Abstract
TAK1 binding protein1 (TAB1) binds and induces autophosphrylation of TGF-β activating kinase (TAK1). TAK1, a mitogen activated kinase kinase kinase, is involving in several distinct signaling pathways including non-Smad pathways for TGF-β superfamily members and inflammatory responses caused by cytokines. Conventional disruption of the murine Tab1 gene results in late gestation lethality showing intraventricular septum defects and under developed lung alveoli. To gain a better understanding of the roles of TAB1 in different tissues at different stages of development and in pathological conditions, we generated Tab1 floxed mice in which loxP sites flank exons 9 and 10 to remove the C-terminal region of TAB1 protein necessary for activation of TAK1. We demonstrate that Cre-mediated recombination using Sox2-Cre, a Cre line expressed in the epiblast during early embryogenesis, results in deletion of the gene and protein. These homozygous Cre-recombined null embryos display an identical phenotype to conventional null embryos. This animal model will be useful to reveal distinct roles of TAB1 in different tissues at different stages.
Introduction
The transforming growth factor-beta (TGF-β) superfamily members including bone morphogenetic proteins (BMPs), growth and differentiation factors (GDFs), nodal /lefties, activins, and Mullerian inhibitory substance, exert a wide range of functions during early patterning of embryos, organogenesis, and physiological regulations after birth (de Caestecker, 2004; Kishigami and Mishina, 2005). Numerous reports indicate that Smad proteins are involving in the signaling pathway activated by members of the TGF-β superfamily (Miyazono et al., 2005; Whitman, 1998). In addition to the canonical signaling pathway mediated by Smad proteins, it is shown that mitogen-activated protein (MAP) kinase pathways are involved in the TGF-β superfamily signaling pathways (Aubin et al., 2004; Kretzschmar et al., 1997). The MAP kinase pathway is a conserved eukaryotic signaling module regulated through a three-tiered kinase cascade composed of MAP kinase kinase kinase (MAPKKK), MAP kinase kinase (MAPKK), and MAP kinase (MAPK) (Johnson and Lapadat, 2002; Pearson et al., 2001).
TGF-β Activation Kinase 1 (TAK1) was originally found as a MAPKKK of which activity is upregulated by TGF-β treatment (Yamaguchi et al., 1995). TAK1 participates in the non-Smad pathway by activating p38 and SnoN degradation (Hanafusa et al., 1999; Kajino et al., 2007). Besides the TGF-β signaling pathway, TAK1 is involved in several innate immune signaling pathways including cytokines TNF and IL-1, Toll-like receptors and intracellular bacterial sensor NOD-like receptor NOD1/2 pathways (Hasegawa et al., 2008; Kim et al., 2008; Ninomiya-Tsuji et al., 1999; Shim et al., 2005; Takaesu et al., 2003). The innate stimuli-activated TAK1 also induces activation of transcription factor AP-1 through MAPKs such as c-Jun N-terminal kinase (JNK) and p38. It has been reported that TAK1 is critically involved in stress-activated cell signaling (Chen et al., 2002; Singhirunnusorn et al., 2005) including osmotic stress-induced JNK activation (Huangfu et al., 2006).
Using TAK1 protein as a bait, TAK1 binding protein 1 (TAB1, Map3k7ip1) (Shibuya et al., 1996) and TAK1 binding protein 2/3 (TAB2/3) (Ishitani et al., 2003; Takaesu et al., 2000) are previously identified. TAB2 and its homolog TAB3 function as an adaptor tethering TAK1 to the IKK complex for ubiquitination (Kanayama et al., 2004; Kishida et al., 2005). In cultured cells, TAB1 is constitutively associated with endogenous TAK1 (Kishimoto et al., 2000). TAB1 induces TAK1 autophosphorylation and thereby catalytically activates TAK1 kinase activity in vitro (Kishimoto et al., 2000). Among a total of 502 amino acid residues of TAB1, only 16 residues in the C-terminal region (480-495 aa.) are essential for TAK1-TAB1 binding, and 68 residues (437-504 aa.) are sufficient for the autophosphorylation/activation of TAK1 following the binding (Ono et al., 2001). It was demonstrated that the TAK1-TAB1 complex interacts with BMP receptors via X-linked inhibitor of apoptosis protein (IAP), supporting further the idea that TAK1-TAB1 is involved in mediating BMP signaling as a non-Smad pathway (Yamaguchi et al., 1999).
TAB1 is encoded by 11 exons on chromosome 15 in mice. Previously, Tab1-deficient mice were generated by deleting exon 9 and 10 of Tab1, which encode the C-terminal region of TAB1 (308-434 aa.) (Komatsu et al., 2002). An alternative splicing between exon 8 and 11, if present, causes flameshift and the aforementioned domain necessary for TAK1 binding and subsequent activation is not produced (Komatsu et al., 2002; Ono et al., 2001). Therefore, TAB1 protein without the C-terminus (TAB1ΔC), if present in the homozygous mutants, should be functionally inactive regarding TAK1-mediated signaling pathways. Homozygous mice for the Tab1-deficient allele were embryonic lethal with several developmental dysregulations including failure of cardiovascular morphogenesis (Komatsu et al., 2002). Until mid gestation, Tab1 mutant embryos develop without overt abnormalities, however, they start to show a bloated appearance as early as E15.5 and die at term (Komatsu et al., 2002). Histological observation reveal that thinner ventricular wall and defects in intra ventricular septum formation in the heart as well as degeneration of terminal bud epithelium and failure of expansion of alveoli in the lung (Komatsu et al., 2002). It is notable that Id2 expression in the lung epithelium is downregulated in the mutant embryos and response to TGF-β treatment is impaired in the Tab1 deficient mouse embryonic fibroblasts (MEFs) suggesting the involvement of TAB1 in TGF-β and BMP pathways (Komatsu et al., 2002).
