Abstract
Objective
Chemokine receptors are G-protein coupled receptors (GPCRs) phosphorylated by G-protein receptor kinases (GRKs) after ligand-mediated activation. We hypothesized that GRK subtypes differentially regulate granulocyte chemotaxis and clinical disease expression in the K/BxN model.
Methods
Clinical, histologic, and cytokine responses in GRK6−/−, GRK5−/−,GRK2+/−, and wildtype mice were evaluated using K/BxN serum transfer. Granulocyte chemotaxis was analyzed by transendothelial migration assays.
Results
Both GRK6−/− and GRK2+/− mice had increased arthritis disease severity (p<0.001); whereas GRK5−/− was not different from controls. Acute weight loss was enhanced in GRK6−/− and GRK2+/− mice (p<0.001, days 3–10). However, GRK6−/− mice uniquely had more weight loss (>10%), elevated serum IL-6, and enhanced migration toward LTB4 and C5a in vitro.
Conclusions
GRK6 and - 2, but not GRK5,are involved in the pathogenesis of acute arthritis in the K/BxN model. In particular, GRK6 may dampen inflammatory responses by regulating granulocyte trafficking toward chemoattractants.
G protein coupled receptors (GPCRs) are seven transmembrane spanning receptors that represent the largest superfamily of membrane-bound receptors in nature. With respect to immune function, GPCR-ligand interactions play critical roles in organ-specific leukocyte trafficking and activation, inflammatory-mediated chemotaxis, effector cell function, and cell survival [1; 2]. Consequently, the regulation of GPCRs and their downstream signaling molecules are attractive therapeutic targets for patients with autoimmune disease.
GPCR-mediated signaling maintains a coordinated balance between receptor activation, desensitization, and subsequent resensitization. G-protein receptor kinases (GRKs) are a family of molecules that play a critical role in the desensitization process of activated GPCRs [3; 4]. Specifically, GRKs phosphorylate only ligand-bound and activated GPCRs, leading to rapid uncoupling of the receptor from heterotrimeric G proteins and subsequent homologous desensitization [4; 5].
There are 7 different subtypes of GRKs [6] that have varying tissue distribution, suggesting some non-overlapping regulation of cellular functions. GRK1 and -7 are exclusively expressed in the retinal rods and cones respectively, and GRK4 has a high level of expression in the testes and a low level in kidney and cerebellum [7]. GRK subtypes -2, -3, -5, and -6 are expressed ubiquitously but have particularly high expression in leukocytes [8; 9; 10]. Moreover, GRK subtypes appear to be differently regulated in inflammatory disease states. Specifically, GRK6 and -2 protein levels are downregulated within peripheral blood mononuclear cells of rheumatoid arthritis patients [11]. In the rat model of adjuvant arthritis, GRK6 and -2 are decreased >50% in lymphoid organs at the peak of the inflammatory process, which subsequently normalized during the remission phase of the disease [12]. These observations suggest that GRKs play an important regulatory role in immune function and could have a more critical role in autoimmune disease states.
In gene deletion studies, deficiencies in GRK6 or -2 have been shown to specifically affect the migration of leukocyte subsets both in vitro and in vivo [13; 14]. In specific, GRK6-deficient (GRK6−/−) mice have increased granulocyte infiltration in the AA-induced ear inflammation model after topical arachadonic acid is applied [14]. GRK6−/− mice also have enhanced granulocyte-mediated inflammation of the gut in a dextran sodium sulfate (DSS)-induced colitis model [15]. In contrast, in vitro work has shown reduced lymphocyte chemotaxis in GRK6−/− T cells to CXCL12 [13]. GRK2 heterozygote mice (GRK2+/−) have been evaluated in the animal model of multiple sclerosis, (experimental allergic encephalomyelitis, EAE) since homozygotes are embryonic lethal due to cardiac dysgenesis [16]. T cells and macrophages were increased in the central nervous system of GRK2+/− mice in the early stages of disease, followed by a reduction in the inflammatory cell infiltrate at later time points [17].
