Populations
While the biosynthesis of ENaC is not discussed here, it is important to note that the delivery of ENaC to the apical surface may involve two or more paths. In model epithelia constitutive ENaC production is required to maintain baseline levels of Na+ absorption, which are observed under nonstimulated conditions. Under basal conditions, apical membrane ENaC is maintained at steady-state by the constitutive delivery of channels from biosynthesis. When protein production is inhibited, a slow rundown in Na+ absorption is observed (25). In functional studies the half-time of current decay was estimated to be ∼240 min. In addition to the constitutive production and surface delivery of ENaC, a proportion of the produced channels appears to enter a regulated subapical intracellular pool. Evidence for this pool has emerged from both epithelial model cells and in vivo tissue studies (25, 106, 107). For example, in recent work using labeled ENaC subunits overexpressed in an MDCK cell line, a large intracellular pool of ENaC was observed which could be trafficked to the surface in response to cAMP stimulation (107). This finding corroborated earlier work using the same cell system which demonstrated an increase in channel density at the apical surface in response to cAMP stimulation (122). The intracellular pool in which ENaC resides was characterized in a mouse CCD cell line which endogenously expresses the channel. In response to cAMP stimulation, ENaC surface membrane density was shown to increase by insertion of ENaC from an intracellular pool (25). The increase in apical membrane channel density in response to cAMP stimulation was detected biochemically and by parallel electrophysiological measurements in which increased apical membrane capacitance reflected an increase in vesicle exocytosis and delivery of ENaC to the cell surface.
The size and/or behavior of this cellular compartment (assessed by cAMP-evoked changes in membrane capacitance) vary directly with ENaC expression, suggesting that the channel itself plays a role in establishing this regulated trafficking pathway. Likely, this occurs by direct or indirect interactions of the channel with traffic regulatory proteins, a form of cargo selection that should be an area of fruitful study. In addition, the literature on other channels/transporters (76, 141, 189) indicates that it would be an error to conclude that ENaC within the regulated compartment is static in the absence of stimulation. It is more likely that ENaC traffic via this pathway turns over continuously, with the kinetics of key steps being under regulation to provide for changes in channel distribution between the interior and the cell surface when conditions warrant.
Similar findings have emerged from studies of insulin-dependent GLUT4 trafficking, which indicate that the transporter turns over constitutively in the absence of stimulation, conditions under which its distribution favors the cell interior. In the absence of insulin stimulation, GLUT4 continues to cycle to the plasma membrane, but insulin alters steps in the GLUT4 recycling pathway, primarily exocytosis, to reorient its distribution in favor of the plasma membrane (76). Recent studies with membrane dyes suggest that a similar model applies to ENaC trafficking regulation by cAMP agonists, (unpublished observations, M. B. Butterworth and C. A. Bertrand). Continuous turnover, as well as the low level of ENaC in systems that endogenously express the channel, will make it difficult to identify the precise location(s) and trafficking pathways of ENaC-containing intracellular compartments. Under steady-state conditions, the channel will be present at various levels throughout endocytic recycling pathways, and measurements of individual steps in the process could be misleading concerning their contribution to channel redistribution during stimulation.
The use of fluorescently tagged proteins may ultimately provide significant advances in our ability to visualize the constitutive and regulated pathways for ENaC trafficking, but this will be particularly challenging in polarized epithelia. Progress in this direction has been achieved with the combined application of total internal reflection microscopy (TIRF) and fluorescence recovery after photobleaching (FRAP) to the process of ENaC movement toward the plasma membrane (139). In their studies of Rho-dependent ENaC trafficking and involvement of the cytoskeleton, Pochynyuk and colleagues (139) combined electrophysiological measurements with these advanced imaging approaches. Following TIRF-based photobleaching of the near membrane signal, they monitored ENaC-GFP movement in relation to a plasma membrane fluorescent marker and found that the channel was organized into tubular vesicles and small labeled patches of membrane. These structures could represent trafficking vesicles or microdomains of membrane containing ENaC on its way to the cell surface. As verification of this, they used a dominant-negative dynamin and exogenous Nedd4-2 expression to show that endocytic processes did not influence their signals, so that this method seems capable of isolating plasma membrane-directed ENaC movements. Manipulations of RhoA indicated that this small GTPase increases ENaC activity by increasing the movement of channels to the membrane and that this process depended on microtubules (see Fig. 2). The approach could be very helpful in further defining the factors contributing to the exocytic flow of ENaC and its regulation, but at present it is not apparent how it could be adapted to the apical membrane domain of epithelial cells, and preferably at lower channel expression levels. It may be necessary to combine studies of ENaC-expressing fibroblasts, with secondary assays carried out using endogenous expression systems and methods (e.g., RNAi) to examine physiological significance.
Intracellular Retention and Exocytosis of ENaC
As indicated by Bernard Rossier in an editorial comment some years ago (150), “… we know much more about the mechanism of [ENaC] membrane retrieval than that of insertion.” Unfortunately, the same statement applies more than 5 years later. We know much less about the intracellular compartments from which ENaC is mobilized to the cell surface and the processes that move the channel into and out of these compartments than we do about the early steps in the endocytosis and recycling of the channel.
Recent work suggests that constitutive and regulated pathways of ENaC traffic to the apical membrane may involve its movement within different membrane domains (74, 75). Several channel and transporter proteins, including those in epithelial cells, can be found in the light, or buoyant, fractions in sucrose density gradients, commonly referred to as lipid rafts. For example, aquaporins, CFTR, NHE3, and Ca-activated K channels, among others, are localized to these cholesterol-rich domains (64, 70, 98, 103, 113, 185, 200, 202). In principle, this partitioning may serve for apical targeting within the biosynthetic pathway and lead to the organization of the channel with receptor and/or signaling complexes at the cell surface. Recent findings suggest also that ENaC may use cholesterol-rich domains to gain access to the apical membrane of CCD cells (75).
It is recognized that ENaC subunits in epithelia are only partially soluble in detergents. Channel subunits exhibit light buoyant properties and colocalize with caveolin-1 on sucrose density gradients (74). Interestingly, SNARE proteins are also present in these fractions, as observed at presynaptic membranes (88, 89, 100, 131, 132), perhaps highlighting the role of these domains in the final stages of apical channel insertion (see also Fig. 2). Overall, 20–30% of ENaC subunits in CCD epithelia were present in raft fractions, and there was no influence of short-term cAMP/PKA stimulation or long-term (18 h) aldosterone treatment on the gradient distribution of ENaC subunits. These findings imply that a raft-associated pathway to the apical membrane may not participate in the regulated redistribution of ENaC at the cell surface. The expression of a dominant-negative caveolin-1 in CCD cells inhibited ENaC currents, implying that raft domains may provide a mechanism for the apical delivery of ENaC or for modulating the regulators of ENaC trafficking. In addition, treatment of cells with the cholesterol disrupting reagent, methyl-cyclodextrin (m-CD), resulted in a slow decline in ENaC current, mimicking the time course of basal current inhibition observed when channel biosynthesis is inhibited with cycloheximide (25). Interestingly, the acute response of ENaC current to cAMP/PKA stimulation, which is mediated by channel insertion from a subapical pool, was not affected by m-CD or expression of the caveolin-1 mutant. These findings imply that the constitutive delivery of ENaC to the apical surface involves organization of the channel into lipid raft domains. Conversely, the regulated insertion and recycling of ENaC appear to be independent of raft, utilizing other pathways, including those dependent on clathrin-mediated endocytosis. The extent to which these pathways communicate under different regulatory conditions, that is, the possibility that ENaC subunits may shift between pathways, is unclear and requires further study.
Events in ENaC Trafficking at the Apical Membrane
The final step of vesicle fusion with the plasma membrane involves cognate interactions between vesicle and target membrane-localized SNARE proteins. R-SNAREs with a single coiled-coil domain are localized to the donor compartment (vesicles). At the target membrane, Q-SNAREs form dimers, with one member from the synataxin family and the second member derived from the SNAP family of proteins. Syntaxins are type 2 membrane proteins with a single coiled domain and single transmembrane segment, while SNAP isoforms are generally comprised of two coiled helices and are attached to the plasma membrane by palmitoylation. The assembly of a four-helix bundle derived from R- and Q-SNAREs constitutes the minimal machinery required for vesicle fusion with the target membrane (7, 27, 37, 47, 56).