It is an interesting contrast that Tak1 conventional knockout embryos are lethal by E9.5 (Jadrich et al., 2006; Sato et al., 2005; Shim et al., 2005), whereas Tab1 knockout embryos are grossly normal by embryonic day 14.5 (E14.5) (Komatsu et al., 2002). These facts prompted us to speculate that TAB1 is essential for some, but not all, of the TAK1-mediated signaling pathways. Indeed, studies using TAB1 deficient MEFs reported that TNF-, and IL-1-induced activation of NF-κB, JNK and p38 are intact in TAB1-deficient MEFs (Shim et al., 2005).
Cell type-specific gene ablation by Cre-loxP technology is particularly useful for determining the distinct tissue-specific function of a gene that is lethal when deleted (Kwan, 2002). To overcome the perinatal lethality occurring with the conventional deletion of the Tab1 locus, and elucidate further the functions of TAB1 in BMP signaling pathways and other cytokine signaling pathways in adult mice, we generated a conditional allele of Tab1 using Cre-loxP technology.
Results and Discussion
Generation of a universal vector for conditional gene targeting
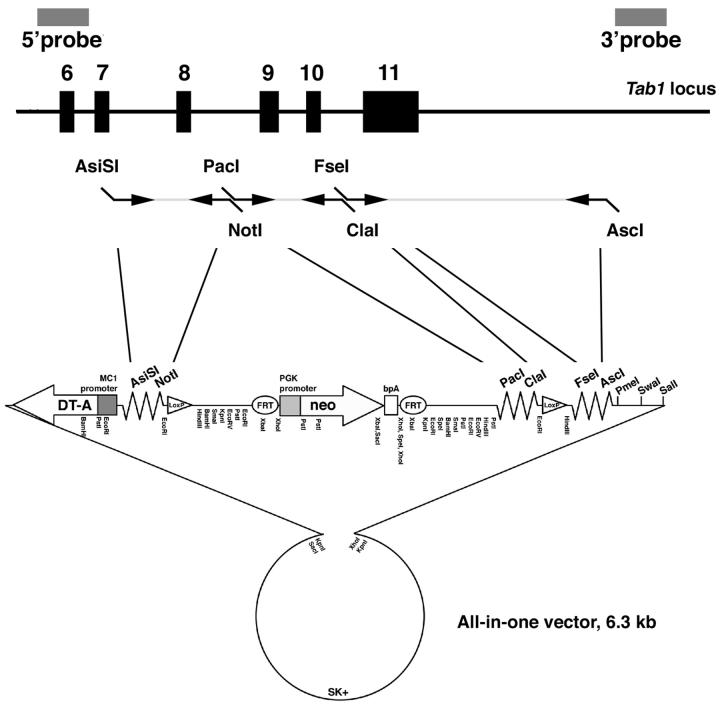
To facilitate vector construction for gene targeting to generate a conditional allele, first we prepared a vector which allows for directional cloning of PCR amplified genomic fragments. A Pgk-neo selection cassette was flanked by FRT sites (Dymecki, 1996) and two loxP sites were placed outside of the FRT-Pgk-neo-FRT cassette. A MC-DTA cassette (Yagi et al., 1993) was also included for negative selection. Multicloning sites for a series of rare-cutting enzymes were added in 3 places; between the DTA cassette and one of the loxP site, the FRT-Pgk-neo-FRT cassette and the other loxP site, and the other loxP site and the vector backbone (Fig. 1). These multicloning sites allow introducing genomic PCR products amplified with primers having corresponding enzyme recognition sequences at the ends of each primer. Each multicloning site has at least 2 cut sites that allow directional cloning, if applicable. Furthermore, we added three more rare cutter sites for linearizing targeting vectors prior to electroporation to ES cells. We named this all-in-one type vector the Multiple Amplicon Insertion Knock Out (MAIKO) vector.
Figure 1. Structure of the all-in-one type Multiple Amplicon Insertion Knock Out (MAIKO) vector.
A Pgk-neo cassette flanked by FRT sites for positive selection, a MC1-DTA cassette for negative selection, two loxP sites were produced with 3 rare cutter multicloning sites with. These configurations allow directional cloning of PCR amplified genomic fragments for both homologous arms and a floxed region. Bold indicates unique enzyme sites. For this study, genomic DNA was PCR amplified with primers designed to produce the enzyme sites indicated, and inserted into multicloning sites as shown.
