Abstract
Obesity is a risk factor for development of insulin resistance, type 2 diabetes, cardiovascular disease, osteoarthritis, and some forms of cancer. Many of the adverse health consequences of excess fat deposition are caused by increased secretion of proinflammatory adipokines by adipose tissue. Reciprocal muscle-to-fat signaling factors, or myokines, are starting to be identified. Interleukin-15 (IL-15) is a cytokine that is highly expressed in muscle tissue and that, on the basis of cell culture experiments, has been proposed to act as a circulating myokine that inhibits adipose tissue deposition. To test this hypothesis in vivo, two lines of transgenic mice that overexpressed IL-15 mRNA and protein in skeletal muscle tissue were constructed. By substitution of the inefficient native IL-15 signal peptide with a more efficient signal peptide, one of the transgenic mouse lines also exhibited elevated secretion of IL-15 in the circulation. Overexpression of IL-15 in muscle tissue without secretion in the bloodstream resulted in no differences in body composition. Elevated circulating levels of IL-15 resulted in significant reductions in body fat and increased bone mineral content, without appreciably affecting lean body mass or levels of other cytokines. Elevated circulating levels of IL-15 also inhibited adiposity induced by consumption of a high-fat/high-energy diet in male, but not female, transgenic mice. Female mice with elevated serum IL-15 exhibited increased deposition of lean body mass on a low-fat/low-energy diet and a high-fat/high-energy diet. These findings indicate that muscle-derived circulating IL-15 can modulate adipose tissue deposition and support addition of IL-15 to the growing list of potential myokines that are increasingly being implicated in regulation of body composition.
Keywords: adipose tissue, obesity, cytokines, adipokines, myokines, bone, skeletal muscle, interleukin-15, body composition
obesity is a major health problem in industrialized countries and increasingly worldwide, accounting for as much as 7.8% of health care expenses in the United States (25). Obesity is a leading risk factor for development of insulin resistance, type 2 diabetes, cardiovascular disease, osteoarthritis, and some forms of cancer (25, 30, 51, 70). Obesity is also a strong predictor of frailty, disability, and skeletal muscle loss (termed sarcopenia) during aging (14, 38). Recent progress in understanding the hormonal control of body composition, the ratio of fat to lean body mass, has been made through identification of circulating adipose-derived factors such as leptin, resistin, and adiponectin (17, 68). These factors, termed “adipokines,” are produced both by adipocytes and by tissue macrophages that infiltrate obese adipose tissue (71). Adipokines regulate both the mass and metabolism of other tissues, including skeletal muscle and bone (17, 68). Adipose tissue mRNA expression and serum levels of proinflammatory adipokines, such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), increase with obesity and are implicated in insulin resistance, muscle wasting, osteoporosis, and bone fractures (13, 17, 27, 30, 57, 59, 68). Conversely, serum levels of adiponectin, an insulin-sensitizing adipokine that has direct effects on skeletal muscle, are lower in obese subjects than in lean animals and humans (17, 60, 68, 70). Reciprocal pathways whereby skeletal muscle secretes circulating factors that affect adipose tissue (“myokines”) are only beginning to be identified (49). Clear identification of potential myokines is confounded by reports that experimental modulation of intramuscular hypertrophic factors, which increase muscle mass and basal metabolic rate, can indirectly decrease fat deposition by reducing energy available for fat deposition, in the absence of a direct endocrine effect on adipose tissue (35, 73).
Interleukin-15 (IL-15) is a 14- to 15-kDa cytokine that is highly expressed at the mRNA and protein levels in skeletal muscle compared with other tissues (23, 55, 56, 65). Moreover, although IL-15 is inefficiently secreted (65), skeletal muscle tissue preferentially expresses the IL-15 mRNA variant that is capable of being secreted (66). Although IL-15 was originally characterized as a stimulator of natural killer (NK) T cell development (6, 23, 65), it exerts hypertrophic and/or anti-atrophic actions on muscle in experimental models (9, 11, 19, 24, 54). Cell culture and short-term in vivo experiments in laboratory rodents indicated that IL-15 inhibits skeletal muscle protein degradation and muscle nuclear apoptosis (9, 11, 19, 54). IL-15 also has metabolic effects, stimulating glucose uptake and lipid oxidation in muscle tissue (2, 10). Additionally, although little or no IL-15 is normally expressed by adipocytes (55), adipose tissue expresses mRNA for all three subunits of the IL-15 receptor (3). Systemically administered IL-15 reduces fat deposition in normal and obese rodents, effects associated with inhibition of lipogenesis in liver and adipose tissue (3, 12). A direct effect of IL-15 on adipose tissue is suggested by cell culture studies that demonstrated that IL-15 inhibits preadipocyte differentiation, stimulates lipolysis, inhibits lipogenesis, and stimulates adiponectin secretion (1, 12, 55). In human subjects, variations in percent body fat (16) correlate with single-nucleotide polymorphisms (SNPs) in the gene for the IL-15-specific α-receptor subunit (IL-15Rα), which controls IL-15 availability and signaling (4, 6, 7, 61). Because IL-15 can also have muscle hypertrophic actions, these observations suggested, but did not prove, that IL-15 secreted from muscle tissue may function as a circulating regulator of body composition.
The present study tested the hypothesis that IL-15 can act as a myokine by analyzing body composition and responses to high-fat/high-energy feeding in two lines of transgenic mice that overexpress and/or oversecrete IL-15 from skeletal muscle tissue. Neither line of transgenic mice exhibited skeletal muscle hypertrophy, whereas reductions in body fat and resistance to diet-induced adiposity were observed, specifically in the transgenic line that exhibited increased circulating levels of IL-15. These findings indicate that IL-15 secretion from muscle tissue can modulate body composition via an endocrine mechanism, supporting the emerging concept that skeletal muscle can function as an endocrine tissue, secreting circulating myokines that affect body composition.
MATERIALS AND METHODS
Animal care.
Animal procedures were carried out following protocols approved by the Veterans Affairs Puget Sound Health Care System and University of Washington Institutional Animal Care and Use Committees. Mice were maintained in the Animal Research Facility at Veterans Affairs Puget Sound Health Care System in specific pathogen-free conditions on a 12:12-h light-dark cycle. For breeding and initial biochemical and phenotypic characterization, mice were fed an irradiated medium fat/medium energy “breeder” diet (4.6 kcal/g) by kilocalories 23% protein, 22% fat, and 55% carbohydrate (PicoLab Mouse Diet 20; Purina Labdiets, St. Louis, MO). Food and water were provided ad libitum. Biochemical and initial phenotypic characterizations were performed at 4 mo of age in mice conventionally housed at a density of 4 mice/cage for females and two to three littermates per cage for males; single-gender group cages contained mixtures of control and transgenic mice from the same line.
For more controlled diet and housing density experiments, mice from the HSA-IL2SP-IL15 line were weaned at 3 wk of age and housed singly for 5 more weeks on a low-fat/low-energy diet (3.8 kcal/g) consisting of (by kcal) 20% protein, 10% fat, and 70% carbohydrate (DIO Series Diet D12450B; Research Diets, New Brunswick, NJ), with food and water provided ad libitum. At 8 wk of age, control and transgenic mice of both genders were randomly assigned to continue on the low-fat diet or were supplied with a high-fat/high-energy diet (5.2 kcal/g) consisting of (by kcal) 20% protein, 60% fat, and 20% carbohydrate (DIO Series Diet D12492; Research Diets), with food and water provided ad libitum, for 21 wk. Mice continued to be singly housed to allow analysis of food consumption and to control for housing density and social interactions. Mice were provided with an excess of food (40 g) weekly. Food consumption was monitored weekly for each mouse by subtracting the weight of food remaining in the cage, including spillage on cage bedding, from the weight of food supplied the previous week. Food spillage/wastage was negligible except for one mouse, which was removed from the analysis. Individual body weights were also determined weekly.
Generation of transgenic mice.