Interestingly, ENaC localized to lipid raft domains was present together with the plasma membrane Q-SNARE proteins, syntaxin 1A (S1A), and SNAP-23, and with the R-SNARE protein, VAMP2, in CCD cells (75). These SNARE components are localized to the apical domain of polarized CCD epithelia by immunolabeling (unpublished observations, M. B. Butterworth). Therefore, these SNAREs are candidates to mediate ENaC-containing vesicle fusion, although information on the presence of other apical candidates, such as syntaxin 3, is also required. For some time, it has been known that the coexpression of ENaC with S1A inhibits ENaC currents in Xenopus laevis oocytes (135), similar to findings made previously for CFTR (125, 126). For both proteins, S1A inhibition resulted from a decrease in channel density at the plasma membrane, although changes in channel open probability have also been reported for CFTR (135). A direct protein interaction of ENaC with S1A was demonstrated in pairwise binding studies, and this appeared to preferentially involve the subunit COOH termini and the H3 domain of S1A (42, 43). To date, the physiological significance of this interaction in renal epithelia endogenously expressing ENaC has not been shown. Investigators suggested that SNAP-23 has an inhibitory effect on ENaC in HT-29 cells (155), an interaction that was augmented by S1A coexpression in oocytes. The interaction of ENaC with S1A and SNAP-23 may play a role in modulating the activity or trafficking of ENaC. The localization of SNARE proteins in lipid microdomains may reflect their role in apical insertion, and data thus far suggest that they likely affect the constitutive delivery of channels to the apical surface. It is interesting that SNARE protein regulators, such as SNAPs, NSF, munc18, and complexin, appear to exist primarily in nonraft domains, and this may indicate that they play a role under some trafficking/regulatory conditions and not others (64, 99). When complexed with SNARES, these proteins are most often found in nonraft domains. Thus, a spatial separation of SNARES and their regulators may facilitate the exocytic function of SNAREs or modulate their localization in raft domains to regulate final steps in ENaC insertion (see Fig. 2).
Hormonal Regulation of ENaC Trafficking
From the descriptions above, it is clear that many targets exist to regulate the trafficking and recycling of ENaC. By changing delivery or retrieval rates or altering the vesicular itinerary of ENaC, cells will be able to shift the localization of ENaC from a predominantly intracellular localization for example to the apical membrane. It is in this way that hormonal regulation can be exercised and examples of this are discussed briefly (see also Fig. 3).
Aldosterone
Aldosterone is the primary mineralocorticoid of vertebrates; its regulation of Na transport involves both early (min-h) and late (h-days) components (8, 44, 48, 62, 71, 95, 133, 147, 159, 188, 205). Aldosterone acts to increase Na+ transport in two ways. Immunohistochemical studies from mouse, rat, and rabbit kidneys demonstrated that upon aldosterone stimulation, ENaC subunits are redistributed from intracellular, cytoplasmic localizations to the apical membranes of principal cells of the distal nephron (4, 8, 15, 106, 115, 127, 147, 205). Manipulations in circulating aldosterone levels are achieved in the rat models either by employing dietary salt restriction or making use of adrenalectomized rats and osmotic pumps or acute aldosterone infusion. It is well established that the long-term action of aldosterone increases the production of ENaC as determined by both RNA and protein expression. More pertinent to this discussion, however, the short-term effects of either salt restriction or aldosterone infusion offer insights into the localization of resident ENaC before the longer-term genomic expressions predominate ENaC regulation. The early aldosterone responses point to trafficking mechanisms that regulate ENaC surface density (101, 102, 106, 117, 124, 171, 188). In a number of studies performed in rat models under salt restriction, no significant increase in the expression of any ENaC subunit was detected within the first 15 h. The only change noted in ENaC expression in these studies was a shift in the apparent size of the γ-ENaC subunit suggestive of a proteolytic cleavage event (49, 62). However, the overall expression of each subunit remained constant. The immunocytochemical and electron microscopic labeling pattern for ENaC expression in distal nephron segments in salt-replete rats exhibited a cytoplasmic distribution for β- and γ-ENaC with a more apical α-ENaC localization. In a separate study to investigate the action of aldosterone on this localization, infusion of aldosterone into adrenalecomized rats causes the redistribution of all three ENaC subunits from the intracellular localization to a more apical localization within 2-4 h (106). In contrast to the findings from salt restriction studies, however, the aldosterone infusion did result in the short-term induction of α-ENaC expression as evidenced by RT-PCR. These studies are in agreement for β- and γ-ENaC as they demonstrate no significant increase in expression with early aldosterone responses, and a redistribution of all subunits to the apical domain of cells in which ENaC was expressed. One of the factors which may have contributed to the apparent discrepancy of α-ENaC production with aldosterone are the more recent studies demonstrating proteolytic cleavage in both α- and γ-ENaC. It is possible that the antibodies used in the earlier studies were unable to distinguish the cleaved (activated) ENaC forms.
Electropysiological measurements from dissected distal nephron tubules in salt-restricted animals confirmed a physiological increase in sodium reabsorption through ENaC (62). This was verified by urine and plasma analysis in a separate study which demonstrated the decrease in urinary Na+ excretion due to increased Na+ absorption. Single-channel analysis of the rats on salt-restricted diets demonstrated the increase in ENaC activity as an increase in NPo (the product of channel number and open probability) further supporting the hypothesis of an increase in channel density at the apical surface with short-term aldosterone action (63). These in vivo studies all suggest that ENaC (or at least some of the ENaC subunits) is held in an intracellular pool under normal, salt-replete, conditions. When dietary changes or external cues necessitate an increase in Na+ absorption, ENaC is mobilized from this intracellular pool and trafficked to the apical membrane to increase channel number and thus Na+ reabsorption.
In addition to the channel translocation process, an increase in mRNA and subsequent protein expression was observed (4, 52, 55). The net result of aldosterone's action is an increase in the abundance of ENaC at the apical membranes of CCD cells which leads to increased Na+ transport. Several proteins are induced by aldosterone to regulate ENaC surface expression. The most significant studied to date, SGK1, negatively modulates the action of Nedd4-2 and the Nedd4-2/ENaC interaction, resulting in an increase in ENaC apical membrane half-life (13).
Insulin and Vasopressin
While the action of aldosterone occurs over hours to days, acute hormonal regulation of ENaC activity is provided by vasopressin, prostaglandins, and insulin (15, 50). It has been demonstrated in several cell, tissue, and animal model systems that the addition of vasopressin or downstream cAMP agonists induces a rapid activation of ENaC activity (93, 114, 122). Binding of vasopressin to its V2 receptor activates adenylate cyclase to convert ATP to cAMP which in turn increases PKA levels. The increase in Na+ transport occurs mainly by increasing channel density at the apical membranes by targeting and fusion of ENaC-containing vesicles to this membrane (28, 41, 51) (Fig. 3). Although this increase in ENaC activity has been described in several model systems, it has not been clear whether AVP impacts sodium uptake similarly in humans. In a study involving healthy volunteers, vasopressin was administered acutely and urinary sodium and osmolarity levels were monitored. In agreement with the analogous animal studies, it was found that sodium and water excretion (as measured in urinary output) was acutely reduced (10).
In cultured cells AVP activation of cAMP results in an increase in sodium current of ∼100% which is fully reversible. We demonstrated that on removal of agonist, ENaC was endocytosed from the membrane surface and reorganized into recycling vesicles to be available for additional rounds of stimulation (25). This mechanism is essentially identical to that described for AQP2 regulation which is found in the same segment of the nephron. The action of PKA to regulate ENaC is not entirely clear. There have been reports of direct ENaC phosphorylation; however, one study linked cAMP to Nedd4-2 directly. Snyder (165) demonstrated that cAMP inhibited Nedd4-2 function and that Nedd4-2 was a substrate for phosphorylation by PKA at serine sites known to be involved with SGK regulation of Nedd4-2. Overexpression of SGK blunted ENaC stimulation by cAMP and conversely inhibition of SGK increased cAMP responses. The authors proposed Nedd4-2 as the convergence point for both the cAMP and SGK pathways.
Prior work demonstrated that vasopressin and aldosterone primarily promote the delivery of additional channels to the apical surface of ENaC-expressing epithelial cells (increased N) (see reviews in Refs. 150, 165). Using an antibody labeling approach developed by Firsov et al. (57) for oocytes, Morris and Schafer (122) provided convincing evidence that cAMP (the mediator of vasopressin action) increases mainly the number of ENaC channels at the surface of MDCK cells. These data confirm earlier patch-clamp findings in amphibian epithelia stimulated with vasopressin or prostaglandins (97, 114).