Generation of the conditional allele of Tab1
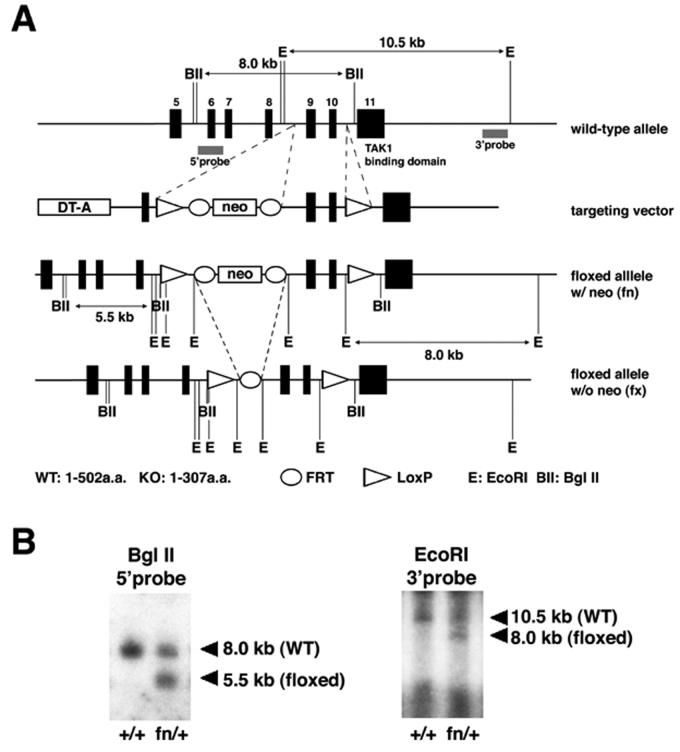
Our targeting vector was designed to introduce one loxP site and an FRT-Pgkneo-FRT cassette to intron 8 and the other loxP to intron 10 (Fig. 1 and 2). By this way, exon 9 and 10, the same exons we previously deleted by a conventional gene knockout method, were flanked by the loxP sites. Cre-dependent DNA recombination results in the deletion of the C-terminus of the TAB1 protein essential for TAK1 binding and subsequent activation (Ono et al., 2001). Therefore, the protein product from the Cre-recombined allele, if present, should be functionally inactive regarding TAK1-mediated signaling pathways. After positive and negative selection with G418 and DT-A, correctly targeted ES cell clones were screened by Southern blot analysis (Fig. 2). Targeted ES clones were injected into blastocysts for germline transmission. Note that we used FRT sites to flank the neo cassette for an option to remove the neo gene with Flp recombinase.
Figure 2. Targeted recombination of ES cells for Tab1 locus.
A. Genomic structure of Tab1 locus and the targeting construct. A loxP site followed by Pgk-neo cassette flanked by FRT sites was inserted into intron 8. Another loxP site with an EcoRI site was inserted into intron 10. Positions of 5′ and 3′ external probes for Southern analyses and the sizes of the restriction fragments detected by these probes are shown. BII, BglII; E, EcoRI.
B. Confirmation of the targeting event in ES cells. Genomic DNA from WT ES cells (left lane, +/+) and correctly targeted cells (right lane, fn/+) were digested with enzymes and blots were hybridized with probes as indicated. EcoRI digestion and use of the 3′ probe confirmed the presence of the loxP site in intron 10.
Confirmation of deletion of Tab1 allele using Cre-loxP approach
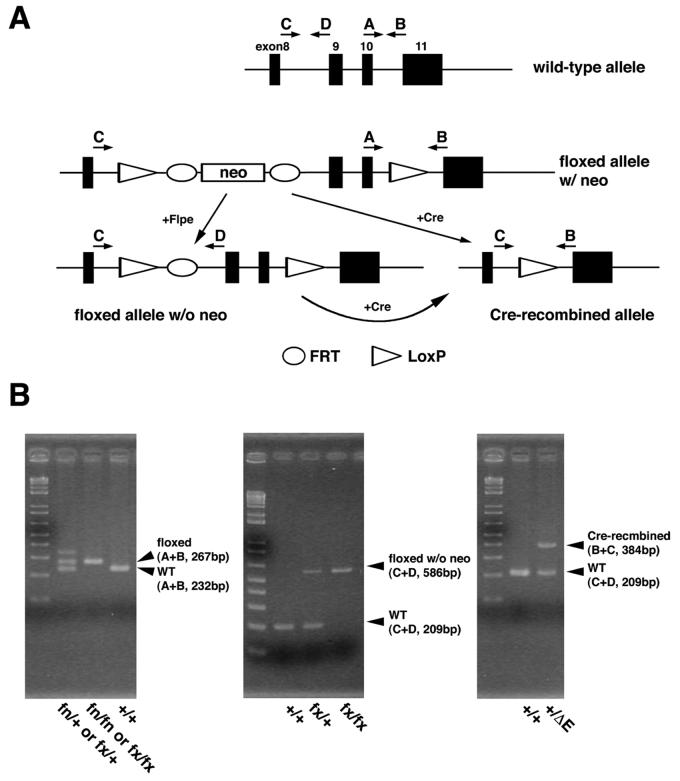
Correct targeting events were confirmed by Southern blotting using 5′ and 3′ external probes, and the presence of the 5′ loxP site was confirmed by the 3′ external probe for the presence of an 8 kb band with EcoRI digestion (Fig. 2A,B). The targeted allele was designated as the floxP+neo (fn) allele. After confirmation of germline transmission, F1 offspring heterozygous for fn were intercrossed to generate mice homozygous for fn. The resulting F2 showed the expected ratio (+/+:fn/+:fn/fn = 22:55:20, 12 litters) of offspring. In addition, F1 offspring heterozygous for fn were bred with ROSA26-Flp mice, containing FLP recombinase, to remove the neo cassette through recombination at the FRT site. Removal of the neo was confirmed by PCR (Fig. 3). The targeted allele without the neo cassette was designated floxP (fx) allele. Intercrosses between mice heterozygous for the fx allele generated the expected ratio of the mice homozygous for fx (+/+:fx/+:fx/fx = 10:20:12). To verify whether the loxP sites are functional, mice heterozygous for fn were bred with Sox2-Cre transgenic mice, producing Cre in the epiblast cells, to remove the floxed exon 9 and 10. Offspring were analyzed by PCR confirmation of Cre-dependent deletion as well as the efficiency of Cre recombination of the floxed allele in vivo (Fig. 3). The Cre-recombined allele was designated as ΔE allele.
