Abstract
Meprin metalloproteases, composed of α and/or β subunits, consist of membrane-bound and secreted forms that are abundantly expressed in proximal tubules of the kidney as well as secreted into the urinary tract. Previous studies indicated that meprin metalloproteases play a role in pathological conditions such as ischemic acute renal failure and urinary tract infection. The aim of this work was to examine the role of meprins in endotoxemic acute renal failure using meprin α knockout (αKO), meprin β knockout (βKO), and wild-type (WT) mice. Differences among the responses of the genotypes were observed as early as 1 h after challenge with 2.5 mg/kg ip Escherichia coli LPS, establishing roles for meprins in the endotoxemic response. Meprin αKO mice displayed lower blood urea nitrogen levels and decreased nitric oxide levels, indicative of a decreased systemic response to LPS compared with WT and meprin βKO mice. Serum cytokine profiles showed lower levels of IL-1β and TNF–α in the meprin αKO mice within 3 h after LPS challenge and confirmed a role for meprins in the early phases of the host response. Meprin αKO mice were also hyporesponsive to LPS administered to the bladder, exhibiting significantly less bladder edema, leukocyte infiltration, and bladder permeability than WT mice. These data indicate that meprin A contributes to the renal and urogenital pathogenesis of endotoxicity.
Keywords: knockout mice, lipopolysaccharide, kidney, metalloproteinase
acute renal failure (ARF) is a frequent complication of sepsis, a systemic response to infection that often leads to shock and multiple organ failure (49). Intraperitoneal (ip) administration of endotoxin or LPS, a glycolipid component of the gram negative bacterial cell wall, generates a robust host response similar to that observed during sepsis, including elevated blood urea nitrogen (BUN) and creatinine levels, extensive production of proinflammatory mediators (e.g., TNF-α, IL-1β), and release of the vasodilator nitric oxide (NO) (15, 20, 29, 54). TNF-α is an important mediator of LPS-induced ARF and is also implicated in induction of hypothermia, a hallmark of the rodent response to endotoxemia and a reliable parameter for evaluating the acute effects of LPS (10, 19, 20, 34, 37). The injurious effects of LPS are also readily apparent on transurethral administration of LPS, which produces a localized inflammatory response that resembles urinary tract infection (UTI) and consists of extensive leukocyte infiltration, edema, and urothelial disruption in the bladder tissue (17, 43).
Meprin metalloproteases are abundantly expressed in the brush-border membranes of kidney proximal tubules as well as secreted into the urinary tract (12, 45). They are also expressed in the intestine, skin, and discrete populations of leukocytes (5, 12, 18, 36). Meprins exist as homo- or hetero-oligomers of two evolutionarily related subunits, α and β. The meprin β subunit is membrane-bound, whereas the meprin α subunit is proteolytically processed during biosynthesis and loses its transmembrane domain. Thus, membrane-bound forms of meprin include meprin B (dimers of β subunits) and heteromeric meprin A (tetramers of α and β subunits); by contrast, homomeric meprin A (multimers of α subunits) is secreted from cells as large multimers (6, 27). In the meprin αKO mouse, only membrane-bound dimeric meprin B is present, whereas in the meprin βKO mouse, only secreted homomeric meprin A is present (40).
Several lines of evidence implicate meprin metalloproteases in inflammatory processes. For example, while the peptide bond specificities of the meprin subunits are substantially different, all forms of meprin A and B are capable of hydrolyzing ECM proteins, including laminin, collagen IV, nidogen-1, and fibronectin, and one study proposed that meprins are responsible for the majority of ECM degrading activity in rodent kidney (7, 31, 50). Additionally, cytokines/chemokines have been identified as meprin substrates in vitro. Meprin A is capable of hydrolyzing the NH2 termini of several cytokines and chemokines, including CCL3 (MIP-1α) and CCL2 (MCP-1), rendering them biologically inactive, whereas meprin B completely degrades TECK/CCL25 and activates pro-IL-1β (2, 24, 41). Recently, meprin β was shown to activate pro-IL-18 in vitro and in vivo in a model of chronic intestinal inflammation (4). Deletion of the meprin β gene decreased the migratory ability of leukocytes in vitro, pointing to a potential role in leukocyte transmigration (18).
Meprins have also been implicated in pathological conditions such as ARF, UTI, and inflammatory bowel disease (12, 14, 16, 18, 47). Previous studies demonstrated that treatment with actinonin, an inhibitor of meprins, attenuates the extent of kidney damage in rodent models of ARF (16, 25, 26). However, actinonin inhibition is not specific for the meprins and can also inhibit aminopeptidases and some matrix metalloproteases (14). In addition, a study of meprin expression in human urine identified a correlation between high meprin A protein levels and active UTI (12). Taken together, the data implicate a role for meprins in renal and urogenital pathologies.
The aim of these studies was to utilize two meprin knockout (KO) mouse models to determine how meprin α and meprin β contribute to the host response to an ip LPS challenge. Plasma BUN, creatinine, body temperature, and several inflammatory mediators were monitored to gain insight into the role of meprin in the response to systemically administered LPS. Another aim was to determine whether the lack of meprin A alters the inflammatory response to a localized LPS challenge to the bladder, as measured by leukocyte infiltration and bladder edema. The effect of meprin A on bladder permeability in vivo was determined to provide insight into the mechanism by which meprins enhance the host response to LPS. These studies are the first to demonstrate a relationship between the inflammatory response and the meprins in a systemic and intravesical LPS challenge and establish a determinative role for meprin A in the host response to LPS.
METHODS
Mice.
Female meprin αKO and meprin βKO were used at 8–9 wk of age for all experiments. Because meprin αKO mice were generated in C57BL/6 mice with 129x1/Sv embryonic stem cells, mixed background C57BL/6 × 129x1/Sv littermates were used as wild-type (WT) controls (35). C57BL/6 mice were used as WT controls for the meprin βKO mice, as these animals were backcrossed for 13 generations on a C57BL/6 strain. Meprin αKO and meprin βKO animals were derived as previously described (3, 40). All mice were maintained in conventional housing and were allowed water and rodent chow ad libitum. All animal protocols were approved by the Penn State Institutional Animal Care and Use Committee.
LPS administration, blood and tissue preparation, and temperature monitoring.
Meprin KO and WT mice were challenged ip with 2.5 mg/kg body wt LPS (Escherichia coli 0111:B4, purified by gel filtration chromatography, Sigma, St. Louis, MO) or sterile saline. Body temperatures were monitored via a rectal thermistor (Cole-Parmer, Vernon Hills, IL) at ambient room temperature. Blood samples were collected from the tail vein into lithium/heparin tubes (Sarstedt, Newton, NC), and plasma was isolated by centrifugation at 10,000 g for 8 min at 14°C.
Immunohistochemistry.