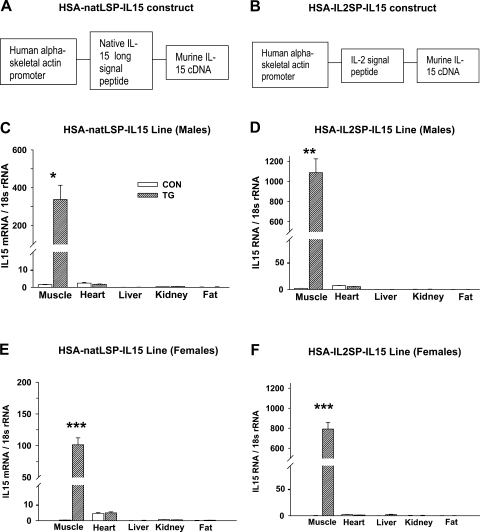
Murine long signal peptide (LSP) isoform IL-15 cDNA (23, 47, 53, 66) was cloned in the pBSX-HSAvpA plasmid containing a modified human skeletal actin (HSA) promoter fragment (5, 15) to generate the HSA-natLSP-IL15 construct (Fig. 1A). For the HSA-IL2SP-IL15 construct (Fig. 1B), a modified murine IL-15 (18) in which the native IL-15 LSP was replaced with the interleukin-2 (IL-2) signal sequence, and in which several posttranscriptional checkpoints [5′-AUGs (adenine-uridine-guanine) and a COOH-terminal retention sequence] blocking efficient IL-15 translation were eliminated, was cloned into the pBSX-HSAvpA plasmid. Linearized transgene DNA was microinjected in C57BL/6 × C3H hybrid oocyte pronuclei and transplanted in pseudopregnant Swiss Webster mice. Founder pups were identified by real-time PCR of tail biopsies using transgene-specific primers. Within each line, transgenic males were bred until individuals with stable single transgene insertion points were identified. Transgene copy number was not determined. HSA-natLSP-IL15 and HSA-IL2SP-IL15 transgenic mouse lines were backcrossed for more than six generations by mating heterozygous male transgenic mice to commercially purchased wild-type C57BL/6 females (The Jackson Laboratory, Bar Harbor, ME). Resulting litters contained both heterozygous transgenic and control (nontransgenic) littermates, allowing analysis of closely related animals. At 3 wk of age, mouse pups were genotyped by real-time PCR analysis of tail biopsies.
Fig. 1.
Validation of human skeletal actin (HSA) natLSP-IL15 and HSA-IL2SP-IL15 transgenic mouse lines. A and B: schematic maps of HSA-natLSP-IL15 (A) and HSA-IL2SP-IL15 (B) transgenes. C–F: interleukin-15 (IL-15) mRNA expression in various tissues of control (CON) and transgenic (TG) mice, determined by real-time PCR. LSP, long signal peptide; SP, signal peptide. Bars show means ± SE; n = 4–5 mice/group; differences between control and transgenic: *P < 0.05, **P < 0.01, and ***P < 0.001.
Real-time PCR for determination of IL-15 mRNA expression.
Tissue samples were submerged in RNAlater RNA Stabilization Reagent (Qiagen, Valencia, CA) and stored frozen at −20°C. RNA was isolated and purified from quadriceps femoris muscle, heart, liver, and kidney using the Qiagen RNeasy Fibrous Tissue Mini Kit and from abdominal visceral fat tissue using the Qiagen RNeasy Lipid Tissue Mini Kit. RNA was quantified and cDNA was reverse transcribed from total RNA with an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) using a Bio-Rad iCycler. Primers for murine IL-15 mRNA were directed at the region coding for the mature IL-15 peptide and detected both endogenous and transgene IL-15 transcripts. Primer sequences were as follows: forward 5′-CAG CAC TCT GTC TTC TAA CAA GAA-3′; reverse 5′-TCT GTG AAG GTT TTC TCC TCC-3′. Primers for 18S ribosomal RNA (used as the “housekeeping” gene) were: forward 5′-GCG AAT GGC TCA TTA AAT CAG TTA-3′; reverse 5′-TTG TTT TGA TCT GAT AAA TGC ACG-3′. Threshold cycles and primer pair efficiencies were used in the 2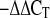 method (39) to yield differences in IL-15 RNA expression as normalized to the 18S housekeeping gene.
method (39) to yield differences in IL-15 RNA expression as normalized to the 18S housekeeping gene.
Assay of serum and muscle cytokines.
Blood was collected from deeply anesthetized mice by cardiac puncture, allowed to clot at room temperature for 30 min, and centrifuged at 1,000 g for 10 min, and serum was removed and stored frozen at −20°C in aliquots. Quadriceps femoris muscle samples were snap-frozen and stored at −80°C. Muscle tissue was weighed and placed in T-PER Tissue Protein Extraction Reagent (Pierce, Rockford, IL) at 100 mg tissue/ml reagent. “Halt” protease inhibitor cocktail (Pierce) was added to 1× and EDTA was added to 5 mM. The tissue was homogenized on ice, and debris and excess lipid were removed by centrifugation at 4°C. Total protein in an aliquot of the protein sample was determined by the BCA Protein Assay (Pierce), and protein content of the tissue piece was calculated. The remainder of the sample was normalized to 1 mg/ml protein content. For transgenic and control mouse samples, muscle IL-15 and serum IL-15, IL-2, IL-6, leptin, and TNF-α concentrations were determined using LINCOplex murine assays (LINCO Research, St. Charles, MO) for each analyte performed concurrently using a BioPlex Protein Array System instrument (Bio-Rad). Standard curves for each analyte provided in the kits were run concurrently for all assays, and all samples were run in triplicate. Intra- and interassay coefficients of variation (CVs) were <20 and 30%, respectively, and cross-reactivity was negligible. The minimum concentration of IL-15 accurately detectable by these assays (as reported by the manufacturer) was 9.3 pg/ml. Serum concentrations of IL-15 were typically below this level in control HSA-IL2SP-IL15 mice and control and transgenic HSA-natLSP-IL15 mice, so reported values were extrapolated from the lower end of the standard curve and should be considered nominal values.
Body composition analysis.
For conventionally housed 4-mo-old mice maintained on the medium-fat (22% by kcal) breeder diet, body composition was determined under deep pentobarbital sodium anesthesia (75 mg/kg ip) using a Lunar PIXImus dual-energy X-ray absorptiometry (DEXA) scanner equipped with fat offset calibration software (GE Healthcare, Madison, WI), with a CV of <1% for lean mass and <3% for fat mass (45). Following DEXA scans, blood was collected for serum cytokine analysis by cardiac puncture under deep anesthesia, followed by a second lethal dose of pentobarbital sodium (150 mg/kg ip) and subsequent tissue collection for cytokine and mRNA analysis. In the low- and high-fat feeding experiment, fat and lean body mass was determined after 21 wk on each diet on conscious mice by quantitative magnetic resonance (QMR) using an Echo MRI-100 rodent whole body composition analyzer (Echo Medical Systems, Houston, TX) with a CV of <2% for fat mass and <4% for lean mass (67). Fat mass measurements by the two methods are similar, while lean mass assessed by QMR is more representative of muscle mass than lean mass assessed by DEXA (67).
Muscle weights and histomorphometry.
Following euthanasia, soleus and extensor digitorum longus muscles were carefully dissected, blotted, and weighed. Intra-abdominal visceral fat was removed, fixed in phosphate-buffered 4% paraformaldehyde, and processed for paraffin sections; resulting sections were stained with hematoxylin and eosin. Mean adipocyte cross-sectional area was determined using a Nikon Optiphot2 microscope equipped with MCI v4.2 image analysis software (Imaging Research, St. Catherines, Ontario, Canada).
Statistical procedures.
All values are expressed as means ± SE. Statistical analyses were performed using SigmaStat 3.0 software (Systat Software, San Jose, CA). To compare IL-15 mRNA expression between control and transgenic mice in each line, significance of differences in this parameter for each tissue was determined by t-tests with correction for multiple comparisons. For all other comparisons, two-way ANOVA was used to assess significance of main genotype and gender, and (where applicable) diet and time effects, and possible interactions of these factors. When considering time effects, repeated-measure procedures were used. Post hoc pairwise multiple comparisons were performed using Student-Newman-Keul's or Holm-Sidak methods. Significance of differences between control and transgenic mice are noted in Figs. 1–6.