Insulin has been shown in both tissues and model cells to increase Na+ transport, again in part by inducing the translocation of ENaC to the apical membrane. A confirmation of these in vitro studies in in vivo models was obtained by acutely introducing insulin into C57BL/CBA mice (184). This resulted in a significant reduction in excreted sodium due to an increase in ENaC activity. Biochemical isolation of the plasma membrane proteins from insulin- and vehicle-treated mouse kidneys demonstrated an increase in the abundance of ENaC subunits in those mice injected with insulin, suggesting that ENaC was trafficked to the apical membrane in response to acute insulin stimulation in agreement with the earlier in vitro studies (168).
Conclusion
As discussed in this review, ENaC trafficking and recycling constitute a central mechanism to exercise physiological regulation of salt and water reapsorption in the distal nephron of the kidney. From disease-related mutations much has been elucidated about the apical membrane residency and half-life of ENaC. However, the mechanisms of ENaC internalization and the importance of channel recycling are becoming increasingly evident. It is clear that a suite of interacting proteins will be implicated in moving ENaC through intracellular vesicular compartments as it traffics through the apical compartments of these polarized epithelial cells. This offers the potential for multiple checkpoints in ENaC regulation, mediated by a complex web of protein associations. By uncovering the mechanics of this regulation, it is hoped that we will gain insight into pathophysiological conditions which arise from defects in this mode of regulation and identify targets for its therapeutic manipulation.
GRANTS
Our work is supported by grants from the Cystic Fibrosis Foundation (BUTTER06G0) and National Institutes of Health (NIH) K99/R00 (DK-78917) to M. B. Butterworth, NIH DK-54814 to R. A. Frizzell, and NIH DK-57718 to J. P. Johnson.
Acknowledgments
We gratefully acknowledge the excellent skills of M. Silvis for assistance in the construction of the figures for this review.
REFERENCES
- 1.Abriel H, Loffing J, Rebhun JF, Pratt JH, Schild L, Horisberger JD, Rotin D, Staub O. Defective regulation of the epithelial Na+ channel by Nedd4 in Liddle's syndrome. J Clin Invest 103: 667–673, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adebamiro A, Cheng Y, Johnson JP, Bridges RJ. Endogenous protease activation of ENaC: effect of serine protease inhibition on ENaC single channel properties. J Gen Physiol 126: 339–352, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adebamiro A, Cheng Y, Rao US, Danahay H, Bridges RJ. A segment of gamma ENaC mediates elastase activation of Na+ transport. J Gen Physiol 130: 611–629, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez de la Rosa D, Li H, Canessa CM. Effects of aldosterone on biosynthesis, traffic, and functional expression of epithelial sodium channels in A6 cells. J Gen Physiol 119: 427–442, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amerik AY, Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta 1695: 189–207, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Anitei M, Baust T, Czupalla C, Parshyna I, Bourel L, Thiele C, Krause E, Hoflack B. Protein networks supporting AP-3 function in targeting lysosomal membrane proteins. Mol Biol Cell 19: 1942–1951, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aroeti B, Okhrimenko H, Reich V, Orzech E. Polarized traffickinig of plasma membrane proteins: emerging roles for coats, SNAREs, GTPases and their link to the cytoskeleton. Biochim Biophys Acta 1376: 57–90, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Asher C, Wald H, Rossier BC, Garty H. Aldosterone-induced increase in the abundance of Na+ channel subunits. Am J Physiol Cell Physiol 271: C605–C611, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Baker EH, Dong YB, Sagnella GA, Rothwell M, Onipinla AK, Markandu ND, Cappuccio FP, Cook DG, Persu A, Corvol P. Association of hypertension with T594M mutation in [beta] subunit of epithelial sodium channels in black people resident in London. Lancet 351: 1388–1392, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Bankir L, Fernandes S, Bardoux P, Bouby N, Bichet DG. Vasopressin-V2 receptor stimulation reduces sodium excretion in healthy humans. J Am Soc Nephrol 16: 1920–1928, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Barbieri AM, Kohn AD, Roth RA, Stahl PD. Protein kinase B/akt and rab5 mediate ras activation of endocytosis. J Biol Chem 273: 19367–19370, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Barile M, Pisitkun T, Yu MJ, Chou CL, Verbalis MJ, Shen RF, Knepper MA. Large scale protein identification in intracellular aquaporin-2 vesicles from renal inner medullary collecting duct. Mol Cell Proteomics 4: 1095–1106, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhalla V, Soundararajan R, Pao AC, Li H, Pearce D. Disinhibitory pathways for control of sodium transport: regulation of ENaC by SGK1 and GILZ. Am J Physiol Renal Physiol 291: F714–F721, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Bhalla V, Daidie D, Li H, Pao AC, LaGrange LP, Wang J, Vandewalle A, Stockand JD, Staub O, Pearce D. Serum- and glucocorticoid-regulated kinase 1 regulates ubiquitin ligase neural precursor cell-expressed, developmentally downregulated protein 4-2 by inducing interaction with 14-3-3. Mol Endocrinol 19: 3073–3084, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Blazer-Yost BL, Liu X, Helman SI. Hormonal regulation of ENaCs: insulin and aldosterone. Am J Physiol Cell Physiol 274: C1373–C1379, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Blazer-Yost BL, Paunescu TG, Helman SI, Lee KD, Vlahos CJ. Phosphoinositide 3-kinase is required for aldosterone-regulated sodium reabsorption. Am J Physiol Cell Physiol 277: C531–C536, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Blazer-Yost BL, Helman SI. The amiloride-sensitive epithelial Na+ channel: binding sites and channel densities. Am J Physiol Cell Physiol 272: C761–C769, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Borgnia M, Nielsen S, Engel A, Agre P. Cellular and molecular biology of the aquaporin water channels. Annu Rev Biochem 68: 425–458, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Boucher RC, Stutts MJ, Knowles MR, Cantley LC, Gatzy JT. Na+ transport in cystic fibrosis respiratory epithelia. J Clin Invest 78: 1245–1252, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooks HL, Ageloff S, Kwon TH, Brandt W, Terris JM, Seth A, Michea L, Nielsen S, Fenton R, Knepper MA. cDNA array identification of genes regulated in rat renal medulla in response to vasopressin infusion. Am J Physiol Renal Physiol 284: F218–F228, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Brown D The ins and outs of aquaporin-2 trafficking. Am J Physiol Renal Physiol 284: F893–F901, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the γ-subunit. J Biol Chem 282: 6153–6160, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 70: 715–728, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Buck TM, Eledge J, Skach WR. Evidence for stabilization of aquaporin-2 folding mutants by N-linked glycosylation in endoplasmic reticulum. Am J Physiol Cell Physiol 287: C1292–C1299, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Butterworth MB, Edinger RS, Johnson JP, Frizzell RA. Acute ENaC stimulation by cAMP in a kidney cell line is mediated by exocytic insertion from a recycling channel pool. J Gen Physiol 125: 81–101, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butterworth MB, Edinger RS, Ovaa H, Burg D, Johnson JP, Frizzell RA. The deubiquitinating enzyme UCH-L3 regulates the apical membrane recycling of the epithelial sodium channel. J Biol