For immunohistochemical analysis, methyl Carnoy's-fixed (60% methanol, 30% chloroform, 10% acetic acid) and paraffin-embedded kidney specimens were cut into 4-μm sections and stained with hematoxylin and eosin (H&E). Acute tubular necrosis was assessed in the outer strip of the outer medulla and cortex using a semiquantitative scale in which the percentage of tubules showing epithelial necrosis, brush-border loss, and vacuolation was assigned a score: 0 = normal, 1 = <10%, 2 = 11–25%, 3 = 26–45%, 4 = 46–75%, and 5 = >76% (42, 52). Ten fields of ×40 magnification were examined and averaged. The individual scoring the slides was blinded to the genotype of the animal.
Quantification of BUN, creatinine, nitrate/nitrite, and cytokine/chemokine levels.
To assess renal function, BUN levels were determined by Vitros DT6011 BUN chemistry slides (Ortho Clinical Diagnostics, Rochester, NY), and creatinine levels were determined by an end-point colorimetric creatinine assay (Diazyme Laboratories, Poway, CA). Serum nitrate/nitrite levels were determined using the Nitrate/Nitrite Colorimetric Assay Kit (Cayman Chemical, Ann Arbor, MI). Serum samples were ultrafiltered through Microcon YM-10 10-kDa microfuge filtration devices (Millipore, St. Charles, MO) before determination of nitrate/nitrite to reduce background. Serum cytokines (IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12, MCP-1, IFN-γ, TNF-α, MIP-1α, GM-CSF, RANTES) were measured using the ELISA-based Quansys Q-Plex Mouse Cytokine Array (Biolegend, San Diego, CA). Imaging of the array was performed on the EC3 Biochemi imaging system equipped with CCD camera (UVP, Upland, CA) and analyzed with Quansys Q-View software. Serum TNF-α levels were confirmed using the Quantikine Mouse TNF-α ELISA assay (R&D Systems, Minneapolis, MN).
Murine model of bladder inflammation.
The protocol for LPS bladder infusion was adapted from Saban et al. and Haugen et al. (23, 43). Mice were anesthetized via isofluorane inhalation and the abdomens were massaged to empty residual urine. Twenty micrograms of LPS in 50 μl of sterile PBS were slowly instilled into the bladder using a 0.5-mm polyethylene catheter (Intramedic PE 10) attached to the hub of a 50-μl #705 syringe (Hamilton) with a 30-gauge blunt-tipped needle. Mice were killed by cervical dislocation following anesthesia by isofluorane inhalation.
Myeloperoxidase assay.
Mouse bladders were homogenized in 10 Vol of potassium phosphate buffer and centrifuged at 20,000 g for 20 min at 4°C. The supernatant fluid was discarded and the sediment was suspended in 0.5% hexa-decyl-trimethyl-ammonium bromide buffer. After five 3-s sonications, the preparation was freeze-thawed three times and incubated on ice for 20 min. The lysate was centrifuged and 50 μl of the supernatant fluid were added to 1.45 ml of potassium phosphate buffer containing o-dianisidine dihydrochloride and hydrogen peroxide, and the change in absorbance at 460 nm was measured over 3 min. Myeloperoxidase (MPO) activity is expressed in MPO units per gram of bladder tissue, where 1 MPO unit is equal to the cleavage of 1 mmol H2O2.
Assessment of bladder permeability after LPS instillation.
The protocol to determine bladder permeability was adapted from Eichel et al. (21). Briefly, 200 μl of 10 mg/ml sodium fluorescein were instilled into the bladders of anesthetized mice at several time points after LPS bladder challenge. After 15 min, serum samples were collected, diluted with saline, and sodium fluorescein concentrations were measured fluorometrically.
Statistical analysis and graphing software.
PRISM GraphPad statistical software was used to plot figures and for data analysis. Results are expressed as means ± SE; a P value <0.05 was considered significantly different.
RESULTS
Meprin A contributes to LPS-induced renal injury.
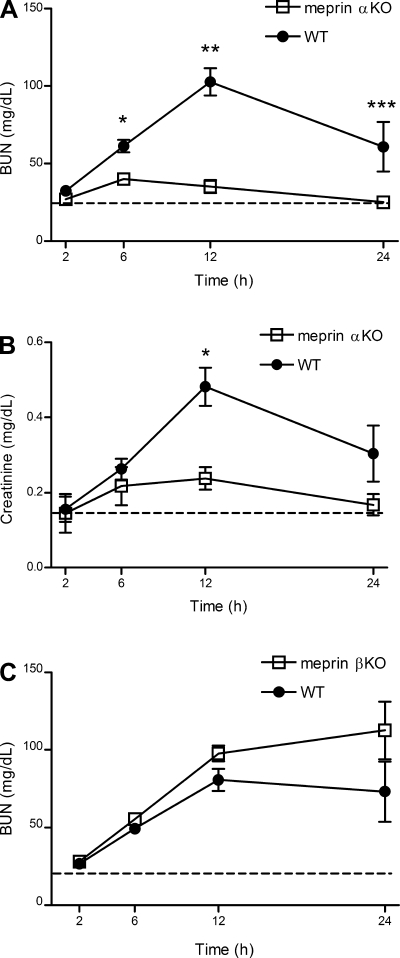
To determine whether meprins participate in LPS-induced kidney injury, meprin αKO and WT mice were challenged ip with a non-lethal dose of 2.5 mg/kg E. coli LPS, and BUN and creatinine levels were measured (Fig. 1A) (42). In LPS-challenged mice, plasma BUN levels were significantly higher than saline controls by 6 h. The BUN response to LPS was markedly different between meprin αKO and WT genotypes. In LPS-challenged WT mice, BUN levels continued to rise until 12 h, remained elevated above saline controls at 24 h, and were significantly higher compared with BUN levels in LPS-challenged meprin αKO mice at 6, 12, and 24 h. For LPS-challenged meprin αKO mice, BUN levels peaked at 6 h and declined to baseline saline levels by 12 h. BUN levels in LPS-challenged WT, C57BL/6, and 129x1/Sv mice were not significantly different (data not shown). No genotypic differences were observed in response to higher LPS challenges of 5 or 10 mg/kg (data not shown). Changes in plasma creatinine levels were congruent with BUN levels and demonstrated attenuated renal damage in LPS-challenged meprin αKO compared with WT mice (Fig. 1B). Meprin βKO and WT mice showed similar BUN profiles for 12 h after challenge with LPS (Fig. 1C) in contrast to the meprin αKO animals. There was a tendency for the BUN levels in meprin βKO mice to be higher than WT levels, but the differences were not significant.
Fig. 1.
Blood urea nitrogen (BUN) and creatinine levels in meprin knockout (KO) vs. wild-type (WT) mice after lipopolysaccharide (LPS) challenge. WT and meprin KO mice were injected intraperitoneal (ip) with 2.5 mg/kg LPS or saline. Blood serum samples were collected at 2, 6, 12, and 24 h postinjection and BUN and creatinine levels were determined. A: meprin αKO mice have lower BUN values compared with WT mice. *P = 0.0002 at 6 h. **P < 0.00001 at 12 h. ***P = 0.04 at 24 h. B: meprin αKO mice have lower creatinine values compared with WT mice. *P < 0.001 at 12 h. C: meprin βKO mice have similar BUN levels vs. WT mice. BUN data are an average of 3 independent experiments, with n = 16–20 mice per genotype. Creatinine data are an average of 2 independent experiments, with n = 11–12 mice per genotype. Dashed lines indicate saline-injected control levels for WT and KO mice.