Figure 3. PCR strategy and genotyping.
A. Schematic representation of structure of each allele and positions of primers. Primers A and B are used to differentiate the conditional allele from WT, whereas primers C and D are used to differentiate the fx allele from fn. Primers B and C are used to identify the Cre-recombined allele.
B. Genotyping results of the conditional allele. Combinations of the primers and sizes of the amplicons are indicated.
Next we examined the functionality of the conditional (fn and fx) alleles by generating heterozygous mice for fn or fx allele over the Cre-recombined allele. Breeding between heterozygous mice for the Cre-recombined allele (ΔE/+) and the allele homozygous for fx (fx/fx) resulted in an expected ratio of pups at the weaning age (fx/+:fx/ΔE = 19:25, 6 litters). However, we failed to get fn/ΔE mice at weaning stage from the breeding between heterozygous mice for Cre-recombined allele (ΔE/+) and homozygous allele for fn (fn/fn) (fn/+:fn/ΔE = 18:0, 4 litters). These results suggest that the presence of the neo cassette in the Tab1 locus makes the gene hypomorphic. Indeed, expression levels of TAB1 from the fn allele is much lower than that from the wild-type allele and the fx allele in keratinocytes isolated from newborn skin (supplemental figure 1). Phenotypic analyses of the fn/ΔE embryos would be informative if they showed less severe phenotypes than these found in previously reported conventional knockout embryos and homozygous mice for Cre-recombined allele (see below).
Similarities of embryonic phenotype in both the conventional Tab1 null and the Cre-recombined Tab1 mice
Mice that were heterozygous for the Cre-recombined allele were intercrossed to obtain mice homozygous for Cre-recombined Tab1 allele (ΔE/ΔE). The phenotype obtained from this cross was compared to that in the conventional Tab1 null mice previously reported (Komatsu et al., 2002).
As is evident in Fig. 4, homozygous mice for the Cre-recombined allele developed similar developmental morphological abnormalities at E16.5 similar to abnormalities of conventional Tab1 null embryos, including edema and hemorrhage (Fig.4 A, B). Western blot using an antibody against C-terminal region of TAB1 failed to detect presence of TAB1 in extracts from ΔE/ΔE embryos (Fig. 4C). Histological examination revealed that embryos homozygous for the Cre recombined allele developed similar intraventricular septum defects (VSD) (Fig. 4D-F) and failure of expansion of alveoli in the lung (Fig. 4G-L) found in embryos homozygous for conventionally targeted allele.
Figure 4. Homozygous embryos for Cre-recombined Tab1 allele show similar developmental abnormalities to those of conventional knockout embryos.
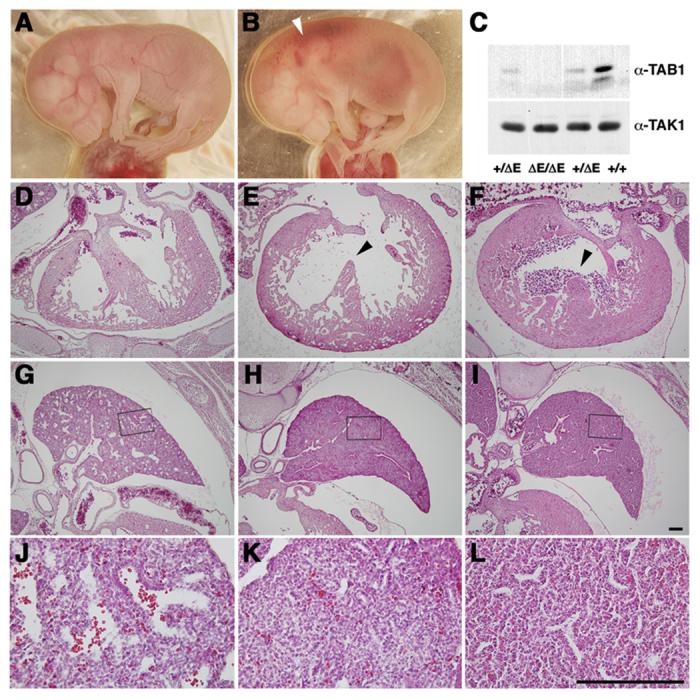
A-B. Whole view of embryos Wild-type (WT) and homozygous for Cre-recombined Tab1 embryos (ΔE/ΔE) at E16.5. Tab1 ΔE/ΔE embryo shows edema and hemorrhage (white arrowhead).