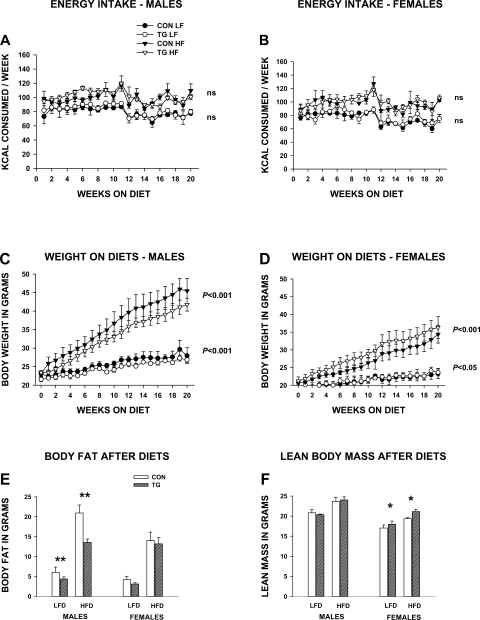
Fig. 6.
Effects of circulating IL-15 on energy intake, weight, and body composition in HSA-IL2SP-IL15 control and transgenic mice fed an extremely low-fat/low-energy diet (LFD) or an extremely high-fat/high-energy (HFD) diet. Bars represent means ± SE; n = 4–5 mice/group for HFD and 3 mice/group for LFD. A and B: energy intake (food consumption) on LFD and HFD, expressed as mean kcal consumed each week by group. C and D: weekly weights on LFD and HFD. Significance of genotype effects are noted. E and F: total body fat (E) and lean body mass (F) following LFD and HFD. Significance of post hoc pairwise comparison of values for control and transgenic mice in each pair are shown: *P < 0.05 and **P < 0.01.
RESULTS
IL-15 mRNA and protein expression in HSA-natLSP-IL15 and HSA-IL2SP-IL15 transgenic mouse lines.
In both mouse and human, the IL-15 gene encodes two IL-15 mRNA variants that utilize different signal sequences but produce identical mature IL-15 proteins following signal peptide cleavage (6, 53, 66). LSP-IL-15 is inefficiently secreted from cells, whereas short signal peptide (SSP)-IL-15 localization is intracellular (6, 47, 66). To produce transgenic mouse models intended to test the hypothesis that secreted IL-15 modulates body composition, two lines of transgenic mice that overexpressed IL-15 mRNA and protein in skeletal muscle tissue were constructed. In the HSA-natLSP-IL15 transgenic line, native LSP-IL-15 was expressed from a strong skeletal muscle-specific promoter based on the HSA promoter (Fig. 1A). In the construct inserted in the HSA-IL2SP-IL15 transgenic line (Fig. 1B), the inefficient native LSP signal peptide was replaced with the more efficient signal peptide for IL-2 (18, 65). The sequence of the mature IL-15 protein was unchanged from native IL-15 in both lines. Transgenic mice in both lines exhibited skeletal muscle-specific overexpression of IL-15 mRNA (Fig. 1, C–F). Because isoform-specific primers were not utilizable for analysis of the HSA-IL2SP-IL15 transgenic line, real-time PCR using primers directed at the IL-15 protein-coding region revealed somewhat elevated levels of IL-15 mRNA in cardiac tissue, which primarily represent the nonsecreted SSP-IL-15 mRNA variant (66). However, IL-15 mRNA expression levels in cardiac tissue did not differ between transgenic and nontransgenic control mice in either line and were considerably lower than skeletal muscle IL-15 mRNA levels in transgenic mice (Fig. 1, C–F).
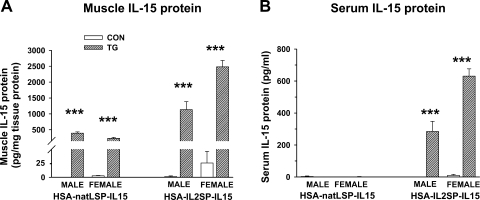
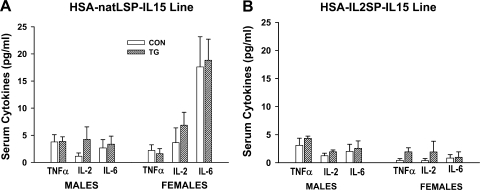
In both the HSA-natLSP-IL15 and HSA-IL2SP-IL15 lines, muscle IL-15 protein levels were significantly higher (P < 0.001) in transgenic mice than in control mice (Fig. 2A). However, circulating IL-15 protein levels were significantly increased (P < 0.001) only in transgenic mice from the HSA-IL2SP-IL15 line (Fig. 2B), consistent with the substitution of the more efficient signal sequence in the IL2SP-IL15 transgene. For the HSA-IL2SP-IL15 line, a significant genotype × gender interaction was detected, with transgenic female mice expressing higher levels of both muscle and serum IL-15 than transgenic males (P < 0.001). Expression of other circulating cytokines was not affected by genotype (Fig. 3, A and B). Main effects of gender on serum TNF-α (P < 0.05) were observed in both lines, with females of both genotypes exhibiting lower circulating levels of this cytokine than males. A significant effect of gender on serum IL-6 (P < 0.01) levels was observed in the HSA-natLSP-IL15 line, which was not dependent on genotype. Over multiple generations, transgenic mice from either construct did not exhibit enlarged spleens, elevated splenic or blood cellularity, or any other apparent abnormalities (data not shown).
Fig. 2.
IL-15 protein expression in muscle (A) and serum (B) in HSA-natLSP-IL15 and HSA-IL2SP-IL15 control and transgenic mice. Bars show means ± SE; n = 4–5 mice/group. ***P < 0.001, significance of post hoc pairwise comparison of values for control and transgenic mice in each pair.
Fig. 3.
Circulating levels of tumor necrosis factor (TNF)-α, IL-2, and IL-6 in HSA-natLSP-IL15 (A) and HSA-IL2SP-IL15 (B) transgenic mouse lines. Bars show means ± SE; n = 4–5 mice/group. No effects of control or transgenic genotype on expression of circulating TNF-α, IL-2, or IL-6 were detected.
Body composition of conventionally housed HSA-natLSP-IL15 and HSA-IL2SP-IL15 transgenic mouse lines.
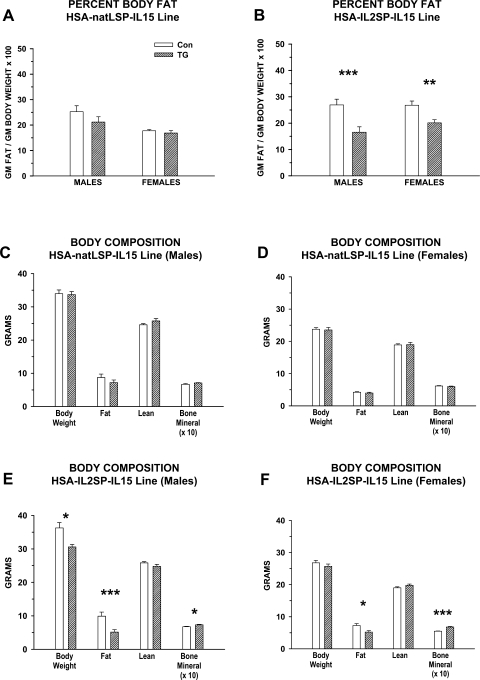
For initial body composition analyses, HSA-natLSP-IL15 and HSA-IL2SP-IL15 control and transgenic mice were conventionally housed in mixed-genotype (control and transgenic), single-gender group cages. Mice were maintained on a moderate-fat (22% of kcal)/moderate-energy (4.6 kcal/g) breeder diet until 4 mo of age, and body composition was determined using DEXA. Main effects of genotype (control vs. transgenic) on percent body fat were detected in the HSA-IL2SP-IL15 line (P < 0.001). Percent body fat in male and female transgenic mice from the HSA-IL2SP-IL15 line, but not the HSA-natLSP-IL15 line, was reduced significantly (Fig. 4, A and B). In the HSA-natLSP-IL15 line, no differences in lean body mass, fat mass, or bone mineral content were observed between transgenic and control mice of either gender (Fig. 4, C and D). In the HSA-IL2SP-IL15 line, the difference in body composition in transgenic mice was due to absolute decreases in body fat (main effect of genotype, P <0.001), with no significant changes in lean body mass (Fig. 4, E and F). Within the HSA-IL2SP-IL15 line, a significant genotype × gender interaction for fat mass was detected (P < 0.01). The magnitude of the decrease in fat mass was larger in HSA-IL2SP-IL15 transgenic males (∼50%) than in HSA-IL2SP-IL15 transgenic females (∼25%). Significant decreases in overall body weight (Fig. 4, E and F) were detected in transgenic HSA-IL2SP-IL15 males but not females (genotype × gender interaction, P < 0.01), which is attributable to the reduced effect of genotype on fat mass in females. In summary, adipose tissue mass was altered in the transgenic mouse line that displayed increased circulating levels of IL-15 (the HSA-IL2SP-IL15 line), but not in the transgenic mouse line that displayed increases in muscle IL-15 protein content only.