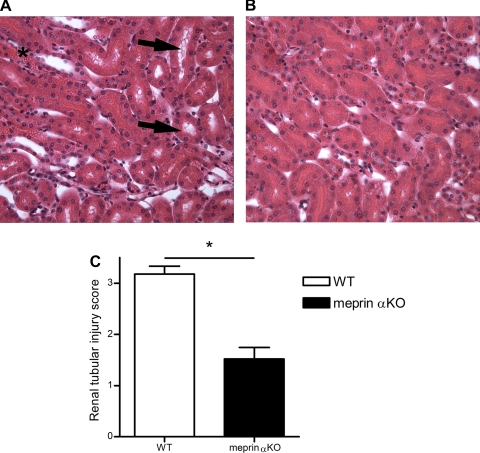
Marked differences in renal histology were present between meprin αKO and WT mice 24 h after LPS challenge (Fig. 2). Semiquantitative scoring of tubule injury was significantly higher in LPS-treated WT kidneys (3.18 ± 0.35) in contrast to LPS-treated meprin αKO kidneys (1.52 ± 0.51; P < 0.0004). The presence of vacuoles and tubule dilation in the cortex was more apparent in kidneys from WT mice (Fig. 2A) than in meprin αKO mice (Fig. 2B). Abnormal vacuole formation was also observed in the outer strip of the outer medulla in WT mice (data not shown). Additionally, brush-border damage and accumulation of red blood cells between the tubules were more severe in WT mice, confirming a greater preservation of renal function in the meprin αKO mice after LPS challenge.
Fig. 2.
Renal histology in meprin αKO vs. WT mice after LPS challenge. Kidneys were harvested from WT and meprin αKO mice 24 h after ip injection of 2.5 mg/kg LPS. Four-micrometer sections were stained with hematoxylin and eosin (H&E) and examined at ×40 magnification. A: meprin WT mice exhibit greater renal injury in contrast to αKO mice (B). Black arrows indicate severe tubule dilation and vacuole formation in WT kidneys. *Red blood cell accumulation. C: acute tubular necrosis was assessed in the juxtamedullary cortex using a semiquantitative scale in which the percentage of tubules showing epithelial necrosis, brush-border loss, and vacuolation was assigned a score: 0 = normal, 1 = <10%, 2 = 11–25%, 3 = 26–45%, 4 = 46–75%, and 5 = >76%. Semiquantitative scoring of tubule injury was significantly higher in LPS-treated WT kidneys (3.18 ± 0.35) in contrast to LPS-treated meprin αKO kidneys (1.52 ± 0.51). *P < 0.0004, with n = 5 kidneys per genotype.
Meprin A contributes to LPS-induced hypothermia.
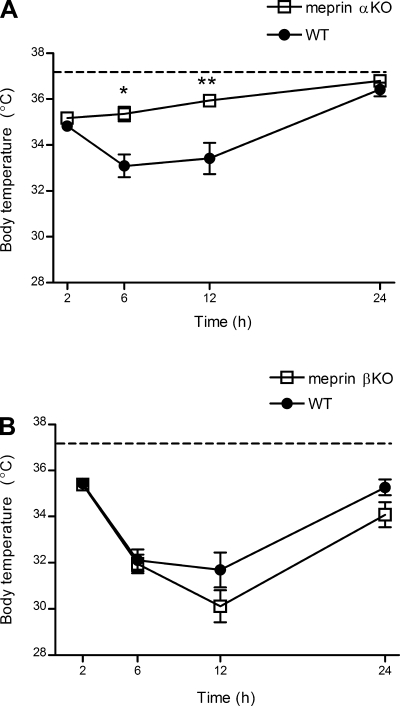
Because ip administration of LPS has been shown to induce prolonged hypothermia in mice, the body temperatures of meprin KO and WT mice were monitored at several time points after LPS challenge (10, 34). The body temperatures of all genotypes of LPS-challenged mice decreased several degrees by 2 h after ip LPS challenge (Fig. 3). WT body temperatures continued to decrease at 6 h, remained low at 12 h, and returned to normal by 24 h. In contrast, body temperatures of the meprin αKO mice began recovery by 6 h, continued to rise at 12 h, and returned to normal by 24 h (Fig. 3A). By 12 h, body temperatures of LPS-challenged meprin αKO were not significantly different from saline-treated controls. Meprin βKO mice demonstrated marked hypothermia upon LPS challenge, and the hypothermic response in the meprin βKO was slightly greater than that of the WT at both 12 and 24 h (Fig. 3B).
Fig. 3.
Body temperature change in meprin KO vs. WT mice after LPS challenge. WT and meprin KO mice were injected ip with 2.5 mg/kg LPS or saline. Body temperatures were monitored via a rectal thermistor at 2, 6, 12, and 24 h postinjection. A: meprin αKO mice have less severe hypothermia vs. WT. *P = 0.003 at 6 h. **P = 0.009 at 12 h. B: meprin βKO and WT mice have a similar hypothermic response to LPS challenge. The data are an average of 3 independent experiments, with n = 16–20 mice per genotype. Dashed lines indicate saline-injected control body temperatures for WT and KO mice.
Meprin A contributes to LPS-induced elevation of serum nitrate/nitrite.
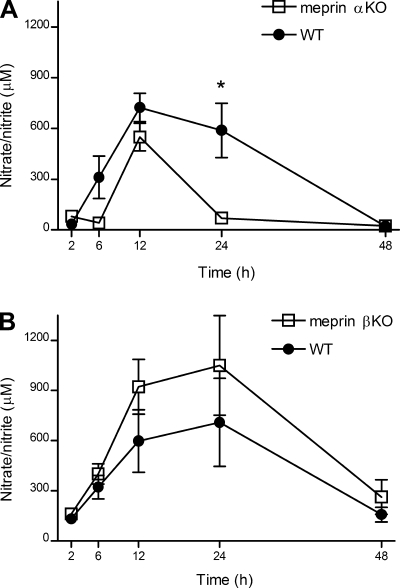
Serum nitrate/nitrite was measured to estimate the levels of NO in the blood after LPS challenge. Nitrate/nitrite levels peaked at 12 h and returned to baseline by 48 h in both meprin αKO and WT mice (Fig. 4A). However, nitrate/nitrite levels declined sharply by 24 h in the meprin αKO mice but remained elevated in the WT mice. No significant difference in serum nitrate/nitrite levels was observed between the meprin βKO and WT animals after LPS challenge, although there was the tendency for greater nitrate/nitrite levels in the meprin βKO mice (Fig. 4B). Nitrate/nitrite levels were low or nondetectable in saline-injected controls (data not shown).