C. Brain extracts from E16.5 embryos were subjected to western blotting using an anti-TAB1 antibody that recognizes the C-terminus of TAB1. TAK1 is shown as a loading control.
D-L. H&E staining of heart (D-F) and lung (G-L) sections from WT embryos (D, G, J), Tab1 ΔE/ΔE embryos (E, H, K), and conventional Tab1-deficient homozygous embryos (−/−) (F, I, L) at E16.5. J-K are higher magnification of G-I (boxed areas). Tab1 ΔE/ΔE embryo shows ventricular septum defects (arrowheads) and under expanded lung alveoli that are similar to these found in −/− embryos. Scale bar (D-L) = 200μm.
It has been reported that TAB1 is involved in BMP signaling for mesoderm induction in Xenopus (Shibuya et al., 1998) and epithelial-mesenchymal interaction of lung morphogenesis in mouse embryos (Komatsu et al., 2002). To further elucidate the involvement of TAB1 in BMP signaling pathways, we examined the expression pattern of Id1 in intestine. Id genes are putative targets of BMP signals (Hollnagel et al., 1999). It is reported that Bmp2 and Bmp4 are expressed in intestinal mesenchyme (Karlsson et al., 2000). Especially, Id1 is known as a target gene of BMP4 expressing in mesenchymal tissues of intestine. Thus, we hypothesized that Id1 gene function may be aberrant in the intestinal mesenchyme of Tab1 mutant embryos. As expected, expression of Id1 in mesenchymal components of intestine was severely reduced in the Tab1 conventional null embryos at E16.5 (Fig. 5A). In addition, Id1 expression level was upregulated by treatment of control MEF cells with BMP4 at 10 ng/ml and 20 ng/ml (Fig. 5B and data not shown), whereas expression of Id1 was not changed when conventional Tab1 null MEFs were treated with BMP4 (Fig. 5B). These results suggest that TAB1/TAK1 is involving in BMP signaling pathways to regulate downstream target genes such as Id1.
Figure 5. TAB1 is involving in BMP signaling cascade.
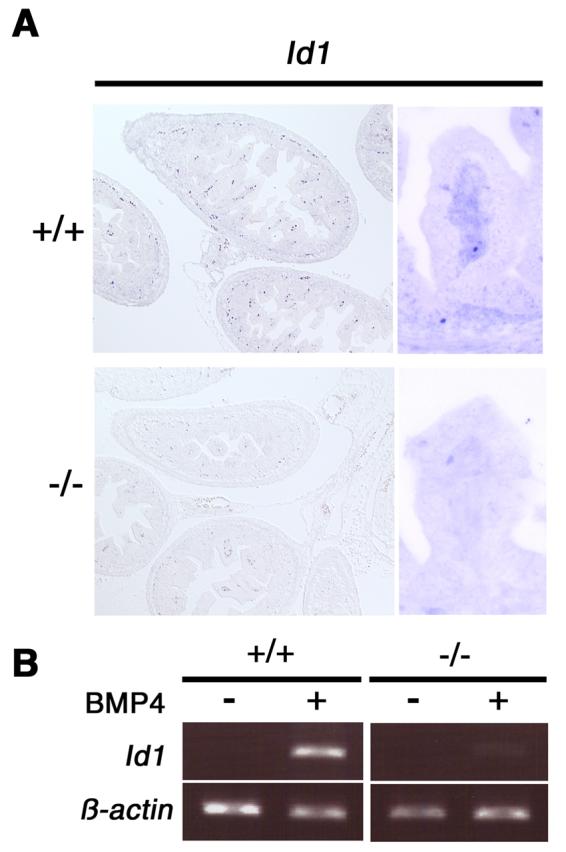
A. Expression analysis of Id1 in Tab1 mutants at E16.5. Id1 expression levels were downregulated in mesenchymal regions of intestine in Tab1 mutant embryos.
B. Wild type and Tab1-deficient (−/−) MEFs were cultured with BMP4 and Id1 expression was determined by RT-PCR. An increase in Id1 expression levels was observed in BMP4-treated control MEFs, however there was no induction of Id1 expression in Tab1-deficient (−/−) MEFs after stimulated by BMP4. β-actin was used as an internal control.
It is a formal possibility that a transcript from exon 1 to exon 8 is present and the N-terminal region of TAB1 encoded by these exons is produced. Indeed, we were able to detect such transcripts by RT-PCR in embryos homozygous for the Cre-recombined allele as well as in embryos homozygous for the conventionally targeted allele (supplemental figure 2 and data not shown, respectively). Unfortunately, the only TAB1 antibody available recognizes the C-terminal region and not the N-terminus of TAB1. However, as mentioned above, domains necessary for TAK1 binding and subsequent activation are located in the C-terminus of TAB1 (Ono et al., 2001) encoded by exon 11, and removal of exon 9 and 10 by Cre-dependent recombination causes a frame shift that prevents expression of a functional C-terminal domain (Fig. 4C). Therefore, it is likely that the floxed mouse line present here can be applied to inactive TAK1-mediated signaling pathways in a tissue-specific manner.