Chem 282: 37885–37893, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Butterworth MB, Frizzell RA, Johnson JP, Peters KW, Edinger RS. PKA-dependent ENaC trafficking requires the SNARE-binding protein complexin. Am J Physiol Renal Physiol 289: F969–F977, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Butterworth MB, Helman SI, Els WJ. cAMP-sensitive endocytic trafficking in A6 epithelia. Am J Physiol Cell Physiol 280: C752–C762, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Caldwell RA, Boucher RC, Stutts MJ. Serine protease activation of near-silent epithelial Na+ channels. Am J Physiol Cell Physiol 286: C190–C194, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Caldwell RA, Boucher RC, Stutts MJ. Neutrophil elastase activates near-silent epithelial Na+ channels and increases airway epithelial Na+ transport. Am J Physiol Lung Cell Mol Physiol 288: L813–L819, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Casanova JE, Wang X, Kumar R, Bhartur SG, Navarre J, Woodrum JE, Altschuler Y, Ray GS, Goldenring JR. Association of rab25 and rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol Biol Cell 10: 47–61, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang SS, Grunder S, Hanukoglu A, Rosler A, Mathew PM, Hanukoglu I, Schild L, Lu Y, Shimkets RA, Nelson-Williams C, Rossier BC, Lifton RP. Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalemic acidosis, pseudoaldosteronism type 1. Nat Genet 13: 248–250, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Chapman RE, Munro S. Retrieval of TGN proteins from the cell surface requires endosomal acidification. EMBO J 13: 2305–2312, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen H, De CP. The association of epsin with ubiquitinated cargo along the endocytic pathway is negatively regulated by its interaction with clathrin. Proc Natl Acad Sci USA 102: 2766–2771, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen W, Feng Y, Chen D, Wandinger-Ness A. Rab11 is required for trans-Golgi network to plasma membrane transport and a preferential target for GDP dissociation inhibitor. Mol Biol Cell 9: 3241–3257, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Zhang B, Fischer JA. A specific protein substrate for a deubiquitinating enzyme: liquid facets is the substrate of Fat facets. Genes Dev 16: 289–294, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol 2: 98–106, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Chraibi A, Vallet V, Firsov D, Hess SK, Horisberger JD. Protease modulation of the activity of the epithelial sodium channel expressed in Xenopus oocytes. J Gen Physiol 111: 127–138, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christensen BM, Marples D, Jensen UB, Frokiær J, Sheikh-Hamad D, Knepper M, Nielsen S. Acute effects of vasopressin V2-receptor antagonist on kidney AQP2 expression and subcellular distribution. Am J Physiol Renal Physiol 275: F285–F297, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Clague MJ, Urbe S. Endocytosis: the DUB version. Trends Cell Biol 16: 551–559, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Coleman RA, Wade JB. ADH-induced recycling of fluid-phase marker from endosomes to the mucosal surface in toad bladder. Am J Physiol Cell Physiol 267: C32–C38, 1994. [DOI] [PubMed] [Google Scholar]
- 42.Condliffe SB, Zhang H, Frizzell RA. Acute inhibition of ENaC function by syntaxin 1A. FASEB J 17: A914, 2003. [Google Scholar]
- 43.Condliffe SB, Zhang H, Frizzell RA. Syntaxin 1A regulates ENaC channel activity. J Biol Chem 279: 10085–10092, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Dahlmann A, Pradervand S, Hummler E, Rossier BC, Frindt G, Palmer LG. Mineralocorticoid regulation of epithelial Na+ channels is maintained in a mouse model of Liddle's syndrome. Am J Physiol Renal Physiol 285: F310–F318, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Di PG, Moskowitz HS, Gipson K, Wenk MR, Voronov S, Obayashi M, Flavell R, Fitzsimonds RM, Ryan TA, De CP. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature 431: 415–422, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Dinudom A, Fotia AB, Lefkowitz RJ, Young JA, Kumar S, Cook DI. The kinase Grk2 regulates Nedd4/Nedd4-2-dependent control of epithelial Na+ channels. Proc Natl Acad Sci USA 101: 11886–11890, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duman JG, Forte JG. What is the role of SNARE proteins in membrane fusion? Am J Physiol Cell Physiol 285: C237–C249, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Eaton DC, Malik B, Saxena NC, Al-Khalili OK, Yue G. Mechanisms of aldosterone's action on epithelial Na+ transport. J Membr Biol 184: 313–319, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Ergonul Z, Frindt G, Palmer LG. Regulation of maturation and processing of ENaC subunits in the rat kidney. Am J Physiol Renal Physiol 291: F683–F693, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Erlij D, De Smet P, van Driessche W. Effect of insulin on area and Na+ channel density of apical membrane of cultured toad kidney cells. J Physiol 481: 533–542, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erlij D, De SP, Mesotten D, Van Driessche W. Forskolin increases apical sodium conductance in cultured toad kidney cells (A6) by stimulating membrane insertion. Pflügers Arch 438: 195–204, 1999. [DOI] [PubMed] [Google Scholar]
- 52.Escoubet B, Coureau C, Bonvalet JP, Farman N. Noncoordinate regulation of epithelial Na channel and Na pump subunit mRNAs in kidney and colon by aldosterone. Am J Physiol Cell Physiol 272: C1482–C1491, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Fakitsas P, Adam G, Daidie D, van Bemmelen MX, Fouladkou F, Patrignani A, Wagner U, Warth R, Camargo SM, Staub O, Verrey F. Early aldosterone-induced gene product regulates the epithelial sodium channel by deubiquitylation. J Am Soc Nephrol 18: 1084–1092, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Falin RA, Cotton CU. Acute downregulation of ENaC by EGF involves the PY motif and putative ERK phosphorylation site. J Gen Physiol 130: 313–328, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farman N, Talbot CR, Boucher R, Fay M, Canessa C, Rossier BC, Bonvalet JP. Noncoordinated expression of α, β, and γ subunit mRNAs of the epithelial sodium channel along the rat respiratory tract. Am J Physiol Cell Physiol 272: C131–C141, 1997. [DOI] [PubMed] [Google Scholar]
- 56.Fasshauer D Structural insights into the SNARE mechanism. Biochim Biophys Acta 1641: 87–97, 2003. [DOI] [PubMed] [Google Scholar]
- 57.Firsov D, Schild L, Gautschi I, Merillat AM, Schneeberger E, Rossier BC. Cell surface expression of the epithelial Na channel and a mutant causing Liddle syndrome: a quantitative approach. Proc Natl Acad Sci USA 93: 15370–15375, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fish EM, Molitoris BA. Alterations in epithelial polarity and the pathogenesis of disease states. N Engl J Med 330: 1580–1588, 1994. [DOI] [PubMed] [Google Scholar]
- 59.Flores SY, Debonneville C, Staub O. The role of Nedd4/Nedd4-like dependant ubiquitylation in epithelial transport processes. Pflügers Arch 446: 334–338, 2003. [DOI] [PubMed] [Google Scholar]
- 60.Ford MG, Mills IG, Peter BJ, Vallis Y, Praefcke GJ, Evans PR, McMahon HT. Curvature of clathrin-coated pits driven by epsin. Nature 419: 361–366, 2002. [DOI] [PubMed] [Google Scholar]
- 61.Ford MG, Pearse BM, Higgins MK, Vallis Y, Owen DJ, Gibson A, Hopkins CR, Evans PR, McMahon HT. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 291: 1051–1055, 2001. [DOI] [PubMed] [Google Scholar]
- 62.Frindt G, Ergonul Z, Palmer LG. Na channel expression and activity in the medullary collecting duct of rat kidney. Am J Physiol Renal Physiol 292: F1190–F1196, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Frindt G, Masilamani S, Knepper MA, Palmer LG. Activation of epithelial Na channels during short-term Na deprivation. Am J Physiol Renal Physiol 280: F112–F118, 2001. [DOI] [PubMed] [Google Scholar]