Fig. 4.
Serum nitrate/nitrite levels in meprin KO vs. WT mice after LPS challenge. WT and meprin KO mice were injected ip with 2.5 mg/kg LPS or saline. Blood serum samples were collected at 2, 6, 12, 24, and 48 h postinjection and nitrate/nitrite levels were determined. A: meprin αKO mice have lower serum nitrate/nitrite vs. WT mice. *P = 0.04 at 24 h. B: meprin βKO mice have similar nitrate/nitrite levels vs. WT mice. The data are an average of 3 independent experiments, with n = 13–16 mice per genotype. Nitrate/nitrite levels in saline-injected controls were low or below detection limits.
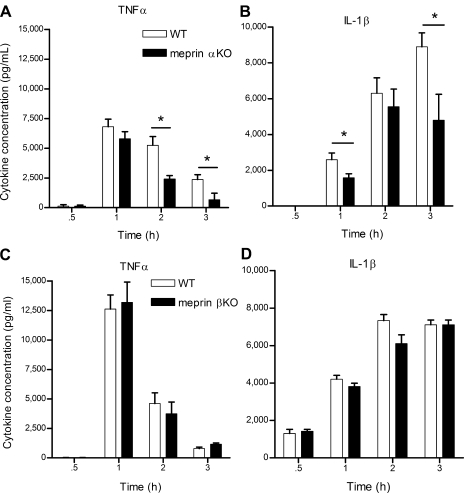
Serum proinflammatory cytokines are significantly elevated in WT vs. meprin αKO animals.
To determine whether differential cytokine expression could be responsible for the observed differences between the WT and meprin KO mice, serum levels of 16 cytokines were analyzed for the first 3 h in both meprin KO genotypes after an ip LPS challenge. Distinct differences in the levels of TNF-α and IL-1β were observed after ip LPS challenge (Fig. 5). TNF-α levels rose sharply 30 min after LPS challenge, peaked at 1 h, and then began to decrease in all genotypes. TNF-α levels decreased more rapidly at 2 and 3 h in the meprin αKO compared with WT mice (Fig. 5A). By contrast, no significant differences were observed in serum TNF-α between the meprin βKO and WT mice (Fig. 5C). Levels of IL-1β rose sharply after 30 min and remained elevated at 3 h in LPS-challenged WT mice. In the meprin αKO mice, IL-1β levels peak at 2 h, with significantly lower levels in the serum at 1 and 3 h (Fig. 5B). No significant differences were observed between the meprin βKO mice and their WT counterparts with respect to IL-1β profiles (Fig. 5D).
Fig. 5.
Serum cytokine levels in KO compared with WT mice after LPS challenge. WT and meprin KO mice were injected ip with 2.5 mg/kg LPS or saline. Blood serum samples were collected at 0.5, 1, 2, and 3 h postinjection and the levels of 16 cytokines were determined by an ELISA-based array. TNF-α levels (A) and IL-1β levels (B) were significantly lower in meprin αKO mice after LPS challenge in contrast to WT counterparts. TNF-α levels (C) and IL-1β levels (D) were similar in meprin βKO and WT mice after LPS challenge. The data are an average of 2 independent experiments, with *P < 0.05 WT vs. KO mice. Results are expressed as means ± SE, with n = 10–12, per genotype, per time point. Cytokine levels in saline-injected controls were low or below detection limits.
Although the cytokines exhibited different temporal expression patterns in response to LPS, no marked genotypic differences were observed after LPS challenge with respect to IL-2, IL-3, IL-9, IL-10, IL-12, IFN-γ, MIP-1α/CCL3, or RANTES/CCL5 (Tables 1 and 2). Statistical differences between the genotypes were observed for IL-1α, IL-5, IL-6, and MCP-1/CCL2, but the numerical differences between the meprin KO and WT mice were marginal (Tables 1–2).
Table 1.
Serum cytokine levels in meprin αKO and WT mice after ip LPS
| 0.5 h | 1 h | 2 h | 3 h | |
|---|---|---|---|---|
| IL-1α | ||||
| WT | 0 | 354±248 | 747±220 | 869±283 |
| KO | 0 | 228±142 | 731±393 | 505±339 |
| IL-1β | ||||
| WT | 0 | 2,593±1,265 | 6,300±3,020 | 8,896±1,919 |
| KO | 0 | 1,586±708 | 5,542±3,286 | 4,788±3,580 |
| IL-2 | ||||
| WT | 281±363 | 285±325 | 446±272 | 777±377 |
| KO | 100±224 | 173±366 | 330±270 | 559±444 |
| IL-3 | ||||
| WT | 1,936±751 | 1,032±959 | 1,636±747 | 3,039±864 |
| KO | 1,538±853 | 1,192±830 | 1,957±1,156 | 2,522±843 |
| IL-4 | ||||
| WT | 1,036±653 | 555±428 | 699±276 | 1,141±585 |
| KO | 583±669 | 451±239 | 703±358 | 972±547 |
| IL-5 | ||||
| WT | 529±528 | 457±109 | 777±311 | 1,095±287 |
| KO | 255±569 | 353±68 | 711±375 | 726±252 |
| IL-6 | ||||
| WT | 3,806±2,112 | 12,456±1,594 | 12,433±2,309 | 11,535±1,503 |
| KO | 4,018±2,461 | 12,670±1,217 | 12,111±2,454 | 11,030±3,999 |
| IL-9 | ||||
| WT | 20,425±14,226 | 14,612±12,700 | 24,681±10,734 | 44,218±13,519 |
| KO | 10,906±13,897 | 8,332±8,833 | 23,210±12,769 | 37,260±9,866 |
| IL-10 | ||||
| WT | 495±932 | 2,936±1,297 | 3,333±1,592 | 4,673±2,183 |
| KO | 0 | 3,803±3,401 | 3,124±1,932 | 2,957±2,878 |
| IL-12 | ||||
| WT | 423±38 | 459±88 | 1,213±317 | 954±183 |
| KO | 408±92 | 477±92 | 1,175±482 | 975±178 |
| MCP-1 | ||||
| WT | 282±690 | 15,842±5,309 | 26,838±8,036 | 23,079±2,912 |
| KO | 0 | 11,995±2,006 | 25,853±5,366 | 21,467±9,960 |
| IFN-γ | ||||
| WT | 11,020±3,433 | 6,271±5,304 | 7,929±3,695 | 15,845±4,209 |
| KO | 10,055±3,769 | 8,335±5,109 | 10,485±6,217 | 13,468±3,716 |
| TNF-α | ||||
| WT | 125±306 | 6,809±2,172 | 5,260±2,523 | 2,358±995 |
| KO | 135±190 | 5,789±2,048 | 2,417±944 | 662±1,238 |
| MIP-1α | ||||
| WT | 0 | 684±865 | 7,843±8,506 | 16,128±4,548 |
| KO | 0 | 255±633 | 8,874±6,356 | 16,701±4,321 |
| GMCSF | ||||
| WT | 1,453±661 | 835±774 | 1,326±393 | 2,401±747 |
| KO | 1,099±892 | 984±565 | 1,706±606 | 2,339±670 |
| RANTES | ||||
| WT | 0 | 0 | 870±940 | 3,904±630 |
| KO | 0 | 0 | 268±357 | 2,689±1,634 |
Results are expressed as means (pg/ml) ± SD, with n = 10–12, per genotype, per time point. Data are an average of two independent experiments. Values in bold indicate P < 0.05 knockout (KO) vs. wild-type (WT). LPS, lipopolysaccharide; ip, intraperitoneal.