In conclusion, we have generated a conditional Tab1 allele and shown that homozygous general deletion of exon 9 and 10 most likely leads to absence of C-terminal region of the TAB1 protein that is essential for binding to TAK1 for its activation, accompanied by embryonic lethality exemplified as VSD. In the future, crossing this floxed line with transgenic mice that express Cre-recombinase in a tissue-specific manner will facilitate studies determining the distinct roles of TAB1 in different tissues where it is normally expressed, and allow determination of relative importance in different signaling pathways mediated by TAK1.
MATERIALS AND METHODS
Generation of a Tab1-floxed allele
A 3.6-kb fragment containing exon 8 was PCR amplified from 129SvEv genomic DNA with Phusion polymerase (New England Biolabs, Inc. MA) using the following primers; 5′-GTGgcgatcgcCTACAGTGGCAGGGTTACCATGGA-3′ and 5′-GTGgcggccgcGCTGTTCAGatCtATCCTTCTGGAACGCACTGA-3′. ASiSl and NotI sites are shown in lower case. A 1.7-kb fragment containing exon 9 and 10 was also PCR amplified using the following primers; 5′- GCGttaattaaCTCAGGTTGCAATTGACTGGCAGA-3′ and 5′-CTCatcgatGAATTCCCACCTTGCCCTGAATCTCCACTT-3′. Pacl and ClaI sites are shown in lower case. A 5.8-kb fragment containing exon 11 was PCR amplified using the following primers; 5′-GTGggccggccCTTTCATCCATGGGCAGCTTCTCT-3′ and 5′-GTGggcgcgccGAGAGGTTGGCTCTAGTTTGTGCT-3′. Fsel and AscI sites are shown in lower case. After amplification, these fragments were digested with restriction enzymes and ligated into the corresponding sites in the MAIKO vector (Fig. 1). The positions of the probes used for Southern analysis are shown in Fig. 2A. The sizes of the restriction fragments detected by these probes in WT and targeted DNA are shown above or below the locus. A 3′ loxP site is marked with an EcoRI site.
Linearized targeting vector was electroporated into 1.0 × 107 AB2.2 ES cells (Lexicon Genetics) and 1.6 × 107 clone A3 of UG347 ES cells, which we recently established from 129SvEv blastocysts. Three hundred G418-resistant ES cell clones from each cell line, a total of 600 clones, were initially screened by Southern blot and 33 correctly targeted ES cell clones were identified. Twenty-four of them were confirmed to possess the loxP site in intron 10 (fn/+). The ratio of these two types of clones obtained without or with the loxP site is close to 1:3 presumably is because the loxP site in intron 10 divides the right homologous arm in about a 1:3 ratio (Cheah et al., 2000). The targeted ES clones were injected into blastocysts from C57BL/6 albino mice. The resulting chimeras were bred to C57BL/6 females and F1 agouti offspring were genotyped by Southern analyses. Five targeted clones were used for injection and two of them underwent germline transmission.
Subsequently, mice heterozygous for Tab1 floxed allele with a Pgk-neo cassette (fn/+) were bred with Flipper mice (Farley et al., 2000) to remove the neo cassette (fx/+). The fn/+ mice also were bred with Sox2-Cre mice (Hayashi et al., 2002) to delete exon 9 and 10 to generate a recombined null allele (ΔE/+).
All animal experiments were conducted according to the U.S. Public Health Service policy on the humane care and use of animals. All animal procedures used in this study were approved by the National Institute of Environmental Health Sciences Institutional Animal Care and Use Committee.
Genotype analyses
Genotypes were determined by Southern blot with 5′ and 3′ external probes as shown in Fig. 2A. For PCR analyses (Fig. 3), primers A and B were used to amplify fragments from wildtype (232 bp) and the Tab1 floxed alleles (267 bp for both fn and fx). Primers C and D were also used to amplify fragments from wildtype (209 bp) and Tab1 floxed allele after removal of the neo cassette (588 bp, fx). Primers B and C were used to detect Cre-dependent deletion of the floxed region (384 bp for ΔE). Primers; A (5′-CCAATTCTCCACCCTCACCTT-3′), B (5′-CTACAGATGCATGAAGCCAGT-3′), C (5′-GTAACCTTGTACCCGTGAGTT-3′), D (5′- GCTGTGTAGGAGACTTAGAGA-3′).
In situ hybridization, Western blot, MEF culture, isolation of keratinocytes
Section in situ hybridization was performed by using standard procedures (Komatsu et al., 2002) with a digoxigenin-labeled RNA probes for Id1 (kindly provided by Dr. Hal Weintraub). Tab1 MEF cells were established from E15.5 homozygous Tab1 mouse embryos. Tab1 MEF cells were grown in Dulbecco's modified Eagle's medium (DMEM) with 10 % fetal bovine serum. Keratinocytes derived from the skin of newborn mice were cultured in Eagle's minimal essential medium supplemented with 4% Chelex-treated bovine growth serum, 10 ug/mL human epidermal growth factor (Invitrogen), and 0.05 mM calcium chloride (Omori et al., JBC). Recombinant human BMP4 was purchased from R&D. Whole protein lysates was extracted from E16.5 embryos and subjected to Western blot using polyclonal antibodies against TAK1 and TAB1 described previously (Ninomiya-Tsuji et al., 1999).