- 64.Fullekrug J, Simons K. Lipid rafts and apical membrane traffic. Ann NY Acad Sci 1014: 164–169, 2004. [DOI] [PubMed] [Google Scholar]
- 65.Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev 77: 359–396, 1997. [DOI] [PubMed] [Google Scholar]
- 66.Gentzsch M, Chang XB, Cui L, Wu Y, Ozols VV, Choudhury A, Pagano RE, Riordan JR. Endocytic trafficking routes of wild type and ΔF508 cystic fibrosis transmembrane conductance regulator. Mol Biol Cell 15: 2684–2696, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gillooly DJ, Raiborg C, Stenmark H. Phosphatidylinositol 3-phosphate is found in microdomains of early endosomes. Histochem Cell Biol 120: 445–453, 2003. [DOI] [PubMed] [Google Scholar]
- 68.Goulet CC, Volk KA, Adams CM, Prince LS, Stokes JB, Snyder PA. Inhibition of the epithelial Na+ channel by interaction of Nedd4 with a PY motif deleted in Liddle's syndrome. J Biol Chem 273: 30012–30017, 1998. [DOI] [PubMed] [Google Scholar]
- 69.Hansson JH, Nelson-Williams C, Suzuki H, Schild L, Shimkets R, Lu Y, Canessa C, Iwasaki T, Rossier B, Lifton RP. Hypertension caused by a truncated epithelial sodium channel gamma subunit: genetic heterogeneity of Liddle syndrome. Nat Genet 11: 76–82, 1995. [DOI] [PubMed] [Google Scholar]
- 70.Hanwell D, Ishikawa T, Saleki R, Rotin D. Trafficking and cell surface stability of the epithelial Na+ channel expressed in epithelial Madin-Darby canine kidney cells. J Biol Chem 277: 9772–9779, 2002. [DOI] [PubMed] [Google Scholar]
- 71.Helman SI, Liu X, Baldwin K, Blazer-Yost BL, Els WJ. Time-dependent stimulation by aldosterone of blocker-sensitive ENaCs in A6 epithelia. Am J Physiol Cell Physiol 274: C947–C957, 1998. [DOI] [PubMed] [Google Scholar]
- 72.Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol 19: 141–172, 2003. [DOI] [PubMed] [Google Scholar]
- 73.Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE 2001: RE19, 2001. [DOI] [PubMed] [Google Scholar]
- 74.Hill WG, An B, Johnson JP. Endogenously expressed epithelial sodium channel is present in lipid rafts in A6 cells. J Biol Chem 277: 33541–33544, 2002. [DOI] [PubMed] [Google Scholar]
- 75.Hill WG, Butterworth MB, Wang H, Edinger RS, Lebowitz J, Peters KW, Frizzell RA, Johnson JP. The epithelial sodium channel (ENaC) traffics to apical membrane in lipid rafts in mouse cortical collecting duct cells. J Biol Chem 282: 37402–37411, 2007. [DOI] [PubMed] [Google Scholar]
- 76.Hou JC, Pessin JE. Ins (endocytosis) and outs (exocytosis) of GLUT4 trafficking. Curr Opin Cell Biol 19: 466–473, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Houle S, Marceau F. Wortmannin alters the intracellular trafficking of the bradykinin B2 receptor: role of phosphoinositide 3-kinase and Rab5. Biochem J 375: 151–158, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, Johnson JP, Stockand JD, Kleyman TR. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem 279: 18111–18114, 2004. [DOI] [PubMed] [Google Scholar]
- 79.Hughey RP, Bruns JB, Kinlough CL, Kleyman TR. Distinct pools of epithelial sodium channels are expressed at the plasma membrane. J Biol Chem 279: 48491–48494, 2004. [DOI] [PubMed] [Google Scholar]
- 80.Hughey RP, Mueller GM, Bruns JB, Kinlough CL, Poland PA, Harkleroad KL, Carattino MD, Kleyman TR. Maturation of the epithelial Na+ channel involves proteolytic processing of the alpha- and gamma-subunits. J Biol Chem 278: 37073–37082, 2003. [DOI] [PubMed] [Google Scholar]
- 81.Hunyady L, Baukal AJ, Gaborik Z, Olivares-Reyes JA, Bor M, Szaszak M, Lodge R, Catt KJ, Balla T. Differential PI 3-kinase dependence of early and late phases of recycling of the internalized AT1 angiotensin receptor. J Cell Biol 157: 1211–1222, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hurley JH, Lee S, Prag G. Ubiquitin-binding domains. Biochem J 399: 361–372, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ichimura T, Yamamura H, Sasamoto K, Tominaga Y, Taoka M, Kakiuchi K, Shinkawa T, Takahashi N, Shimada S, Isobe T. 14-3-3 Proteins modulate the expression of epithelial Na+ channels by phosphorylation-dependent interaction with Nedd4-2 ubiquitin ligase. J Biol Chem 280: 13187–13194, 2005. [DOI] [PubMed] [Google Scholar]
- 84.Inoue T, Terris J, Ecelbarger CA, Chou CL, Nielsen S, Knepper MA. Vasopressin regulates apical targeting of aquaporin-2 but not of UT1 urea transporter in renal collecting duct. Am J Physiol Renal Physiol 276: F559–F566, 1999. [DOI] [PubMed] [Google Scholar]
- 85.Ismailov II, Berdiev BK, Benos DJ. Biochemical status of renal epithelial Na+ channels determines apparent channel conductance, ion selectivity and amiloride sensitivity. Biophys J 69: 1789–1800, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Itoh T, Koshiba S, Kigawa T, Kikuchi A, Yokoyama S, Takenawa T. Role of the ENTH domain in phosphatidylinositol-4,5-bisphosphate binding and endocytosis. Science 291: 1047–1051, 2001. [DOI] [PubMed] [Google Scholar]
- 87.Ivetac I, Munday AD, Kisseleva MV, Zhang XM, Luff S, Tiganis T, Whisstock JC, Rowe T, Majerus PW, Mitchell CA. The type Ialpha inositol polyphosphate 4-phosphatase generates and terminates phosphoinositide 3-kinase signals on endosomes and the plasma membrane. Mol Biol Cell 16: 2218–2233, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jahn R, Lang T, Sudhof TC. Membrane fusion. Cell 112: 519–533, 2003. [DOI] [PubMed] [Google Scholar]
- 89.Jahn R, Sudhof TC. Membrane fusion and exocytosis. Annu Rev Biochem 68: 863–911, 1999. [DOI] [PubMed] [Google Scholar]
- 90.Joly M, Kazlauskas A, Corvera S. Phosphatidylinositol 3-kinase activity is required at a postendocytic step in platelet-derived growth factor receptor trafficking. J Biol Chem 270: 13225–13230, 1995. [DOI] [PubMed] [Google Scholar]
- 91.Kabra R, Knight KK, Zhou R, Snyder PM. Nedd4-2 induces endocytosis and degradation of proteolytically cleaved epithelial Na+ channels. J Biol Chem In press. [DOI] [PubMed]
- 92.Kim SW, Wang W, Nielsen J, Praetorius J, Kwon TH, Knepper MA, Frokiaer J, Nielsen S. Increased expression and apical targeting of renal ENaC subunits in puromycin aminonucleoside-induced nephrotic syndrome in rats. Am J Physiol Renal Physiol 286: F922–F935, 2004. [DOI] [PubMed] [Google Scholar]
- 93.Kleyman TR, Ernst SA, Coupaye-Gerard B. Arginine vasopressin and forskolin regulate apical cell surface expression of epithelial Na+ channels in A6 cells. Am J Physiol Renal Fluid Electrolyte Physiol 266: F506–F511, 1994. [DOI] [PubMed] [Google Scholar]
- 94.Kleyman TR, Myerburg MM, Hughey RP. Regulation of ENaCs by proteases: an increasingly complex story. Kidney Int 70: 1391–1392, 2006. [DOI] [PubMed] [Google Scholar]
- 95.Knepper MA, Kim GH, Masilamani S. Renal tubule sodium transporter abundance profiling in rat kidney: response to aldosterone and variations in NaCl intake. Ann NY Acad Sci 986: 562–569, 2003. [DOI] [PubMed] [Google Scholar]
- 96.Knight KK, Olson DR, Zhou R, Snyder PM. Liddle's syndrome mutations increase Na+ transport through dual effects on epithelial Na+ channel surface expression and proteolytic cleavage. Proc Natl Acad Sci USA 103: 2805–2808, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kokko KE, Matsumoto PS, Ling BN, Eaton DC. Effects of prostaglandin E2 on amiloride-blockable Na+ channels in a distal nephron cell line (A6). Am J Physiol Cell Physiol 267: C1414–C1425, 1994. [DOI] [PubMed] [Google Scholar]
- 98.Kowalski MP, Pier GB. Localization of cystic fibrosis transmembrane conductance regulator to lipid rafts of epithelial cells is required for pseudomonas aeruginosa-induced cellular activation. J Immunol 172: 418–425, 2004. [DOI] [PubMed] [Google Scholar]