Fig. 4.
Body composition of conventionally housed HSA-natLSP-IL15 and HSA-IL2SP-IL15 mice maintained on a medium-fat (22% of kcal) diet. Body composition was determined in transgenic and control mice in each line at 4 mo of age. Bars represent means ± SE; n = 4–5 mice/group. Significance of post hoc pairwise comparison of values for control and transgenic mice in each pair are shown: *P < 0.05, **P < 0.01, and ***P < 0.001. A and B: %body fat; C–F: total body weight, lean body mass, body fat, and bone mineral content. Numerical values for bone mineral content (in grams) were multiplied by 10 to facilitate viewing on the same scale as other body composition parameters.
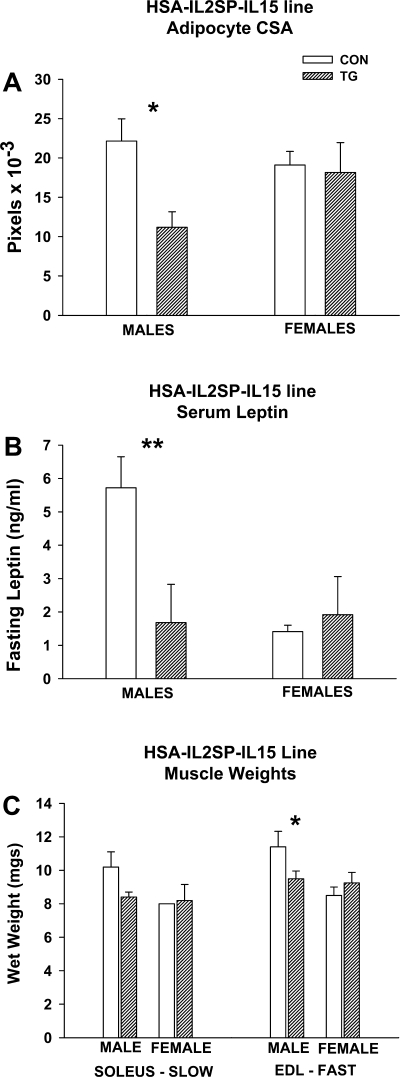
Mean intra-abdominal (visceral) fat cell area in transgenic HSA-IL2SP-IL15 male mice was also approximately one-half that of controls (genotype effect, P < 0.05), whereas the same parameter did not differ significantly between female HSA-IL2SP-IL15 control and transgenic mice (Fig. 5A). Similarly, fasting serum leptin levels were significantly lower in male, but not female, transgenic mice in the HSA-IL2SP-IL15 line (genotype × gender interaction, P < 0.01; Fig. 5B). Wet weight of the slow-twitch soleus muscle did not differ significantly between control and transgenic mice in the HSA-IL2SP-IL15 line (Fig. 5C). Wet weight of the fast-twitch extensor digitorum longus muscle was significantly lower in transgenic male, but not female, HSA-IL2SP-IL15 mice compared with controls (genotype × gender interaction, P < 0.05; Fig. 5C).
Fig. 5.
Ancillary measures of adipose and muscle tissue in male and female HSA-IL2SP-IL15 control and transgenic mice. Bars represent mean ± SE; n = 4–6 mice/group. Significance of post hoc pairwise comparison of values for control and transgenic mice in each pair are shown: *P < 0.05 and **P < 0.01. A: mean abdominal adipocyte cross-sectional area (CSA). B: mean fasting serum leptin levels (ng/ml). C: wet weights of selected muscles.
Although a small component of total body mass, bone mineral content was also increased significantly in transgenic mice from the HSA-IL2SP-IL15 line (genotype effect, P < 0.001; Fig. 4, E and F). Gender differences were observed in this parameter as well (gender effect, P < 0.001). Bone mineral content was elevated 8% in male HSA-IL2SP-IL15 transgenic mice and 24% in female HSA-IL2SP-IL15 transgenic mice compared with the respective controls. Bone mineral content did not differ significantly between control and transgenic mice of either gender in the HSA-natLSP-IL15 line (Fig. 4, C and D).
Effects of low- and high-fat feeding on body composition in singly housed HSA-IL2SP-IL15 mice.
A controlled feeding and housing density study was conducted to explore differences in body composition between control and transgenic mice in the HSA-IL2SP-IL15 line in more detail. Mice were weaned on low-fat (10% of kcal)/low-energy (3.8 kcal/g) diets and were housed singly. Beginning at 8 wk of age, mice were challenged with low-fat or high-fat (60% of kcal)/high-energy (5.2 kcal/g) diets for 21 wk. Mean body weights at 8 wk did not differ significantly among groups within each gender (data not shown).
Energy intake (Fig. 6, A and B) was significantly higher in mice exposed to the high-fat diet but did not differ significantly between control and transgenic mice on either diet (diet effect, P < 0.001). A significant effect of time on energy intake (P < 0.05) was observed for both diets, with a spike in consumption at 11 wk in all groups followed by a decline, which coincided with delivery of fresh diet stocks at that time point. Significant effects of time (P < 0.001), diet (P < 0.001), and genotype (P < 0.05) on weight were detected for both genders (Fig. 6, C and D). There was no significant time × genotype interaction, indicating that the temporal pattern of weight gain did not differ between control and transgenic HSA-IL2SP-IL15 mice. For males on both the low-fat and high-fat diets, control mice gained significantly (P < 0.001) more weight than transgenic mice (Fig. 6C). In females, this pattern was reversed, with transgenic females gaining significantly more weight than control females on both diets (Fig. 6D).
Body composition was determined after 21 wk on the diets using QMR, which does not quantify bone mineral content, but which yields values for lean mass that some studies indicate are more reflective of muscle mass than DEXA (67). For males, main effects of genotype (P < 0.01) and diet (P < 0.001) on fat mass were observed. For females, main effects of diet (P < 0.001), but not genotype, on fat mass were observed. Transgenic male, but not female, HSA-IL2SP-IL15 mice had significantly (P < 0.01) lower fat mass than controls following both high- and low-fat feeding (Fig. 6E). Secreted IL-15 therefore conferred significant resistance to diet-induced adiposity in males but not in females.
In contrast, main effects of both genotype (P < 0.05) and diet (P < 0.001) on lean mass were observed for female mice, whereas for male mice effects of diet only (P < 0.01) were observed. Transgenic female, but not male, HSA-IL2SP-IL15 mice had significantly (P < 0.05) higher amounts of lean body mass than controls following both high- and low-fat feeding (Fig. 6F). Thus, although transgenic female HSA-IL2SP-IL15 mice gained more weight than control females, this was preferentially deposited as lean body mass.
Inasmuch as body weight differed between transgenic and control mice, but energy consumption did not, energy consumed per gram body weight therefore differed. In males, weekly energy consumption per gram body weight was significantly higher in transgenic mice compared with controls (P < 0.001). In females, this pattern was reversed (P < 0.05). These gender-specific effects correlate with the gender-specific pattern of fat and lean body mass accretion as a function of genotype. Feed efficiency (weight gained/energy consumed), a measure of the efficiency of energy metabolism, was significantly lower in transgenic males than control males on high-fat diets (P < 0.001). This parameter did not differ in males on the low-fat diet or females on either diet.
DISCUSSION
This study demonstrated that release of IL-15 in the circulation by skeletal muscle tissue can modulate remote tissues such as bone and adipose tissue. In this study, IL-15 mRNA and protein were overexpressed in two lines of transgenic mice, the HSA-natLSP-IL15 line and the HSA-IL2SP-IL15 line. However, no effects of overexpressed muscle IL-15 protein on body composition were observed unless the IL-15 was released into the circulation, as occurred in the HSA-IL2SP-IL15 transgenic mouse line. Additionally, the lack of change in lean body mass in males of the HSA-IL2SP-IL15 line indicates the IL-15-induced declines in adipose tissue mass were due neither to generalized cachexia nor to increases in metabolic rate caused by massive muscle or lean tissue growth. Therefore, the data presented here constitute evidence that IL-15 can function as a muscle-derived endocrine factor, or myokine. However, inasmuch as most of the findings presented here relate to an artificial system involving enhanced expression of IL-15 from a synthetic promoter as well as enhanced secretion of IL-15 using the signal peptide from another cytokine, it is important to note that these constructs are consistent with many observations concerning endogenous IL-15 expression and action in human subjects and experimental animals, such as high expression of IL-15 in skeletal muscle (23, 55, 56, 65) and correlation of human genetic polymorphisms in the IL-15 system with body composition (16, 52, 58).