Table 2.
Serum cytokine levels in meprin βKO and WT mice after ip LPS
| 0.5 h | 1 h | 2 h | 3 h | ||
|---|---|---|---|---|---|
| IL-1α | |||||
| WT | 0 | 459±223 | 1,079±166 | 1,032±122 | |
| KO | 55±42 | 412±101 | 891±487 | 1,295±548 | |
| IL-1β | |||||
| WT | 1,300±499 | 4,204±634 | 7,331±1,026 | 7,107±790 | |
| KO | 1,412±250 | 3,813±555 | 6,102±1,484 | 7,107±790 | |
| IL-2 | |||||
| WT | 1±254 | 789±137 | 1,068±182 | 919±83 | |
| KO | 121±178 | 630±226 | 909±172 | 899±122 | |
| IL-3 | |||||
| WT | 643±74 | 777±381 | 1,556±384 | 1,153±199 | |
| KO | 535±279 | 750±346 | 1,637±1,136 | 1,180±262 | |
| IL-4 | |||||
| WT | 467±156 | 851±128 | 1,072±284 | 979±165 | |
| KO | 444±64 | 723±180 | 1,002±180 | 933±133 | |
| IL-5 | |||||
| WT | 107±127 | 616±109 | 909±95 | 963±80 | |
| KO | 150±129 | 573±69 | 817±148 | 887±119 | |
| IL-6 | |||||
| WT | 2,780±895 | 21,690±2,805 | 19,559±2,670 | 18,121±942 | |
| KO | 1,075±652 | 20,172±1,969 | 19,426±1,625 | 17,589±1,434 | |
| IL-9 | |||||
| WT | 11,528±4,234 | 27,417±8,565 | 31,084±4,609 | 24,239±4,373 | |
| KO | 10,061±1,339 | 24,972±4,923 | 30,839±8,503 | 29,128±7,000 | |
| IL-10 | |||||
| WT | 0 | 8,240±6,118 | 9,120±3,015 | 10,440±1,254 | |
| KO | 540±3,444 | 6,040±1,656 | 7,690±3,755 | 7,580±2,626 | |
| IL-12 | |||||
| WT | 140±17 | 352±83 | 1,452±232 | 1,619±42 | |
| KO | 111±61 | 373±50 | 1,506±273 | 1,530±88 | |
| MCP-1 | |||||
| WT | 939±370 | 23,602±6,248 | 36,634±4,186 | 35,220±1,265 | |
| KO | 1,469±916 | 22,409±7,336 | 36,855±3,807 | 33,895±2,712 | |
| IFN-γ | |||||
| WT | 4,795±736 | 6,289±1,966 | 8,137±2,419 | 6,377±1,391 | |
| KO | 4,265±2,295 | 5,761±2,753 | 14,825±10,521 | 8,841±3,650 | |
| TNF-α | |||||
| WT | 0 | 12,854±4,274 | 7,226±3,003 | 3,747±667 | |
| KO | 0 | 13,212±5,849 | 6,356±3,529 | 3,747±560 | |
| MIP-1α | |||||
| WT | 0 | 2,187±1,951 | 24,088±10,318 | 22,899±4,146 | |
| KO | 0 | 2,385±1,688 | 21,412±8,705 | 19,331±4,239 | |
| GMCSF | |||||
| WT | 349±167 | 885±313 | 1,665±292 | 1,320±146 | |
| KO | 298±214 | 949±490 | 1,614±451 | 1,524±271 | |
| RANTES | |||||
| WT | 0 | 0 | 1,526±953 | 3,077±908 | |
| KO | 0 | 0 | 1,840±949 | 3,789±618 |
Results are expressed as means (pg/ml) ± SD, with n = 10–12, per genotype, per time point. Data are an average of two independent experiments. Values in bold indicate P < 0.05 KO vs. WT.
To confirm the genotypic differences observed in the levels of serum TNF-α determined by cytokine array, ELISA was performed (data not shown). The ELISA data were congruent with the cytokine array data and confirm that TNF-α levels fell more rapidly in mice lacking meprin A. This trend was also consistent at later time points after LPS challenge, with decreased TNF-α levels in the meprin αKO mice at 12 h (αKO 53 ± 32 pg/ml vs. WT 173 ± 55 pg/ml; P = 0.002).
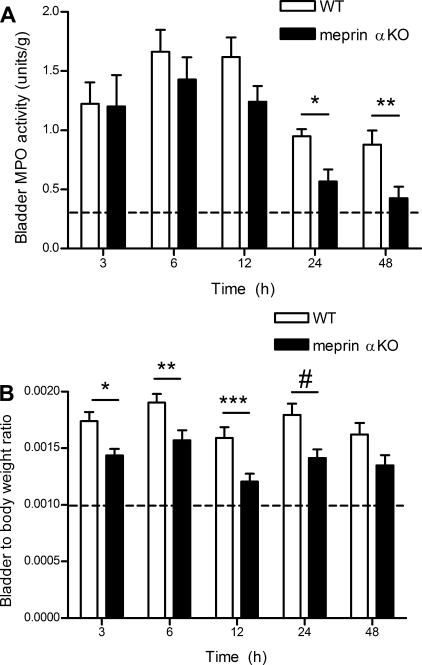
Meprin A contributes to the leukocyte response and edema in a model of bladder inflammation.
Because the most marked differences were observed in the meprin αKO genotype, which lacks meprin A, subsequent studies focused on these mice. To relate the systemic studies to a specific target organ, meprin αKO and WT mice were challenged with transurethral administration of LPS. MPO activity was measured to determine the extent of leukocyte infiltration into the bladder, and bladder weights were utilized to determine the degree of edema (53). Both bladder edema and MPO activity rose sharply within 3 h after transurethral administration of LPS (Fig. 6). Substantially more MPO activity was observed 24 and 48 h after LPS treatment in WT vs. meprin αKO bladders, indicating an attenuated host response in the meprin αKO mice (Fig. 6A). In addition, LPS-treated bladders were significantly heavier in WT vs. meprin αKO mice as early as 3 h after LPS instillation, indicating more extensive edema in the WT bladders (Fig. 6B). Serum BUN levels at 24 h after LPS instillation into the bladder were normal and not significantly different between WT and meprin αKO genotypes (data not shown), confirming that the LPS challenge did not induce a systemic host response.
Fig. 6.