- 99.Lafont F, Verkade P, Galli T, Wimmer C, Louvard D, Simons K. Raft association of SNAP receptors acting in apical trafficking in Madin-Darby canine kidney cells. Proc Natl Acad Sci USA 96: 3734–3738, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lang T, Bruns D, Wenzel D, Riedel D, Holroyd P, Thiele C, Jahn R. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J 20: 2202–2213, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Le MC, Cluzeaud F, Fay M, Blot-Chabaud M. The early nongenomic aldosterone-induced increase in sodium transport is a membrane-initiated event that requires protein carboxyl methylation in renal cells. Cell Mol Biol (Noisy-le-grand) 50: 833–840, 2004. [PubMed] [Google Scholar]
- 102.Le MC, Ouvrard-Pascaud A, Capurro C, Cluzeaud F, Fay M, Jaisser F, Farman N, Blot-Chabaud M. Early nongenomic events in aldosterone action in renal collecting duct cells: PKCalpha activation, mineralocorticoid receptor phosphorylation, and cross-talk with the genomic response. J Am Soc Nephrol 15: 1145–1160, 2004. [PubMed] [Google Scholar]
- 103.Li X, Galli T, Leu S, Wade JB, Weinman EJ, Leung G, Cheong A, Louvard D, Donowitz M. Na+-H+ exchanger 3 (NHE3) is present in lipid rafts in the rabbit ileal brush border: a role for rafts in trafficking and rapid stimulation of NHE3. J Physiol 537: 537–552, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liang X, Peters KW, Butterworth MB, Frizzell RA. 14-3-3 Isoforms are induced by aldosterone and participate in its regulation of epithelial sodium channels. J Biol Chem 281: 16323–16332, 2006. [DOI] [PubMed] [Google Scholar]
- 105.Liddle GW, Bledsoe T, Coppage WS. A familial renal disorder simulating primary aldosteronism but with negligible aldosterone secretion. Trans Assoc Am Physicians 76: 199–213, 1963. [Google Scholar]
- 106.Loffing J, Zecevic M, Feraille E, Kaissling B, Asher C, Rossier BC, Firestone GL, Pearce D, Verrey F. Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK. Am J Physiol Renal Physiol 280: F675–F682, 2001. [DOI] [PubMed] [Google Scholar]
- 107.Lu C, Pribanic S, Debonneville A, Jiang C, Rotin D. The PY motif of ENaC, mutated in Liddle syndrome, regulates channel internalization, sorting and mobilization from subapical pool. Traffic 8: 1246–1264, 2007. [DOI] [PubMed] [Google Scholar]
- 108.Ma HP, Saxena S, Warnock DG. Anionic phospholipids regulate native and expressed epithelial sodium channel (ENaC). J Biol Chem 277: 7641–7644, 2002. [DOI] [PubMed] [Google Scholar]
- 109.Malik B, Price SR, Mitch WE, Yue Q, Eaton DC. Regulation of epithelial sodium channels by the ubiquitin-proteasome proteolytic pathway. Am J Physiol Renal Physiol 290: F1285–F1294, 2006. [DOI] [PubMed] [Google Scholar]
- 110.Malik B, Yue Q, Yue G, Chen X, Price SR, Mitch WE, Eaton DC. Role of Nedd4-2 and polyubiquitination in epithelial sodium channel degradation in untransfected renal A6 cells expressing endogenous ENaC subunits. Am J Physiol Renal Physiol 289: F107–F116, 2005. [DOI] [PubMed] [Google Scholar]
- 111.Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med 10: 487–493, 2004. [DOI] [PubMed] [Google Scholar]
- 112.Martel JA, Michael D, Fejes-Toth G, Naray-Fejes-Toth A. Melanophilin, a novel aldosterone-induced gene in mouse cortical collecting duct cells. Am J Physiol Renal Physiol 293: F904–F913, 2007. [DOI] [PubMed] [Google Scholar]
- 113.Martens JR, Sakamoto N, Sullivan SA, Grobaski TD, Tamkun MM. Isoform-specific localization of voltage-gated K+ channels to distinct lipid raft populations. Targeting of Kv15 to caveolae. J Biol Chem 276: 8409–8414, 2001. [DOI] [PubMed] [Google Scholar]
- 114.Marunaka Y, Eaton DC. Effects of vasopressin and cAMP on single amiloride-blockable Na channels. Am J Physiol Cell Physiol 260: C1071–C1084, 1991. [DOI] [PubMed] [Google Scholar]
- 115.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC α, β, and γ subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Matalon S, Lazrak A, Jain L, Eaton DC. Invited review: biophysical properties of sodium channels in lung alveolar epithelial cells. J Appl Physiol 93: 1852–1859, 2002. [DOI] [PubMed] [Google Scholar]
- 117.May A, Puoti A, Gaeggeler HP, Horisberger JD, Rossier BC. Early effect of aldosterone on the rate of synthesis of the epithelial sodium channel alpha subunit in A6 renal cells. J Am Soc Nephrol 8: 1813–1822, 1997. [DOI] [PubMed] [Google Scholar]
- 119.McCullough J, Clague MJ, Urbe S. AMSH is an endosome-associated ubiquitin isopeptidase. J Cell Biol 166: 487–492, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Millard SM, Wood SA. Riding the DUBway: regulation of protein trafficking by deubiquitylating enzymes. J Cell Biol 173: 463–468, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Milosevic I, Sorensen JB, Lang T, Krauss M, Nagy G, Haucke V, Jahn R, Neher E. Plasmalemmal phosphatidylinositol-4,5-bisphosphate level regulates the releasable vesicle pool size in chromaffin cells. J Neurosci 25: 2557–2565, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Morris RG, Schafer JA. cAMP increases density of ENaC subunits in the apical membrane of MDCK cells in direct proportion to amiloride-sensitive Na+ transport. J Gen Physiol 120: 71–85, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mousavi SA, Malerod L, Berg T, Kjeken R. Clathrin-dependent endocytosis. Biochem J 377: 1–16, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Muller OG, Parnova RG, Centeno G, Rossier BC, Firsov D, Horisberger JD. Mineralocorticoid effects in the kidney: correlation between alphaENaC, GILZ, and Sgk-1 mRNA expression and urinary excretion of Na+ and K+. J Am Soc Nephrol 14: 1107–1115, 2003. [DOI] [PubMed] [Google Scholar]
- 125.Naren AP, Di A, Cormet-Boyaka E, Boyaka PN, McGhee JR, Zhou W, Akagawa K, Fujiwara T, Thome U, Engelhardt JF, Nelson DJ, Kirk KL. Syntaxin 1A is expressed in airway epithelial cells, where it modulates CFTR Cl− currents. J Clin Invest 105: 377–386, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Naren AP, Quick MW, Collawn JF, Nelson DJ, Kirk KL. Syntaxin 1A inhibits CFTR chloride channels by means of domain-specific protein-protein interactions. Proc Natl Acad Sci USA 95: 10972–10977, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nielsen J, Kwon TH, Frøkiær J, Knepper MA, Nielsen S. Maintained ENaC trafficking in aldosterone-infused rats during mineralocorticoid and glucocorticoid receptor blockade. Am J Physiol Renal Physiol 292: F382–F394, 2007. [DOI] [PubMed] [Google Scholar]
- 128.Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, Bernards R. A genomic and functional inventory of deubiquitinating enzymes. Cell 123: 773–786, 2005. [DOI] [PubMed] [Google Scholar]
- 129.Noda Y, Sasaki S. Trafficking mechanism of water channel aquaporin-2. Biol Cell 97: 885–892, 2005. [DOI] [PubMed] [Google Scholar]
- 130.Overstreet E, Fitch E, Fischer JA. Fat facets and liquid facets promote delta endocytosis and delta signaling in the signaling cells. Development 131: 5355–5366, 2004. [DOI] [PubMed] [Google Scholar]
- 131.Pabst S, Hazzard JW, Antonin W, Sudhof TC, Jahn R, Rizo J, Fasshauer D. Selective interaction of complexin with the neuronal SNARE complex. Determination of the binding regions. J Biol Chem 275: 19808–19818, 2000. [DOI] [PubMed] [Google Scholar]
- 132.Pabst S, Margittai M, Vainius D, Langen R, Jahn R, Fasshauer D. Rapid and selective binding to the synaptic SNARE complex suggests a modulatory role of complexins in neuroexocytosis. J Biol Chem 277: 7838–7848, 2002. [DOI] [PubMed] [Google Scholar]
- 133.Pacha J, Frindt G, Antonian L, Silver RB, Palmer LG. Regulation of Na channels of the rat cortical collecting tubule by aldosterone. J Gen Physiol 102: 25–42, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Palmer LG Epithelial Na channels: function and diversity. Annu Rev Physiol 54: 51–66, 1992. [DOI] [PubMed] [Google Scholar]