In multiple tissue analyses, which include lymphoid tissues, the highest levels of IL-15 mRNA expression have been reported in skeletal muscle, placenta, and heart (23, 65, 66). Skeletal muscle expresses high levels of the potentially secreted LSP-IL-15 mRNA isoform, whereas cardiac muscle expresses the nonsecreted SSP-IL-15 mRNA isoform (66). The low levels of IL-15 protein that have been observed in most cell types and biological fluids have been attributed to IL-15 translational blocks, inefficient IL-15 secretion, and instability of the IL-15 protein (4, 6, 7, 61, 65). Indeed, in this study, serum levels of IL-15 in control HSA-IL2SP-IL15 mice and HSA-natLSP-IL15 line mice were generally below the sensitivity levels of our assays, and the reported nominal values were extrapolated from the standard curves. Blocks to both translation and secretion of IL-15 were removed in the HSA-IL2SP-IL15 line, resulting in significantly elevated circulating IL-15 protein levels. Given the rapid rate of IL-15 degradation, it is likely that elevated secretion of IL-15 in transgenic HSA-IL2SP-IL15 mice resulted in a steady stream of bioavailable IL-15 to remote tissues, which activated IL-15 signaling in target tissues via existing but unsaturated cell-associated IL-15 receptors.
In human subjects, IL-15 mRNA expression is higher in muscles composed mainly of type 2 fibers than in muscles composed predominantly of type 1 fibers; however, IL-15 protein content is similar across muscle types (46). In the transgenic mouse lines used in this study, IL-15 mRNA and protein expression were enhanced using a strong skeletal muscle-specific promoter, derived from the HSA promoter, which does not display fiber-type specificity (5, 15). In light of recent evidence that many of the proinflammatory factors secreted from adipose tissue are in fact derived from immune infiltrating cells such as macrophages, rather than adipocytes (71, 72), it is also important to note that published evidence indicates IL-15 is in fact normally expressed at both the mRNA and protein level by muscle fibers (40, 55, 56, 63), and IL-15 protein has been immunolocalized to human skeletal muscle fibers (63). IL-15 mRNA and protein are expressed in primary human myogenic cultures (63) and human rhabodomyosarcoma-derived cell lines (40). IL-15 mRNA expression is low, but detectable, in mouse C2C12 skeletal myogenic cultures at the myoblast (muscle precursor cell) stage but is induced ∼10-fold upon differentiation into immature muscle fibers called myotubes (55). Therefore, published evidence indicates that IL-15 is highly expressed by skeletal muscle fibers, as opposed to vascular, connective tissue or lymphoid infiltrating cells present in muscle and in primary cultures. It may seem counterintuitive that IL-15, which was first identified on the basis of its ability to support NK T lymphocytes (6, 23, 65), is highly expressed in muscle tissue and that IL-15 has been proposed to regulate body composition (3, 12, 55, 56). However, like NK cells, skeletal muscle is now recognized as part of the innate immune system, responding to environmental and immune stresses partly by modulating expression of cytokines such as TNF-α and IL-6 (20, 29). Moreover, IL-15 belongs to the same structural class of molecules as growth hormone and leptin, the class-I α-helical cytokines (6, 28). Both growth hormone and leptin regulate body composition, and both are principally expressed in nonlymphoid tissues (pituitary and adipose tissue, respectively). Moreover, growth hormone and leptin have been shown to regulate immunity, and leptin in particular regulates NK T lymphocyte activity (31, 48). The adipokine visfatin is expressed in skeletal muscle and other tissues but was originally identified as a regulator of pre-B lymphocyte development (22). Thus the concept of a skeletal muscle-derived cytokine with roles in both immunity and body composition is consistent with other recent findings linking the immune and endocrine systems (20, 29, 31).
As observed in this study, IL-15 mRNA is undetectable in adipose tissue under normal physiological conditions by sensitive real-time PCR. Cultured 3T3-L1 adipocytes and preadipocytes also do not express IL-15 mRNA detectable above background levels by real-time PCR (55). However, one study using suspension-cultured primary porcine adipocytes indicated that these cultures expressed low levels of IL-15 mRNA under basal conditions, which increased following stimulation with interferon-γ (1). Because of the methodology utilized, it was unclear which isoform of IL-15 was induced in porcine adipocyte cultures, and IL-15 protein production was not examined in that study. Also, although enriched for adipocytes, it is unclear if the primary cultures in the study contained other cell types (such as macrophages) and/or if the acute isolation procedure induced oxidative or other stresses in the primary cells. Thus, at least under normal physiological conditions, skeletal muscle appears to be a principal depot for both LSP-IL-15 mRNA and IL-15 protein, which may be translationally activated or released, respectively, upon appropriate physiological stimuli.
Because release of IL-15 from muscle in the HSA-IL2SP-IL15 transgenic mouse line was facilitated by replacement of the inefficient native IL-15 LSP signal sequence, the question of how secretion of endogenous IL-15 from skeletal muscle is regulated was not addressed in this study. Our study clearly shows that, by itself, upregulation of intramuscular IL-15, as in the HSA-natLSP-IL15 line, was not sufficient to cause release of IL-15 from muscle in the circulation. However, several putative myokines, including IL-15, have been reported to be released from muscle by physical activity and/or muscular contractions (46, 49, 50, 58). The HSA-natLSP-IL15 transgenic mouse model described here, with elevated intramuscular, but not serum, IL-15 levels, may be useful for future investigation of the hypothesis that IL-15 release from muscle is facilitated by physical activity.
Although not addressed in this study, it is likely that the effects of IL-15 on adipose tissue observed in HSA-IL2SP-IL15 transgenic mice were direct, since other studies have shown direct effects of IL-15 on both adipogenic cell lines and primary adipocyte cultures, including inhibition of preadipocyte differentiation, inhibition of lipogenesis, dose-dependent stimulation of adiponectin secretion, and dose-dependent stimulation of lipolysis (1, 55). Additionally, white adipose tissue harvested from mice and rats expresses mRNA for all three IL-15 receptor subunits (3). Moreover, although systemic IL-15 administration induced reductions in adiposity in rodent obesity models in which IL-15 receptor expression levels in adipose tissue were robust, IL-15 administration was ineffective in causing reductions in adiposity in strains that expressed lower levels of IL-15 receptor mRNA in adipose tissue (3). Thus expression of IL-15 receptor subunit mRNA in adipose tissue correlated with the ability of IL-15 to inhibit adipose tissue deposition in these systems, strongly suggesting a direct effect of IL-15 on adipose tissue (3). In this study, alterations in adipose tissue mass specifically in the transgenic mouse line in which circulating levels of IL-15 were elevated likewise support the hypothesis that IL-15 modulated fat mass by an endocrine effect.
IL-15, like other cytokines, can cross the blood-brain barrier, and inasmuch as IL-15 and IL-15 receptors are expressed in brain tissue (34), the present study does not rule out additional, central, mechanisms of IL-15 on body composition such as regulation of activity levels. Although the results of this study suggest that IL-15 does not modulate food consumption per mouse, energy intake per gram body weight was altered in a gender-specific fashion. Additionally, this study did not examine activity levels. Differences in activity levels in male HSA-IL2SP-IL15 transgenic mice could account for their lower energetic efficiency and decreased fat content compared with controls. One study showed that intracerebroventricular administration of IL-15 can enhance non-rapid eye movement sleep in a rabbit model (34). This effect is similar to other cytokines and is consistent with the role of IL-15 in innate immunity (34). Increases in sleep would, however, be expected to decrease activity. Therefore, the effects of IL-15 on activity levels and metabolism remain unclear, and warrant further investigation.