Less bladder myeloperoxidase (MPO) activity and edema in meprin αKO vs. WT after transurethral administration of LPS. LPS (20 μg) was instilled into the bladder via catheter. Mice were necropsied at multiple time points, and leukocyte infiltration was measured by MPO assay. A: bladders from WT mice had significantly more MPO U/g bladder tissue vs. meprin αKO. *P = 0.01 at 24 h. **P < 0.015 at 48 h. MPO activity was low in saline controls (0.3 ± 0.18 MPO U/g). B: bladder-to-body weight ratios were higher in WT vs. αKO, indicating more bladder edema. *P = 0.007 at 3 h. **P < 0.01 at 6 h. ***P < 0.005 at 12 h. #P < 0.02 at 24 h. The data are an average of 2 independent experiments, with n = 8–12 mice per genotype, per time point. Dashed lines indicate average value for saline-treated control animals.
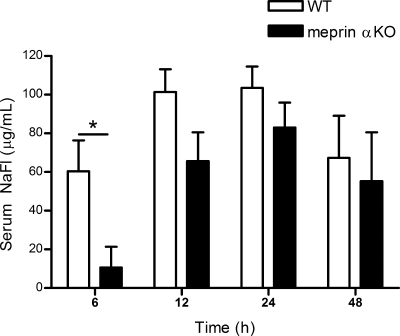
Meprin A contributes to loss of bladder permeability after an LPS bladder challenge.
To determine the extent of bladder damage inflicted by LPS-induced bladder inflammation, bladder permeability after LPS challenge was determined by measurement of sodium fluorescein leakage from the bladder into the serum (21). At several time points after transurethral LPS administration, sodium fluorescein instilled into the bladder appeared in the blood (Fig. 7). Significantly more sodium fluorescein was detected in the blood of WT samples than in meprin αKO samples at 6 h after LPS challenge. By 12 h, both meprin αKO and WT mice displayed substantial loss of barrier function, with WT affected to a greater extent than meprin αKO mice.
Fig. 7.
Less bladder permeability in meprin αKO mice after LPS challenge. After LPS bladder challenge, sodium fluorescein (NaFl) was instilled into bladders at 6, 12, 24, or 48 h and serum was analyzed after 15 min. Leakage of NaFl into the serum was significantly higher in WT vs. αKO mice. *P = 0.02. The data are an average of 2 independent experiments, with n = 14 mice per genotype. Serum NaFl levels of saline-treated control WT and meprin αKO mice were below detection limits at all time points.
DISCUSSION
These studies with the meprin KO mice are the first to demonstrate that meprin A has a determinative role in host response to challenge with LPS. The data establish that the presence of meprin A contributes to LPS-induced renal damage, hypothermia, and proinflammatory cytokine production. Taken together, the data indicate that the meprin α subunit enhances the host response to LPS and that the absence of meprin A protects against LPS-induced injury. These results were confirmed and extended in an LPS-induced model of bladder inflammation, which highlights participation of meprin A in increased leukocyte infiltration and edema in bladder tissue. The observed breakdown in the bladder barrier could be attributed to the ability of active meprin A to disrupt cellular tight junctions, as bladder permeability after LPS challenge was markedly decreased in mice lacking meprin A.
The BUN and creatinine data indicate that WT mice were more susceptible to LPS-induced renal injury than the meprin αKO mice. Several studies implicate early release of proinflammatory cytokines into the circulation in mediating LPS-induced kidney damage, and increased levels of TNF-α in plasma have been correlated with severity of outcome (37, 39, 44). Furthermore, data indicate that maintaining a proper balance among proinflammatory cytokines is critical and that short-term disturbances in inflammatory mediators can have long-term detrimental effects (38, 51). For example, it has been shown that an imbalance between levels of TNF-α and the TNF receptor 1–4 h after an initial immune insult results in persistence of biochemical and hemodynamic perturbations for as long as 18 h despite endogenous correction at 8–12 h (1). This is likely due to the ability of freely circulating TNF-α to induce other factors that perpetuate the dysregulated immune response. Differential regulation of TNF-α 1–3 h after ip LPS challenge could thus account for the marked differences observed 12 and 24 h after LPS challenge in the meprin αKO and WT mice.
The release of cytotoxic TNF-α as a consequence of LPS challenge has been associated with renal failure, and treatment with anti-TNF antibody affords LPS-challenged mice protection from septic shock (8, 22, 29). Furthermore, TNF-α acts synergistically with IL-1β in the endotoxemic response (34). Serum levels of TNF-α and IL-1β are lower in mice lacking meprin A after LPS challenge. Thus, it is likely that the prolonged elevation of cytokine levels in WT mice contributes to the more extensive kidney damage observed compared with the meprin αKO mice. Meprin βKO mice produce only homomeric meprin A, whereas the WT mice produce all three meprin isoforms. The marked difference between the meprin αKO mice and their WT counterparts corroborates the proposition that homomeric meprin A contributes to LPS-induced kidney injury. Consistent with these results, previous work with C3H/He mice that have low levels of kidney meprin A shows less kidney damage when subjected to ischemia reperfusion in contrast to C57BL/6 mice with high levels of meprin A (9, 13, 47).
The body temperature data are congruent with the BUN data and indicate a marked genotypic difference in the response to LPS, with significantly less hypothermia in the meprin αKO mice compared with WT. TNF-α, along with IL-1β, modulates the hypothermia observed in mice after LPS challenge (34). Specifically, data indicate that TNF-α injection induces hypothermia, and LPS-induced hypothermia can be reversed with soluble TNF receptor (10, 30). The dramatic hypothermia observed in the WT mice might be due in part to higher levels of TNF-α at 3 h, whereas the attenuated hypothermic response observed in the mice lacking meprin A could be attributed to the lower levels of TNF-α and IL-1β.
LPS triggers the synthesis of iNOS, which results in an increase in production of NO, a potent vasodilator and mediator of organ failure in endotoxemia (29). TNF-α and IL-1β have redundant activities, including eliciting NO production (28, 32, 44). Studies by Holly et al. (26) identified elevated nitrate levels at 8 and 24 h after induction of sepsis in a rodent model, with greater nitrate levels in animals also exhibiting severe ARF. This is consistent with our observations of LPS-challenged meprin αKO mice, which demonstrate both attenuated renal failure and lower serum nitrate levels compared with WT counterparts.
The data indicate a decreased inflammatory response to bladder LPS challenge in mice lacking meprin A compared with WT counterparts. The inflammatory cascade that LPS sets in motion is amplified by leukocyte recruitment, and the data herein indicate that fewer leukocytes are infiltrating into the inflamed meprin αKO bladders compared with WT (48). These results are consistent with work by Herzog et al. (25) which demonstrates decreased leukocyte infiltration after treatment with the meprin inhibitor actinonin in a model of cisplatin-induced kidney failure. The differential response of meprin genotypes may reflect either the rate at which TNF-α-producing leukocytes migrate to the site of injury or the modulation of TNF-α functionality.