- 135.Peters KW, Qi J, Johnson JP, Watkins SC, Frizzell RA. Role of snare proteins in CFTR and ENaC trafficking. Pflügers Arch 443, Suppl 1: S65–S69, 2001. [DOI] [PubMed] [Google Scholar]
- 136.Pfeffer S, Aivazian D. Targeting Rab GTPases to distinct membrane compartments. Nat Rev Mol Cell Biol 5: 886–896, 2004. [DOI] [PubMed] [Google Scholar]
- 137.Picciano JA, Ameen N, Grant BD, Bradbury NA. Rme-1 regulates the recycling of the cystic fibrosis transmembrane conductance regulator. Am J Physiol Cell Physiol 285: C1009–C1018, 2003. [DOI] [PubMed] [Google Scholar]
- 138.Planes C, Blot-Chabaud M, Matthay MA, Couette S, Uchida T, Clerici C. Hypoxia and beta 2-agonists regulate cell surface expression of the epithelial sodium channel in native alveolar epithelial cells. J Biol Chem 277: 47318–47324, 2002. [DOI] [PubMed] [Google Scholar]
- 139.Pochynyuk O, Staruschenko A, Bugaj V, LaGrange L, Stockand JD. Quantifying RhoA facilitated trafficking of the epithelial Na+ channel toward the plasma membrane with total internal reflection fluorescence-fluorescence recovery after photobleaching. J Biol Chem 282: 14576–14585, 2007. [DOI] [PubMed] [Google Scholar]
- 140.Pratt JH Central role for ENaC in development of hypertension. J Am Soc Nephrol 16: 3154–3159, 2005. [DOI] [PubMed] [Google Scholar]
- 141.Procino G, Caces DB, Valenti G, Pessin JE. Adipocytes support cAMP-dependent translocation of aquaporin-2 from intracellular sites distinct from the insulin-responsive GLUT4 storage compartment. Am J Physiol Renal Physiol 290: F985–F994, 2006. [DOI] [PubMed] [Google Scholar]
- 142.Prydz K, Hansen SH, Sandvig K, van Deurs B. Effects of Brefeldin A on endocytosis, transcytosis and transport to the Golgi complex in polarized MDCK cells. J Cell Biol 119: 259–272, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Raiborg C, Bache KG, Gillooly DJ, Madshus IH, Stang E, Stenmark H. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat Cell Biol 4: 394–398, 2002. [DOI] [PubMed] [Google Scholar]
- 144.Raiborg C, Bache KG, Mehlum A, Stang E, Stenmark H. Hrs recruits clathrin to early endosomes. EMBO J 20: 5008–5021, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Raiborg C, Rusten TE, Stenmark H. Protein sorting into multivesicular endosomes. Curr Opin Cell Biol 15: 446–455, 2003. [DOI] [PubMed] [Google Scholar]
- 146.Record RD, Froelich L, Vlahos CJ, Blazer-Yost BL. Phosphatidylinositol 3-kinase activation is required for insulin-stimulated sodium transport in A6 cells. Am J Physiol Endocrinol Metab 274: E611–E617, 1998. [DOI] [PubMed] [Google Scholar]
- 147.Reif MC, Troutman SL, Schafer JA. Sodium transport by rat cortical collecting tubule. Effects of vasopressin and desoxycorticosterone. J Clin Invest 77: 1291–1298, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Roosterman D, Cottrell GS, Schmidlin F, Steinhoff M, Bunnett NW. Recycling and resensitization of the neurokinin 1 receptor. Influence of agonist concentration and Rab GTPases. J Biol Chem 279: 30670–30679, 2004. [DOI] [PubMed] [Google Scholar]
- 149.Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annu Rev Physiol 64: 877–897, 2002. [DOI] [PubMed] [Google Scholar]
- 150.Rossier BC Hormonal regulation of the epithelial sodium channel ENaC: N or Po? J Gen Physiol 120: 67–70, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rossier BC Negative regulators of sodium transport in the kidney: key factors in understanding salt-sensitive hypertension? J Clin Invest 111: 947–950, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rotin D, Kanelis V, Schild L. Trafficking and cell surface stability of ENaC. Am J Physiol Renal Physiol 281: F391–F399, 2001. [DOI] [PubMed] [Google Scholar]
- 153.Rotin D, Staub O, Haguenauer-Tsapis R. Ubiquitination and endocytosis of plasma membrane proteins: role of Nedd4/Rsp5p family of ubiquitin-protein ligases. J Membr Biol 176: 1–17, 2000. [DOI] [PubMed] [Google Scholar]
- 154.Sanchez-Perez A, Kumar S, Cook DI. GRK2 interacts with and phosphorylates Nedd4 and Nedd4-2. Biochem Biophys Res Commun 359: 611–615, 2007. [DOI] [PubMed] [Google Scholar]
- 155.Saxena S, Singh M, Engisch K, Fukuda M, Kaur S. Rab proteins regulate epithelial sodium channel activity in colonic epithelial HT-29 cells. Biochem Biophys Res Commun 337: 1219–1223, 2005. [DOI] [PubMed] [Google Scholar]
- 156.Saxena SK, Horiuchi H, Fukuda M. Rab27a regulates epithelial sodium channel (ENaC) activity through synaptotagmin-like protein (SLP-5) and Munc13-4 effector mechanism. Biochem Biophys Res Commun 344: 651–657, 2006. [DOI] [PubMed] [Google Scholar]
- 157.Saxena SK, Kaur S. Regulation of epithelial ion channels by Rab GTPases. Biochem Biophys Res Commun 351: 582–587, 2006. [DOI] [PubMed] [Google Scholar]
- 158.Saxena SK, Singh M, Shibata H, Kaur S, George C. Rab4 GTP/GDP modulates amiloride-sensitive sodium channel (ENaC) function in colonic epithelia. Biochem Biophys Res Commun 340: 726–733, 2006. [DOI] [PubMed] [Google Scholar]
- 159.Shahedi M, Laborde K, Bussieres L, Sachs C. Acute and early effects of aldosterone on Na-K-ATPase activity in Madin-Darby canine kidney epithelial cells. Am J Physiol Renal Fluid Electrolyte Physiol 264: F1021–F1026, 1993. [DOI] [PubMed] [Google Scholar]
- 160.Sheng S, Carattino MD, Bruns JB, Hughey RP, Kleyman TR. Furin cleavage activates the epithelial Na+ channels by relieving Na+ self-inhibition. Am J Physiol Renal Physiol 290: F1488–F1496, 2006. [DOI] [PubMed] [Google Scholar]
- 161.Shenoy SK, Lefkowitz RJ. Trafficking patterns of beta-arrestin and G protein-coupled receptors determined by the kinetics of beta-arrestin deubiquitination. J Biol Chem 278: 14498–14506, 2003. [DOI] [PubMed] [Google Scholar]
- 162.Shimkets RA, Lifton RP, Canessa CM. The activity of the epithelial sodium channel is regulated by clathrin-mediated endocytosis. J Biol Chem 272: 25537–25541, 1997. [DOI] [PubMed] [Google Scholar]
- 163.Shimkets RA, Warnock DG, Bositis CM, Nelson-Williams C, Hansson JH, Schambelan M, Gill JR Jr, Ulick S, Milora RV, Findling JW, Canessa CM, Rossier BC, Lifton RP. Liddle's syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell 79: 407–414, 1994. [DOI] [PubMed] [Google Scholar]
- 164.Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci USA 102: 2760–2765, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Snyder PM The epithelial Na+ channel: cell surface insertion and retrieval in Na+ homeostasis and hypertension. Endocr Rev 23: 258–275, 2002. [DOI] [PubMed] [Google Scholar]
- 167.Soboleva TA, Baker RT. Deubiquitinating enzymes: their functions and substrate specificity. Curr Protein Pept Sci 5: 191–200, 2004. [DOI] [PubMed] [Google Scholar]
- 168.Song J, Hu X, Riazi S, Tiwari S, Wade JB, Ecelbarger CA. Regulation of blood pressure, the epithelial sodium channel (ENaC), and other key renal sodium transporters by chronic insulin infusion in rats. Am J Physiol Renal Physiol 290: F1055–F1064, 2006. [DOI] [PubMed] [Google Scholar]
- 169.Soundararajan R, Wang J, Melters D, Pearce D. Differential activities of glucocorticoid-induced leucine zipper protein isoforms. J Biol Chem 282: 36303–36313, 2007. [DOI] [PubMed] [Google Scholar]
- 170.Soundararajan R, Zhang TT, Wang J, Vandewalle A, Pearce D. A novel role for glucocorticoid-induced leucine zipper protein in epithelial sodium channel-mediated sodium transport. J Biol Chem 280: 39970–39981, 2005. [DOI] [PubMed] [Google Scholar]
- 171.Spindler B, Mastroberardino L, Custer M, Verrey F. Characterization of early aldosterone-induced RNAs identified in A6 kidney epithelia. Pflügers Arch 434: 323–331, 1997. [DOI] [PubMed] [Google Scholar]