Reverse-transcription PCR
Total RNA was prepared from MEFs using the RNeasy protect mini-kit (Qiagen). cDNA was synthesized using TaqMan reverse transcription reagents (Applied Biosystems). PCR analysis was performed using the following primers: Tab1 N-terminus specific primers, 5′-CCACTCTGTCACCTCTCTGGA-3′ (located in exon 2) and 5′-CAGTGTGCTCAGTGTTGAGCT-3′ (located in exon 3), which produce a 232 bp fragment; Tab1 C-terminus specific primers, 5′-GGCTGTGGTAGACCGTGTAAA-3′ and 5′-GTGCTCTGGGCACTTGAGTAG-3′ (both located in exon 9), which produce a 210 bp fragment.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the Intramural Research Program of the NIEHS, NIH to Y. M. and by a grant from NIH to J. N-T. Y. K. is supported, in part, by a fellowship from the Japan Society for the Promotion of Science.
REFERENCES
- Aubin J, Davy A, Soriano P. In vivo convergence of BMP and MAPK signaling pathways: impact of differential Smad1 phosphorylation on development and homeostasis. Genes Dev. 2004;18:1482–1494. doi: 10.1101/gad.1202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah SS, Kwan KM, Behringer RR. Requirement of LIM domains for LIM1 function in mouse head development. Genesis. 2000;27:12–21. doi: 10.1002/1526-968x(200005)27:1<12::aid-gene30>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Chen W, White MA, Cobb MH. Stimulus-specific requirements for MAP3 kinases in activating the JNK pathway. J Biol Chem. 2002;277:49105–49110. doi: 10.1074/jbc.M204934200. [DOI] [PubMed] [Google Scholar]
- de Caestecker M. The transforming growth factor-beta superfamily of receptors. Cytokine Growth Factor Rev. 2004;15:1–11. doi: 10.1016/j.cytogfr.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Dymecki SM. A modular set of Flp, FRT and lacZ fusion vectors for manipulating genes by site-specific recombination. Gene. 1996;171:197–201. doi: 10.1016/0378-1119(96)00035-2. [DOI] [PubMed] [Google Scholar]
- Farley FW, Soriano P, Steffen LS, Dymecki SM. Widespread recombinase expression using FLPeR (flipper) mice. Genesis. 2000;28:106–110. [PubMed] [Google Scholar]
- Hanafusa H, Ninomiya-Tsuji J, Masuyama N, Nishita M, Fujisawa J, Shibuya H, Matsumoto K, Nishida E. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-beta-induced gene expression. J Biol Chem. 1999;274:27161–27167. doi: 10.1074/jbc.274.38.27161. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Fujimoto Y, Lucas PC, Nakano H, Fukase K, Nunez G, Inohara N. A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-kappaB activation. Embo J. 2008;27:373–383. doi: 10.1038/sj.emboj.7601962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, Lewis P, Pevny L, McMahon AP. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Gene Expr Patterns. 2002;2:93–97. doi: 10.1016/s0925-4773(02)00292-7. [DOI] [PubMed] [Google Scholar]
- Hollnagel A, Oehlmann V, Heymer J, Rüther U, Nordheim A. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem. 1999;274:19838–19845. doi: 10.1074/jbc.274.28.19838. [DOI] [PubMed] [Google Scholar]
- Huangfu WC, Omori E, Akira S, Matsumoto K, Ninomiya-Tsuji J. Osmotic stress activates the TAK1-JNK pathway while blocking TAK1-mediated NF-kappaB activation: TAO2 regulates TAK1 pathways. J Biol Chem. 2006;281:28802–28810. doi: 10.1074/jbc.M603627200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani T, Takaesu G, Ninomiya-Tsuji J, Shibuya H, Gaynor RB, Matsumoto K. Role of the TAB2-related protein TAB3 in IL-1 and TNF signaling. Embo J. 2003;22:6277–6288. doi: 10.1093/emboj/cdg605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadrich JL, O'Connor MB, Coucouvanis E. The TGF beta activated kinase TAK1 regulates vascular development in vivo. Development. 2006;133:1529–1541. doi: 10.1242/dev.02333. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Kajino T, Omori E, Ishii S, Matsumoto K, Ninomiya-Tsuji J. TAK1 MAPK kinase kinase mediates transforming growth factor-beta signaling by targeting SnoN oncoprotein for degradation. J Biol Chem. 2007;282:9475–9481. doi: 10.1074/jbc.M700875200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama A, Seth RB, Sun L, Ea CK, Hong M, Shaito A, Chiu YH, Deng L, Chen ZJ. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol Cell. 2004;15:535–548. doi: 10.1016/j.molcel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Karlsson L, Lindahl P, Heath J, Betsholtz C. Abnormal gastrointestinal development in PDGF-A and PDGFR-(alpha) deficient mice implicates a novel mesenchymal structure with putative instructive properties in villus morphogenesis. Development. 2000;127:3457–3466. doi: 10.1242/dev.127.16.3457. [DOI] [PubMed] [Google Scholar]