In cell culture studies, IL-15 has been shown to act as a hypertrophic factor, increasing the size and myofibrillar protein content of cultured myotubes by stimulating protein synthesis as well as by inhibiting protein degradation (54). The hypertrophic effect of IL-15 in muscle cultures was a direct action on protein dynamics and, in contrast to the hypertrophic action of the insulin-like growth factors, occurred without stimulation of skeletal myoblast proliferation and differentiation (54). However, in isolated muscle preparations or short-term in vivo studies in mature rodents, the main effect of IL-15 on muscle protein dynamics was to lower protein degradation rates (9, 11). Thus, as was observed in this study, systemically administered IL-15 had little or no effect on muscle mass in healthy rodents in these studies but nevertheless could inhibit muscle wasting in cachectic rats (11) and improve diaphragm muscle strength, muscle fiber area, and muscle architecture in dystrophic mice (24). Likewise, transgenic mice with targeted deletion of IL-15 did not display reduced muscle mass or muscle weakness, but were not exposed to stress, exercised, or followed into aging (32). These findings support the idea that IL-15 is not involved in normal muscle growth or hypertrophy but rather may be part of a systemic response to stress that inhibits the breakdown of muscle. This is consistent with the generally low levels of IL-15 observed in tissues and serum in normal physiological situations.
In this study, the lack of change in muscle or lean body mass in the HSA-IL2SP-IL15 line in most conditions indicates that the IL-15-induced declines in adipose tissue mass were not an indirect effect of muscle hypertrophy. In contrast, massive muscle hypertrophy and concomitant reductions in adipose tissue deposition were observed in transgenic mice with skeletal muscle-specific conditional activation of the intracellular signaling molecule Akt/protein kinase B, and reductions in fat mass were attributed to the metabolic demands of muscle hypertrophy, which reduce energy available for fat deposition, rather than to an endocrine mechanism (35). Similarly, the transforming growth factor-β superfamily member myostatin has been suggested to be a potential myokine, since myostatin is predominantly expressed in muscle tissue and is detectable in the circulation (42, 74). Mice and cattle with induced or natural deletion of functional myostatin have decreased adipose tissue mass (43, 44). However, evidence for an endocrine effect of muscle-derived myostatin to stimulate adipose tissue deposition has been weak, since adipose tissue also expresses myostatin mRNA (42) and systemically administered myostatin reduces, rather than increases, fat mass (74). Some studies have suggested that, similar to the mechanism demonstrated from the muscle Akt activation study, the mechanism of myostatin deletion on fat mass is due to preferential utilization of metabolic energy by massively hypertrophied skeletal muscle (73). IL-6 has also been suggested to function as a myokine that reduces fat deposition and improves insulin sensitivity (49, 50). IL-6 is expressed in muscle, and it is clear that IL-6 can be released from muscle by physical activity to increase circulating IL-6 levels significantly (26, 50). However, IL-6 also has paracrine muscle hypertrophic actions via stimulation of satellite cell proliferation (62); paradoxically, systemic IL-6 also stimulates muscle atrophy and muscle protein degradation (21, 69). Moreover, IL-6 is also highly expressed by adipocytes, hepatocytes, and several other cell types, and the role of IL-6 as pro- or anti-inflammatory, insulin sensitizing or desensitizing, paracrine or endocrine, has been a subject of dispute (26, 33, 49, 50). The findings reported here regarding IL-15 stand in contrast to those reported for IL-6 and myostatin and suggest that IL-15 may function as an authentic myokine.
Although elevated circulating IL-15 inhibited adipose tissue deposition in both male and female mice maintained on the medium-fat breeder diet, the depression of fat deposition was greater in male mice. Additionally, circulating IL-15 significantly reduced both adipocyte size and serum leptin levels in male, but not female, mice. Following high-fat feeding, elevated serum IL-15 levels inhibited adipose tissue deposition in male, but not female, mice. In contrast, the effect of circulating IL-15 to increase bone mineral content was greater in female mice compared with male mice. Furthermore, in female, but not male, mice, IL-15 stimulated deposition of lean mass on both the high- and low-fat diets. We did not examine whether this gender-related increase in lean body mass was due to increases in skeletal muscle specifically. However, significant differences in deposition of lean mass between control and transgenic females were not detected by DEXA in the initial phenotypic analyses of group-housed mice. In the low- and high-fat diet experiments, body composition was assessed by QMR, which some reports indicate yields values for lean body mass that are more representative of muscle mass than those derived from DEXA (67). Alternatively, differences between the two experiments could be caused by differences in housing conditions. Indeed, muscle weights in the experiment conducted with group housing did not differ significantly between transgenic and control female mice. The increase in lean body mass in transgenic females detected in the second experiment (conducted with single housing) is consistent with reports that IL-15 enhances fat oxidation by skeletal muscle (2). Gender differences in the regulation and effects of adipokines, including leptin, as well as in the regulation of bone mass and adiposity, have been well-documented (37, 41, 59, 68); thus, gender differences in the response of these parameters to a putative myokine are not unexpected but could be an avenue for further investigation. In contrast to the situation with humans, male mice generally exhibit higher serum leptin levels than female mice (37), as was observed in our study. In this study, female control mice generally exhibited lower fat mass and higher circulating endogenous IL-15 levels than control male mice, suggesting the lower effects of elevating serum IL-15 on fat mass in females could be due to higher baseline levels of IL-15 in that gender. However, inasmuch as our measurement of IL-15 in control mice was inaccurate, this point needs further investigation using more sensitive assays. Nevertheless, one study indicated that administration of a synthetic progestin to elderly male human subjects increased circulating IL-15 levels (36), suggesting gender difference in endogenous IL-15 expression may be due to sex steroids. Another study demonstrated gender differences in the correlation of several SNPs in the human IL-15 and IL-15Rα genes with muscle mass and circulating markers associated with adiposity and risk for metabolic syndrome (52). Gender differences in expression or regulation of the IL-15Rα or other components of the IL-15 receptor complex are therefore possible but were not addressed in this study because of the complexity of IL-15Rα biochemistry (4, 6, 7, 61).
The positive effect of increased circulating IL-15 on bone mineral content was not anticipated before this study. Although other measures of skeletal mass and quality were not examined here, a very recent study indicated SNPs in the human IL-15Rα gene correlated with bone mass and density and also noted gender differences in the association of certain SNPs with bone deposition (52). There are several potential mechanisms for the effect of IL-15 on bone, including inhibition of marrow adiposity, a factor proposed to inhibit osteogenesis (59). Additionally, one of the established actions of IL-15 is inhibition of TNF-α signaling (6, 8). Because TNF-α has been implicated in bone loss, as well as muscle wasting and obesity-induced insulin resistance (13, 17, 57), it is possible that the actions of IL-15 on cachectic muscle, adipose tissue, and bone may be due to interference with TNF-α signaling. Confirmation of the effects of IL-15 on bone parameters and underlying mechanisms will require further investigation.
Three studies have identified partially overlapping SNPs in the human IL-15 gene, and particularly in the human IL-15Rα gene, which correlate with baseline muscle mass (52), the ability to build muscle by training (58), bone deposition (52), abdominal adiposity (16), and serum predictors of metabolic syndrome (52) in human subjects. The aforementioned SNPs identified in the IL-15 gene are located in the 5′- and 3′-regulatory regions, suggesting an effect on expression levels (52). Likewise, many of the reported polymorphisms in the human IL-15Rα gene that affect body composition are located at exon/intron borders (16, 52, 58). IL-15Rα has numerous mRNA splice variants, one of which encodes a soluble form that stabilizes IL-15, enhances IL-15 secretion in the circulation, and increases the biological activity of IL-15 (4, 6, 7, 61). The IL-15Rα SNPs may affect expression of the soluble IL-15Rα variant and thereby modulate IL-15 bioavailability and consequent effects on fat and muscle mass. These genetic correlations indicate that IL-15 expression and signaling are relevant to control of fat and lean mass in humans and are consistent with the results of the present study that show IL-15 must be released in the circulation to exert effects on muscle and bone tissue.
Many aspects of the control of IL-15 release and expression, its interactions with adipokines and classic endocrine hormones, as well as the mechanism of IL-15 action remain to be explored. Inasmuch as this study indicates that IL-15 functions as an authentic myokine with significant effects on body composition, further investigation of the role of IL-15 in muscle, adipose tissue, and possibly bone is warranted. IL-15 and other cytokines comprise part of the innate immune system, involved in the response to immune and environmental stress (20, 29). The reported actions of IL-15, such as mobilization of fatty acids from adipose depots, stimulation of glucose uptake and lipid oxidation by muscle, and stabilization of muscle protein, may function as a regulatory response to stress to facilitate utilization of fat for muscular energy and maintenance of muscle mass (1, 29, 56, 64). This pathway might therefore be pharmacologically exploitable to control body composition parameters in abnormal states such as obesity, sarcopenic obesity, or cachexia. Modulation of IL-15 secretion, stability, or signaling pathways might be explored as a therapeutic avenue for improving body composition and combating the associated decrements in health and quality of life.