- 172.Staruschenko A, Patel P, Tong Q, Medina JL, Stockand JD. Ras activates the epithelial Na+ channel through phosphoinositide 3-OH kinase signaling. J Biol Chem 279: 37771–37778, 2004. [DOI] [PubMed] [Google Scholar]
- 173.Staruschenko A, Pochynyuk O, Stockand JD. Regulation of epithelial Na+ channel activity by conserved serine/threonine switches within sorting signals. J Biol Chem 280: 39161–39167, 2005. [DOI] [PubMed] [Google Scholar]
- 174.Staruschenko A, Pochynyuk OM, Tong Q, Stockand JD. Ras couples phosphoinositide 3-OH kinase to the epithelial Na+ channel. Biochim Biophys Acta 1669: 108–115, 2005. [DOI] [PubMed] [Google Scholar]
- 175.Staub O, Abriel H, Plant P, Ishikawa T, Kanelis V, Saleki K, Horisberger JD, Schild L, Rotin D. Regulation of the epithelial Na+ channel by Nedd4 and ubiquitination. Kidney Int 57: 609–615, 2000. [DOI] [PubMed] [Google Scholar]
- 176.Staub O, Gautschi I, Ishikawa T, Breitschopf K, Ciechanover A, Schild L, Rotin D. Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination. EMBO J 16: 6325–6336, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Staub O, Yeger H, Plant PJ, Kim H, Ernst SA, Rotin D. Immunolocalization of the ubiquitin-protein ligase Nedd4 in tissues expressing the epithelial Na+ channel. Am J Physiol Cell Physiol 272: C1871–C1880, 1997. [DOI] [PubMed] [Google Scholar]
- 178.Strazzullo P, Galletti F, Barba G. Altered renal handling of sodium in human hypertension: short review of the evidence. Hypertension 41: 1000–1005, 2003. [DOI] [PubMed] [Google Scholar]
- 179.Swiatecka-Urban A, Brown A, Moreau-Marquis S, Renuka J, Coutermarsh B, Barnaby R, Karlson KH, Flotte TR, Fukuda M, Langford GM, Stanton BA. The short apical membrane half-life of rescued ΔF508-cystic fibrosis transmembrane conductance regulator (CFTR) results from accelerated endocytosis of ΔF508-CFTR in polarized human airway epithelial cells. J Biol Chem 280: 36762–36772, 2005. [DOI] [PubMed] [Google Scholar]
- 180.Swiatecka-Urban A, Talebian L, Kanno E, Moreau-Marquis S, Coutermarsh B, Hansen K, Karlson KH, Barnaby R, Cheney RE, Langford GM, Fukuda M, Stanton BA. Myosin Vb is required for trafficking of the cystic fibrosis transmembrane conductance regulator in Rab11a-specific apical recycling endosomes in polarized human airway epithelial cells. J Biol Chem 282: 23725–23736, 2007. [DOI] [PubMed] [Google Scholar]
- 181.Swift PA, MacGregor GA. The epithelial sodium channel in hypertension: genetic heterogeneity and implications for treatment with amiloride. Am J Pharmacogenomics 4: 161–168, 2004. [PubMed] [Google Scholar]
- 182.Taguchi N, Niisato N, Sawabe Y, Miyazaki H, Hirai Y, Marunaka Y. Benzamil, a blocker of epithelial Na+ channel-induced upregulation of artery oxygen pressure level in acute lung injury rabbit ventilated with high frequency oscillation. Biochem Biophys Res Commun 327: 915–919, 2005. [DOI] [PubMed] [Google Scholar]
- 183.Tian X, Hansen D, Schedl T, Skeath JB. Epsin potentiates Notch pathway activity in Drosophila and C. elegans. Development 131: 5807–5815, 2004. [DOI] [PubMed] [Google Scholar]
- 184.Tiwari S, Nordquist L, Halagappa VKM, Ecelbarger CA. Trafficking of ENaC subunits in response to acute insulin in mouse kidney. Am J Physiol Renal Physiol 293: F178–F185, 2007. [DOI] [PubMed] [Google Scholar]
- 185.Toselli M, Biella G, Taglietti V, Cazzaniga E, Parenti M. Caveolin-1 expression and membrane cholesterol content modulate N-type calcium channel activity in NG108–15 cells. Biophys J 89: 2443–2457, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Valenti G, Procino G, Tamma G, Carmosino M, Svelto M. Minireview: aquaporin 2 trafficking. Endocrinology 146: 5063–5070, 2005. [DOI] [PubMed] [Google Scholar]
- 187.Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature 389: 607–610, 1997. [DOI] [PubMed] [Google Scholar]
- 188.Verrey F Early aldosterone action: toward filling the gap between transcription and transport. Am J Physiol Renal Physiol 277: F319–F327, 1999. [DOI] [PubMed] [Google Scholar]
- 189.Vicogne J, Vollenweider D, Smith JR, Huang P, Frohman MA, Pessin JE. Asymmetric phospholipid distribution drives in vitro reconstituted SNARE-dependent membrane fusion. Proc Natl Acad Sci USA 103: 14761–14766, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vuagniaux G, Vallet V, Jaeger NF, Hummler E, Rossier BC. Synergistic activation of ENaC by three membrane-bound channel-activating serine proteases (mCAP1, mCAP2, and mCAP3) and serum- and glucocorticoid-regulated kinase (Sgk1) in Xenopus oocytes. J Gen Physiol 120: 191–201, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vuagniaux G, Vallet V, Jaeger NF, Pfister C, Bens M, Farman N, Courtois-Coutry N, Vandewalle A, Rossier BC, Hummler E. Activation of the amiloride-sensitive epithelial sodium channel by the serine protease mCAP1 expressed in a mouse cortical collecting duct cell line. J Am Soc Nephrol 11: 828–834, 2000. [DOI] [PubMed] [Google Scholar]
- 192.Waldmann R, Champigny G, Bassilana F, Voilley N, Lazdunski M. Molecular cloning and functional expression of a novel amiloride-sensitive Na+ channel. J Biol Chem 270: 27411–27414, 1995. [DOI] [PubMed] [Google Scholar]
- 193.Wang H, Traub LM, Weixel KM, Hawryluk MJ, Shah N, Edinger RS, Perry CJ, Kester L, Butterworth MB, Peters KW, Kleyman TR, Frizzell RA, Johnson JP. Clathrin-mediated endocytosis of the epithelial sodium channel. Role of epsin. J Biol Chem 281: 14129–14135, 2006. [DOI] [PubMed] [Google Scholar]
- 194.Wang W, Struhl G. Drosophila epsin mediates a select endocytic pathway that DSL ligands must enter to activate Notch. Development 131: 5367–5380, 2004. [DOI] [PubMed] [Google Scholar]
- 195.Weisz OA, Wang JM, Edinger RS, Johnson JP. Noncoordinate regulation of endogenous epithelial sodium channel (ENaC) subunit expression at the apical membrane of A6 cells in response to various transporting conditions. J Biol Chem 275: 39886–39893, 2000. [DOI] [PubMed] [Google Scholar]
- 196.Weixel KM, Edinger RS, Kester L, Guerriero CJ, Wang H, Fang L, Kleyman TR, Welling PA, Weisz OA, Johnson JP. Phosphatidylinositol 4-phosphate 5-kinase reduces cell surface expression of the epithelial sodium channel (ENaC) in cultured collecting duct cells. J Biol Chem 282: 36534–36542, 2007. [DOI] [PubMed] [Google Scholar]
- 197.Wendland B Round-trip ticket: recycling to the plasma membrane requires RME-1. Nat Cell Biol 3: E133–E135, 2001. [DOI] [PubMed] [Google Scholar]
- 198.Wendland B Epsins: adaptors in endocytosis? Nat Rev Mol Cell Biol 3: 971–977, 2002. [DOI] [PubMed] [Google Scholar]
- 199.Wiemuth D, Ke Y, Rohlfs M, McDonald FJ. Epithelial sodium channel (ENaC) is multi-ubiquitinated at the cell surface. Biochem J 405: 147–155, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yarbrough TL, Lu T, Lee HC, Shibata EF. Localization of cardiac sodium channels in caveolin-rich membrane domains: regulation of sodium current amplitude. Circ Res 90: 443–449, 2002. [DOI] [PubMed] [Google Scholar]
- 201.Yue G, Malik B, Yue G, Eaton DC. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J Biol Chem 277: 11965–11969, 2002. [DOI] [PubMed] [Google Scholar]
- 202.Zheng X, Lu D, Sadler JE. Apical sorting of bovine enteropeptidase does not involve detergent-resistant association with sphingolipid-cholesterol rafts. J Biol Chem 274: 1596–1605, 1999. [DOI] [PubMed] [Google Scholar]
- 203.Zhou R, Patel SV, Snyder PM. Nedd4-2 catalyzes ubiquitination and degradation of cell surface ENaC. J Biol Chem 282: 20207–20212, 2007. [DOI] [PubMed] [Google Scholar]
- 204.Zhou R, Snyder PM. Nedd4-2 phosphorylation induces serum and glucocorticoid-regulated kinase (SGK) ubiquitination and degradation. J Biol Chem 280: 4518–4523, 2005. [DOI] [PubMed] [Google Scholar]
- 205.Zhou ZH, Bubien JK. Nongenomic regulation of ENaC by aldosterone. Am J Physiol Cell Physiol 281: C1118–C1130, 2001. [DOI] [PubMed] [Google Scholar]