Maintenance of proper barrier function is crucial in protection from endotoxemia, and the barrier between urine and blood constituents is maintained by the apical membrane as well as tight junctions in the uppermost epithelial cell layer of the bladder (33, 44, 48). Loss of barrier function causes leaking of urine into the lamina propria of the bladder and leads to inflammation of the underlying musculature. Thus, the significant increase in bladder permeability observed in WT compared with meprin αKO bladders could account for the enhanced bladder edema present in LPS-challenged WT bladders (33). Several lines of evidence implicate metalloproteases in disruption of junctional proteins, but specific enzymes have not been identified (11, 46). Meprins could be among the proteases actively involved in breakdown of tight junction proteins, as initial studies indicate that meprin treatment of Madin-Darby canine kidney cells in culture resulted in disruption of the tight junctions between these cells (data not shown).
Meprin A's role in the host response to LPS has been shown to involve modulation of serum chemokine levels as well as epithelial barrier function. These studies demonstrate that meprin A exacerbates LPS-induced kidney damage, contributes to increased levels of LPS-elicited TNF-α and IL-1β, and disrupts tight junctions in vitro and in vivo, leading to increased epithelial permeability, greater leukocyte infiltration, and enhanced bladder edema.
GRANTS
This work was supported in part by National Institutes of Health Grant DK-19691, a Dean's Feasibility Grant from the Pennsylvania State University, and Tobacco Settlement Funds to J. S. Bond, and National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1-DK-063120 to W. B. Reeves.
Acknowledgments
The authors thank the laboratory of J. Kim for the use of the Biochemi imaging system for cytokine array visualization. We also express gratitude to the laboratory of R. Welch, especially B. Haugen, A. Anfora, and S. Pellett, for instruction on the murine model of catheterization.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aderka D, Sorkine P, Abu-Abid S, Lev D, Setton A, Cope AP, Wallach D, Klausner J. Shedding kinetics of soluble tumor necrosis factor (TNF) receptors after systemic TNF leaking during isolated limb perfusion. Relevance to the pathophysiology of septic shock. J Clin Invest 101: 650–659, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambort D, Stalder D, Lottaz D, Huguenin M, Oneda B, Heller M, Sterchi EE. A novel 2D-based approach to the discovery of candidate substrates for the metalloendopeptidase meprin. FEBS J 275: 4490–4509, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee S Meprin metalloproteases modulate the intestinal host response (Online). Pennsylvania State University. http://etda.libraries.psu.edu/theses/approved/WorldWideIndex/ETD-2541/index.html (2008).
- 4.Banerjee S, Bond JS. Prointerleukin-18 is activated by meprin beta in vitro and in vivo in intestinal inflammation. J Biol Chem 283: 31371–31377, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker-Pauly C, Howel M, Walker T, Vlad A, Aufenvenne K, Oji V, Lottaz D, Sterchi EE, Debela M, Magdolen V, Traupe H, Stocker W. The alpha and beta subunits of the metalloprotease meprin are expressed in separate layers of human epidermis, revealing different functions in keratinocyte proliferation and differentiation. J Invest Dermatol 127: 1115–1125, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Bertenshaw GP, Norcum MT, Bond JS. Structure of homo- and hetero-oligomeric meprin metalloproteases. Dimers, tetramers, and high molecular mass multimers. J Biol Chem 278: 2522–2532, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Bertenshaw GP, Turk BE, Hubbard SJ, Matters GL, Bylander JE, Crisman JM, Cantley LC, Bond JS. Marked differences between metalloproteases meprin A and B in substrate and peptide bond specificity. J Biol Chem 276: 13248–13255, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science 229: 869–871, 1985. [DOI] [PubMed] [Google Scholar]
- 9.Beynon RJ, Bond JS. Deficiency of a kidney metalloproteinase activity in inbred mouse strains. Science 219: 1351–1353, 1983. [DOI] [PubMed] [Google Scholar]
- 10.Blanque R, Meakin C, Millet S, Gardner CR. Hypothermia as an indicator of the acute effects of lipopolysaccharides: comparison with serum levels of IL1 beta, IL6 and TNF alpha. Gen Pharmacol 27: 973–977, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Bojarski C, Weiske J, Schoneberg T, Schroder W, Mankertz J, Schulzke JD, Florian P, Fromm M, Tauber R, Huber O. The specific fates of tight junction proteins in apoptotic epithelial cells. J Cell Sci 117: 2097–2107, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Bond JS, Matters GL, Banerjee S, Dusheck RE. Meprin metalloprotease expression and regulation in kidney, intestine, urinary tract infections and cancer. FEBS Lett 579: 3317–3322, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Bond JS, Shannon JD, Beynon RJ. Certain mouse strains are deficient in a kidney brush-border metallo-endopeptidase activity. Biochem J 209: 251–255, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bylander J, Li Q, Ramesh G, Zhang B, Reeves WB, Bond JS. Targeted disruption of the meprin metalloproteinase beta gene protects against renal ischemia/reperfusion injury in mice. Am J Physiol Renal Physiol 294: F480–F490, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Camussi G, Ronco C, Montrucchio G, Piccoli G. Role of soluble mediators in sepsis and renal failure. Kidney Int Suppl 66: S38–S42, 1998. [PubMed] [Google Scholar]
- 16.Carmago S, Shah SV, Walker PD. Meprin, a brush-border enzyme, plays an important role in hypoxic/ischemic acute renal tubular injury in rats. Kidney Int 61: 959–966, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Chen LM, Wang C, Chen M, Marcello MR, Chao J, Chao L, Chai KX. Prostasin attenuates inducible nitric oxide synthase expression in lipopolysaccharide-induced urinary bladder inflammation. Am J Physiol Renal Physiol 291: F567–F577, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Crisman JM, Zhang B, Norman LP, Bond JS. Deletion of the mouse meprin beta metalloprotease gene diminishes the ability of leukocytes to disseminate through extracellular matrix. J Immunol 172: 4510–4519, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Cunningham PN, Dyanov HM, Park P, Wang J, Newell KA, Quigg RJ. Acute renal failure in endotoxemia is caused by TNF acting directly on TNF receptor-1 in kidney. J Immunol 168: 5817–5823, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Cunningham PN, Wang Y, Guo R, He G, Quigg RJ. Role of Toll-like receptor 4 in endotoxin-induced acute renal failure. J Immunol 172: 2629–2635, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Eichel L, Scheidweiler K, Kost J, Shojaie J, Schwarz E, Messing E, Wood R. Assessment of murine bladder permeability with fluorescein: validation with cyclophosphamide and protamine. Urology 58: 113–118, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Glauser MP, Zanetti G, Baumgartner JD, Cohen J. Septic shock: pathogenesis. Lancet 338: 732–736, 1991. [DOI] [PubMed] [Google Scholar]