GRANTS
This work was supported by National Research Initiative Competitive Grant no. 2005-35206-15264 from the United States Department of Agriculture Cooperative State Research, Education, and Extension Service Animal Growth and Nutrient Utilization Program to L. S. Quinn, National Institute on Aging (NIA) Grant RO1AG-024136 (L. S. Quinn), the Transgenic Resource Core of the University of Washington Nathan Shock Center of Excellence in the Basic Biology of Aging (NIA Grant 5P30AG-013280), the Molecular Genetics Core of the University of Washington Diabetes Endocrinology Research Center (National Institute of Diabetes and Digestive and Kidney Diseases Grant 5P30DK-17047), and by use of resources and facilities at the Veterans Affairs Puget Sound Health Care System, Seattle/Tacoma, WA.
Acknowledgments
We thank T. Fehniger (Ohio State University) for the IL2SP-IL15 construct, Immunex for the natLSP-IL-15 cDNA, and J. S. Chamberlain (University of Washington) for the HSA promoter, originally developed by E. Hardeman (Children's Hospital, Westmead, Australia).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ajuwon KM, Spurlock ME. Direct regulation of lipolysis by interleukin-15 in primary pig adipocytes. Am J Physiol Regul Integr Comp Physiol 287: R608–R611, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Almendro V, Busquets S, Ametller E, Carbó N, Figueras M, Fuster G, Argilés JM, López-Soriano FJ. Effects of interleukin-15 on lipid oxidation. Disposal of an oral [14C]-triolein load. Biochem Biophys Acta 1761: 37–42, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez B, Carbó N, López-Soriano J, Drivdahl RH, Busquets S, López-Soriano FJ, Argilés JM, Quinn LS. Effects of interleukin-15 (IL-15) on adipose tissue mass in rodent obesity models: evidence for direct IL-15 action on adipose tissue. Biochem Biophys Acta 1570: 33–37, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Bergamaschi C, Rosati M, Jalah R, Valentin A, Kulkarni V, Alicea C, Zhang GM, Patel V, Felber BK, Pavlakis GN. Intracellular interaction of interleukin-15 with its receptor α during production leads to mutual stabilization and increased bioactivity. J Biol Chem 283: 4189–4199, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Brennan KJ, Hardeman EC. Quantitative analysis of the human α-skeletal actin gene in transgenic mice. J Biol Chem 268: 719–725, 1993. [PubMed] [Google Scholar]
- 6.Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev 17: 259–280, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Bulanova E, Budagian V, Duitman E, Orinska Z, Krause H, Ruckert R, Reiling N, Bulfone-Paus S. Soluble interleukin-15Ralpha is generated by alternative splicing or proteolytic cleavage and forms functional complexes with IL-15. J Biol Chem 282: 13167–13179, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Bulfone-Paus S, Bulanova E, Pohl T, Budagian V, Durkop H, Ruckert R, Kunzendorf U, Paus R, Krause H. Death deflected: IL-15 inhibits TNF-α-mediated apoptosis in fibroblasts by TRAF2 recruitment to the IL-15Rα chain. FASEB J 13: 1575–1585, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Busquets S, Figueras MT, Meijsing S, Carbó N, Quinn LS, Almendro V, Argilés JM, López-Soriano FJ. Interleukin-15 decreases proteolysis in skeletal muscle: A direct effect. Int J Mol Med 16: 471–476, 2005. [PubMed] [Google Scholar]
- 10.Busquets S, Figueras M, Almendro V, López-Soriano FJ, Argilés JM. Interleukin-15 increases glucose uptake in skeletal muscle. An antidiabetogenic effect of the cytokine. Biochem Biophys Acta 1760: 1613–1617, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Carbó N, López-Soriano J, Costelli P, Busquets S, Alvarez B, Baccino FM, Quinn LS, López-Soriano FJ, Argilés JM. Interleukin-15 antagonizes muscle protein waste in tumour-bearing rats. Br J Cancer 83: 526–531, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carbó N, López-Soriano J, Costelli P, Alvarez B, Busquets S, Baccino FM, Quinn LS, López-Soriano FJ, Argilés JM. Interleukin-15 mediates reciprocal regulation of adipose and muscle mass: a potential role in body weight control. Biochem Biophys Acta 1526: 17–24, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Cauley JA, Danielson ME, Boudreau RM, Forest KYZ, Zmuda JM, Pahor M, Tylavsky FA, Cummings SR, Harris TB, Newman AB. Inflammatory markers and incident fracture risk in older men and women: the Health Aging and Body Composition study. J Bone Miner Res 22: 1088–1095, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Cesari M, Kritchevsky SB, Baumgartner RN, Atkinson HH, Penninx BWHJ, Lenchik L, Palla SL, Ambrosius WT, Tracy RP, Pahor M. Sarcopenia, obesity, and inflammation: results from the Trial of Angiotension Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr 82: 428–434, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Crawford GE, Faulkner JA, Crosbie RH, Campbell KP, Froehner SC, Chamberlain JS. Assembly of the dystrophin-associated protein complex does not require the dystrophin COOH-terminal domain. J Cell Biol 150: 1399–1410, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Renzo L, Bigioni M, Bottini FG, Del Grobbo V, Premrov MG, Cianci R, De Lorenzo A. Normal Weight Obese syndrome: role of single nucleotide polymorphism of IL-15Rα and MTHFR 677→T genes in the relationship between body composition and resting metabolic state. Eur Rev Med Pharm Sci 10: 235–245, 2006. [PubMed] [Google Scholar]
- 17.Dyck DJ, Heignehauser GJF, Bruce CR. The role of adipokines as regulators of skeletal muscle fatty acid metabolism and insulin sensitivity. Acta Physiol 186: 5–16, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Fehniger TA, Suzuki K, Ponnappan A, VanDeusen JB, Cooper MA, Florea SM, Freud AG, Robinison ML, Durbin J, Caliguiri MA. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J Exptl Med 193: 219–231, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Figueras M, Busquets S, Carbó N, Barreiro E, Almendro V, Argilés JM, López-Soriano FJ. Interleukin-15 is able to suppress the increased DNA fragmentation associated with muscle wasting in tumour-bearing rats. FEBS Lett 569: 201–206, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Frost RA, Lang CH. Skeletal muscle cytokines: regulation by pathogen-associated molecules and catabolic hormones. Curr Opin Clin Nutr Metab Care 8: 255–263, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Goodman MN Interleukin-6 induces skeletal muscle protein breakdown in rats. Proc Soc Exp Biol Med 205: 182–185, 1994. [DOI] [PubMed] [Google Scholar]
- 22.Gosset M, Berenbaum F, Salat C, Sautet A, Pigenet A, Tahiri K, Jacques C. Crucial role of visfatin/pre-B cell colony-enhancing factor in matrix degradation and prostaglandin E2 synthesis in chrondrocytes. Arthr Rheum 58: 1399–1409, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M, Johnson L, Alderson MR, Watson JD, Anderson DM, Giri J. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science 264: 965–968, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Harcourt LJ, Holmes AG, Gregorevic P, Schertzer JD, Stupka N, Plant DR, Lynch GS. Interleukin-15 administration improves diaphragm muscle pathology and function in dystrophic mdx mice. Am J Pathol 166: 1131–1141, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill JA, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science 299: 853–855, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Hoene M, Weigert C. The role of inteleukin-6 in insulin resistance, body fat distribution and energy balance. Obes Rev 9: 20–29, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Hotamisligil GS Inflammation and metabolic disorders. Nature 444: 860–867, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Huising MO, Kruiswijk CP, Flik G. Phylogeny and evolution of class-I helical cytokines. J Endocrinol 189: 1–25, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Jacobi SK, Gabler NK, Ajuwon KM, Davis JE, Spurlock ME. Adipocytes, myofibers, and cytokine biology: new horizons in the regulation of growth and body composition. J Anim Sci 84: E140–E149, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444: 840–846, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Kelley KW, Weigent DA, Kooijman R. Protein hormones and immunity. Brain Behav Immun 21: 384–392, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JCL, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin-15-deficient mice. J Exp Med 191: 771–780, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klover PJ, Clementi AH, Mooney RA. Interleukin-6 depletion selectively improves hepatic insulin action in obesity. Endocrinol 146: 3417–3427, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Kubota T, Brown RA, Fang J, Krueger JM. Interleukin-15 and interleukin-2 enhance non-REM sleep in rabbits. Am J Physiol Regul Integr Comp Physiol 281: R1004–R10012, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Lai KMV, Gonzalez M, Poueymirou WT, Kline WO, Na E, Zlotchenko E, Stitt TN, Economides AR, Yancopoulos GD, Glass DJ. Conditional activation of Akt in adult skeletal muscle induces rapid hypertrophy. Molec Cell Biol 24: 9295–9304, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lambert CP, Flynn MG, Sullivan DH, Evans WJ. Effects of megestrol acetate on circulating interleukin-15 and interleukin-18 concentrations in healthy elderly men. J Gerontol 59A: 855–858, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Landt M, Gingerich RL, Havel PJ, Mueller WM, Schoner B, Hale JE, Heiman ML. Radioimmunoassay of rat leptin: sexual dimorphism reversed from humans. Clin Chem 44: 565–570, 1998. [PubMed] [Google Scholar]