- Kim JY, Omori E, Matsumoto K, Nunez G, Ninomiya-Tsuji J. TAK1 is a central mediator of NOD2 signaling in epidermal cells. J Biol Chem. 2008;283:137–144. doi: 10.1074/jbc.M704746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida S, Sanjo H, Akira S, Matsumoto K, Ninomiya-Tsuji J. TAK1-binding protein 2 facilitates ubiquitination of TRAF6 and assembly of TRAF6 with IKK in the IL-1 signaling pathway. Genes Cells. 2005;10:447–454. doi: 10.1111/j.1365-2443.2005.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishigami S, Mishina Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005;16:265–278. doi: 10.1016/j.cytogfr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Kishimoto K, Matsumoto K, Ninomiya-Tsuji J. TAK1 mitogen-activated protein kinase kinase kinase is activated by autophosphorylation within its activation loop. J Biol Chem. 2000;275:7359–7364. doi: 10.1074/jbc.275.10.7359. [DOI] [PubMed] [Google Scholar]
- Komatsu Y, Shibuya H, Takeda N, Ninomiya-Tsuji J, Yasui T, Miyado K, Sekimoto T, Ueno N, Matsumoto K, Yamada G. Targeted disruption of the Tab1 gene causes embryonic lethality and defects in cardiovascular and lung morphogenesis. Mech Dev. 2002;119:239–249. doi: 10.1016/s0925-4773(02)00391-x. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Massague J. Opposing BMP and EGF signalling pathways converge on the TGF-beta family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- Kwan KM. Conditional alleles in mice: practical considerations for tissue-specific knockouts. Genesis. 2002;32:49–62. doi: 10.1002/gene.10068. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16:251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- Ono K, Ohtomo T, Sato S, Sugamata Y, Suzuki M, Hisamoto N, Ninomiya-Tsuji J, Tsuchiya M, Matsumoto K. An evolutionarily conserved motif in the TAB1 C-terminal region is necessary for interaction with and activation of TAK1 MAPKKK. J Biol Chem. 2001;276:24396–24400. doi: 10.1074/jbc.M102631200. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- Shibuya H, Iwata H, Masuyama N, Gotoh Y, Yamaguchi K, Irie K, Matsumoto K, Nishida E, Ueno N. Role of TAK1 and TAB1 in BMP signaling in early Xenopus development. Embo J. 1998;17:1019–1028. doi: 10.1093/emboj/17.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya H, Yamaguchi K, Shirakabe K, Tonegawa A, Gotoh Y, Ueno N, Irie K, Nishida E, Matsumoto K. TAB1: an activator of the TAK1 MAPKKK in TGF-beta signal transduction. Science. 1996;272:1179–1182. doi: 10.1126/science.272.5265.1179. [DOI] [PubMed] [Google Scholar]
- Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, Yamada G, Akira S, Matsumoto K, Ghosh S. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhirunnusorn P, Suzuki S, Kawasaki N, Saiki I, Sakurai H. Critical roles of threonine 187 phosphorylation in cellular stress-induced rapid and transient activation of transforming growth factor-beta-activated kinase 1 (TAK1) in a signaling complex containing TAK1-binding protein TAB1 and TAB2. J Biol Chem. 2005;280:7359–7368. doi: 10.1074/jbc.M407537200. [DOI] [PubMed] [Google Scholar]
- Takaesu G, Kishida S, Hiyama A, Yamaguchi K, Shibuya H, Irie K, Ninomiya-Tsuji J, Matsumoto K. TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol Cell. 2000;5:649–658. doi: 10.1016/s1097-2765(00)80244-0. [DOI] [PubMed] [Google Scholar]
- Takaesu G, Surabhi RM, Park KJ, Ninomiya-Tsuji J, Matsumoto K, Gaynor RB. TAK1 is critical for IkappaB kinase-mediated activation of the NF-kappaB pathway. J Mol Biol. 2003;326:105–115. doi: 10.1016/s0022-2836(02)01404-3. [DOI] [PubMed] [Google Scholar]
- Whitman M. Smads and early developmental signaling by the TGFbeta superfamily. Genes Dev. 1998;12:2445–2462. doi: 10.1101/gad.12.16.2445. [DOI] [PubMed] [Google Scholar]
- Yagi T, Nada S, Watanabe N, Tamemoto H, Kohmura N, Ikawa Y, Aizawa S. A novel negative selection for homologous recombinants using diphtheria toxin A fragment gene. Anal Biochem. 1993;214:77–86. doi: 10.1006/abio.1993.1459. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Nagai S, Ninomiya-Tsuji J, Nishita M, Tamai K, Irie K, Ueno N, Nishida E, Shibuya H, Matsumoto K. XIAP, a cellular member of the inhibitor of apoptosis protein family, links the receptors to TAB1-TAK1 in the BMP signaling pathway. Embo J. 1999;18:179–187. doi: 10.1093/emboj/18.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.