- 23.Haugen BJ, Pellett S, Redford P, Hamilton HL, Roesch PL, Welch RA. In vivo gene expression analysis identifies genes required for enhanced colonization of the mouse urinary tract by uropathogenic Escherichia coli strain CFT073 dsdA. Infect Immun 75: 278–289, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herzog C, Kaushal GP, Haun RS. Generation of biologically active interleukin-1beta by meprin B. Cytokine 31: 394–403, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Herzog C, Seth R, Shah SV, Kaushal GP. Role of meprin A in renal tubular epithelial cell injury. Kidney Int 71: 1009–1018, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Holly MK, Dear JW, Hu X, Schechter AN, Gladwin MT, Hewitt SM, Yuen PS, Star RA. Biomarker and drug target discovery using proteomics in a new rat model of sepsis-induced acute renal failure. Kidney Int 70: 496–506, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishmael FT, Norcum MT, Benkovic SJ, Bond JS. Multimeric structure of the secreted meprin A metalloproteinase and characterization of the functional protomer. J Biol Chem 276: 23207–23211, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Kielian T, Bearden ED, Baldwin AC, Esen N. IL-1 and TNF-alpha play a pivotal role in the host immune response in a mouse model of Staphylococcus aureus-induced experimental brain abscess. J Neuropathol Exp Neurol 63: 381–396, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Knotek M, Rogachev B, Wang W, Ecder T, Melnikov V, Gengaro PE, Esson M, Edelstein CL, Dinarello CA, Schrier RW. Endotoxemic renal failure in mice: role of tumor necrosis factor independent of inducible nitric oxide synthase. Kidney Int 59: 2243–2249, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Kozak W, Conn CA, Klir JJ, Wong GH, Kluger MJ. TNF soluble receptor and antiserum against TNF enhance lipopolysaccharide fever in mice. Am J Physiol Regul Integr Comp Physiol 269: R23–R29, 1995. [DOI] [PubMed] [Google Scholar]
- 31.Kruse MN, Becker C, Lottaz D, Kohler D, Yiallouros I, Krell HW, Sterchi EE, Stocker W. Human meprin alpha and beta homo-oligomers: cleavage of basement membrane proteins and sensitivity to metalloprotease inhibitors. Biochem J 378: 383–389, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuemmerle JF Synergistic regulation of NOS II expression by IL-1 beta and TNF-alpha in cultured rat colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 274: G178–G185, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Lavelle JP, Apodaca G, Meyers SA, Ruiz WG, Zeidel ML. Disruption of guinea pig urinary bladder permeability barrier in noninfectious cystitis. Am J Physiol Renal Physiol 274: F205–F214, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Leon LR Invited review: cytokine regulation of fever: studies using gene knockout mice. J Appl Physiol 92: 2648–2655, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Linder CC Genetic variables that influence phenotype. ILAR J 47: 132–140, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Lottaz D, Hahn D, Muller S, Muller C, Sterchi EE. Secretion of human meprin from intestinal epithelial cells depends on differential expression of the alpha and beta subunits. Eur J Biochem 259: 496–504, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Lowell CA, Berton G. Resistance to endotoxic shock and reduced neutrophil migration in mice deficient for the Src family kinases Hck and Fgr. Proc Natl Acad Sci USA 95: 7580–7584, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mallen-St CJ, Pham CT, Villalta SA, Caughey GH, Wolters PJ. Mast cell dipeptidyl peptidase I mediates survival from sepsis. J Clin Invest 113: 628–634, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mira JP, Cariou A, Grall F, Delclaux C, Losser MR, Heshmati F, Cheval C, Monchi M, Teboul JL, Riche F, Leleu G, Arbibe L, Mignon A, Delpech M, Dhainaut JF. Association of TNF2, a TNF-alpha promoter polymorphism, with septic shock susceptibility and mortality: a multicenter study. JAMA 282: 561–568, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Norman LP, Jiang W, Han X, Saunders TL, Bond JS. Targeted disruption of the meprin beta gene in mice leads to underrepresentation of knockout mice and changes in renal gene expression profiles. Mol Cell Biol 23: 1221–1230, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norman LP, Matters GL, Crisman JM, Bond JS. Expression of meprins in health and disease. Curr Top Dev Biol 54: 145–166, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Ramesh G, Kimball SR, Jefferson LS, Reeves WB. Endotoxin and cisplatin synergistically stimulate TNF-α production by renal epithelial cells. Am J Physiol Renal Physiol 292: F812–F819, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Saban MR, Hellmich H, Nguyen NB, Winston J, Hammond TG, Saban R. Time course of LPS-induced gene expression in a mouse model of genitourinary inflammation. Physiol Genomics 5: 147–160, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Sriskandan S, Altmann DM. The immunology of sepsis. J Pathol 214: 211–223, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Sterchi EE, Stocker W, Bond JS. Meprins, membrane-bound and secreted astacin metalloproteinases. Mol Aspects Med (August 22, 2008). doi: 10.1016/j.mam.2008.08.002. [DOI] [PMC free article] [PubMed]
- 46.Tanaka Y, Irie K, Hirota T, Sakisaka T, Nakanishi H, Takai Y. Ectodomain shedding of nectin-1alpha by SF/HGF and TPA in MDCK cells. Biochem Biophys Res Commun 299: 472–478, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Trachtman H, Valderrama E, Dietrich JM, Bond JS. The role of meprin A in the pathogenesis of acute renal failure. Biochem Biophys Res Commun 208: 498–505, 1995. [DOI] [PubMed] [Google Scholar]
- 48.Turler A, Schwarz NT, Turler E, Kalff JC, Bauer AJ. MCP-1 causes leukocyte recruitment and subsequently endotoxemic ileus in rat. Am J Physiol Gastrointest Liver Physiol 282: G145–G155, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Van Amersfoort ES, Van Berkel TJ, Kuiper J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin Microbiol Rev 16: 379–414, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker PD, Kaushal GP, Shah SV. Meprin A, the major matrix degrading enzyme in renal tubules, produces a novel nidogen fragment in vitro and in vivo. Kidney Int 53: 1673–1680, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Wang W, Faubel S, Ljubanovic D, Mitra A, Falk SA, Kim J, Tao Y, Soloviev A, Reznikov LL, Dinarello CA, Schrier RW, Edelstein CL. Endotoxemic acute renal failure is attenuated in caspase-1-deficient mice. Am J Physiol Renal Physiol 288: F997–F1004, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Wang W, Li C, Summer SN, Falk S, Wang W, Ljubanovic D, Schrier RW. Role of AQP1 in endotoxemia-induced acute kidney injury. Am J Physiol Renal Physiol 294: F1473–F1480, 2008. [DOI] [PubMed] [Google Scholar]
- 53.Warren JW Interstitial cystitis as an infectious disease. Urol Clin North Am 21: 31–39, 1994. [PubMed] [Google Scholar]
- 54.Zhang C, Walker LM, Mayeux PR. Role of nitric oxide in lipopolysaccharide-induced oxidant stress in the rat kidney. Biochem Pharmacol 59: 203–209, 2000. [DOI] [PubMed] [Google Scholar]