- 38.Lebrun CEI, van der Schouw YT, de Jong FH, Grobbee DE, Lamberts SW. Fat mass rather than muscle strength is the major determinant of physical function and disability in postmenopausal women younger than 75 years of age. Menopause 13: 474–481, 2006. [DOI] [PubMed] [Google Scholar]
-
39.Livak K, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the
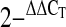 method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar] - 40.Lollini PL, Palmieri G, De Giovanni C, Landuzzi L, Nicoletti G, Rossi I, Griffoni C, Frabetti F, Scotland K, Benini S, Baldini N, Santoni A, Nanni P. Expression of interleukin 15 (IL-15) in human rhabdomyosarcoma, osteosarcoma and Ewing's sarcoma. Intl J Cancer 71: 732–736, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Maynes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obesity Rev 5: 197–216, 2004. [DOI] [PubMed] [Google Scholar]
- 42.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 387: 83–90, 1997. [DOI] [PubMed] [Google Scholar]
- 43.McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA 94: 12457–12461, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest 109: 595–601, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagy TR, Clair AL. Precision and accuracy of dual-enery X-ray absorptiometry for determining in vivo body composition of mice. Obes Res 8: 392–398, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Nielsen AR, Mounier R, Plomgaard P, Mortensen OH, Penkowa M, Speerschneider T, Pilegaard H, Pedersen BK. Expression of interleukin-15 in human skeletal muscle: effect of exercise and muscle fibre type composition. J Physiol 584: 305–312, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishimura H, Fujimoto A, Tamura N, Yajima T, Wajjwalku W, Yoshikai Y. A novel autoregulatory mechanism for transcriptional activation of the IL-15 gene by a nonsecretable isoform of IL-15 generated by alternative splicing. FASEB J 19: 19–28, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Otero M, Lago R, Lago F, Casanueva FF, Dieguez C, Gómez-Reino JJ, Gualillo O. Leptin, from fat to inflammation: old questions and new insights. FEBS Lett 579: 295–301, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol 103: 1093–1098, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Pedersen BK, Fischer CP. Physiological roles of muscle-derived interleukin-6 in response to exercise. Curr Opin Clin Nutr Metab Care 10: 265–271, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Pischon T, Nothlings U, Boeing H. Obesity and cancer. Proc Nutr Soc 67: 128–145, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Pistilli EE, Devaney JM, Gordish-Dressman H, Bradbury MK, Seip RL, Thompson PD, Angelopoulos TJ, Clarkson PM, Moyna NM, Pescatello LS, Visich PS, Zoeller RF, Gordon PM, Hoffman EP. Interleukin-15 and insterleukin-15Ralpha SNPs and associations with muscle, bone, and predictors of the metabolic syndrome. Cytokine doi: 10.1016/j.cyto.2008.04.008.PMID18514540.2008. [DOI] [PMC free article] [PubMed]
- 53.Prinz M, Hanisch UK, Kettenmann H, Kirchohoff F. Alternative splicing of mouse IL-15 is due to the use of an internal splice site in exon 5. Mol Brain Res 63: 155–162, 1998. [DOI] [PubMed] [Google Scholar]
- 54.Quinn LS, Anderson BG, Drivdahl RH, Alvarez B, Argilés JM. Overexpression of interleukin-15 induces skeletal muscle hypertrophy in vitro: implications for treatment of muscle wasting disorders. Exp Cell Res 280: 55–63, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Quinn LS, Strait-Bodey L, Anderson BG, Argilés JM, Havel PJ. Interleukin-15 stimulates adiponectin secretion by 3T3-L1 adipocytes: evidence for a skeletal muscle-to-fat signaling pathway. Cell Biol Intl 29: 449–457, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Quinn LS Interleukin-15: a muscle-derived cytokine regulating fat-to-lean body composition. J Anim Sci 86: E75–E83, 2008. [DOI] [PubMed] [Google Scholar]
- 57.Reid MB, Li YP. Tumor necrosis factor-alpha and muscle wasting: a cellular perspective. Respir Res 2: 269–272, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Riechman SE, Balasekaran G, Roth SM, Ferrell RE. Association of interleukin-15 protein and interleukin-15 receptor genetic variation with resistance exercise training responses. J Appl Physiol 97: 2214–2219, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Rosen C, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol 2: 35–43, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444: 847–853, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schluns KS, Stoklasek T, Lefrançois L. The roles of interleukin-15 receptor α: trans-presentation, receptor component, or both? Intl J Biochem Cell Biol 37: 1567–1571, 2005. [DOI] [PubMed] [Google Scholar]
- 62.Serrano AL, Baeza-Raja B, Perdiguero E, Jardí M, Munoz-Cánoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 7: 33–44, 2008. [DOI] [PubMed] [Google Scholar]
- 63.Sugiura T, Harigai M, Kawaguchi Y, Takagi K, Fukasawa C, Ohsako-Higami S, Ohta S, Tanaka M, Masako H, Kamatani N. Increased IL-15 production of muscle cells in polymyositis and dermatomyositis. Intl Immunol 14: 917–924, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Sun X, Zemel MB. Leucine and calcium regulate fat metabolism and energy partitioning in murine adipocytes and muscle cells. Lipids 42: 297–305, 2007. [DOI] [PubMed] [Google Scholar]
- 65.Tagaya Y, Bamford RN, DeFilippis AP, Waldmann TA. IL-15: A pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity 4: 329–336, 1996. [DOI] [PubMed] [Google Scholar]
- 66.Tagaya Y, Kurys G, Thies TA, Losi JM, Azimi N, Hanover JA, Bamford RN, Waldmann TA. Generation of secretable and nonsecretable interleukin 15 isoforms through alternate usage of signal peptides. Proc Natl Acad Sci USA 94: 14444–14449, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tinsley FC, Taicher GZ, Heiman ML. Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis. Obesity Res 12: 150–160, 2004. [DOI] [PubMed] [Google Scholar]
- 68.Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocrine Rev 27: 762–778, 2006. [DOI] [PubMed] [Google Scholar]
- 69.Tsujinaka T, Ebisui C, Fujita J, Kishibuchi M, Morimoto T, Ogawa A, Katsume A, Ohsugi Y, Kominami E, Monden M. Muscle undergoes atrophy in association with increase of lysosomal cathepsin activity in interleukin-6 transgenic mouse. Biochem Biophys Res Commun 207: 168–174, 1995. [DOI] [PubMed] [Google Scholar]
- 70.Van Gaal LF, Mertens IL, De Block. Mechanisms linking obesity with cardiovascular disease. Nature 444: 875–880, 2006. [DOI] [PubMed] [Google Scholar]
- 71.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou Sole J CJ, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang J, Zhao B. Postnatal expression of myostatin propeptide cDNA maintained high muscle growth and normal adipose tissue mass in transgenic mice fed a high-fat diet. Mol Reprod Dev 73: 462–469, 2006. [DOI] [PubMed] [Google Scholar]
- 74.Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ. Induction of cachexia in mice by systemically administered myostatin. Science 296: 1486–1488, 2002. [DOI] [PubMed] [Google Scholar]