Given the aforementioned data supporting GRK involvement in inflammatory disease expression in vivo and in leukocyte trafficking in vitro, we hypothesized that GRK6 and -2 may be involved in the pathogenesis of inflammatory arthritis via effects on chemotaxis. To test this, we performed studies using the K/BxN serum transfer mouse model of acute inflammatory arthritis [18] where the serum from K/BxN mice can transfer disease to naive recipients [19], resulting in bony erosions similar to human rheumatoid arthritis [20]. The K/BxN serum transfer model is notably independent of both B and T cells, whereas granulocytes are critical for disease [21]. Consequently, this model enables us to examine the regulatory effects of GRK2, -5, and -6 gene deletions that are independent of the lymphocytic induction phase of disease and more reflective of the effector immune response.
Materials and Methods
Animals
Animals used in these experiments were backcrossed ≥9 generations onto the C57/Bl6 strain with littermate controls. All animals were bred, housed, and cared for in DLAM facilities under the approved IACUC protocol number 03–265.0 in pathogen free specific conditions.
Induction and evaluation of K/BxN arthritis
Male 6 week old mice heterozygous for GRK2 or deficient in GRK5 or -6 and wild-type littermates were injected on days 0 and 2 with a dose of 5 µl K/BxN serum per gram of mouse weight intraperitoneally. Mice were measured daily for endpoints of paw swelling, clinical disease index, and weight. Paw swelling was represented as a change in the mean thickness of the mouse’s fore- and hindlimbs (mm) from its baseline average and was obtained by a blinded measurer. Clinical index was assessed as the consensus of 2 blinded observers with the following scoring system: 0=normal paw; 1=mild but definite swelling of either the ankle or digits; 2=moderate redness and swelling of an ankle ± any number of digits; 3=maximal redness and swelling of the entire paw and digits. The maximum score per paw obtainable being 3 with a total of 12 per mouse. Results for paw swelling and clinical index are reported as an average in the total score ± SEM for the group. At experiment termination, hindlimbs were fixed for 24h in 4% paraformaldehyde, decalcified using formic acid for 24–48h, and embedded in paraffin for histopathology.
Histopathology
Histopathology sections of the hindlimbs were stained with hematoxylin and eosin and were scored by a blinded observer for the severity of inflammatory cell infiltrate and the presence of erosions using the following scoring system: 0=normal paw; 1=mild inflammatory cell infiltrate with minimal synovial thickening and no architectural distortion; 2=moderate inflammatory cell infiltrate with enhanced synovial proliferation without erosions; 3=severe inflammatory cell infiltrate with erosions.
Transendothelial migration assays
Transendothelial migration (TEM) assays were performed as previously described [13]. Briefly, 104 Ea.hy 926 endothelial cells were plated onto 24-well (5 µm) Transwells and incubated for 7 days. Monolayer integrity was determined by [14C] mannitol diffusion. For migration studies, the medium in the bottom chamber was replaced with medium or chemoattractant (10 nM LTB4,100 nM C5a; Sigma-Aldrich, St. Louis, MO, or hIL-8 100ng/ml; R&D Systems, Minneapolis, MN) and primary bone marrow cells isolated from individual mice of the respective GRK genotypes were added to the top chamber. Following 4 h of incubation at 37°C, cells in the lower chamber were harvested and stained with anti-mouse Gr-1 conjugated to PE (BD Biosciences, San Diego, CA). The number of migrated granulocytes was determined by flow cytometry.
Serum cytokine analysis
Mouse blood was collected by tail vein nick on days 0, 4, and 8 after immunization with K/BxN mouse serum on days 0 and 2. Cytokine measurements were performed using a Millipore Beadlyte Multiplex assay (Millipore Corporation, Billerica, MA), and analyzed on a Luminex 100 Total System v.1.7 (Luminex Corporation, Austin, Texas) according to the manufacturer’s protocol. Data were analyzed using Starstation v.2.0 (Applied Cytometry Systems, Sacramento, CA).
Data analysis
For clinical index, paw swelling, and % weight loss curves, a statistical curve-fit was used to determine whether significant differences existed in the course of the disease over time between the GRK deficient mice versus wildtype controls. A backward selection (α=.05) procedure was used to select a linear mixed model with the best fit for the individual curves. Variables considered in the statistical analysis included group, time, and experiment effect. The overall group effect was assessed using a likelihood ratio test (LRT) (α=.05). The best fit curves were plotted using predicted values calculated using the fixed effects from the models, averaging across experiment, which was controlled for if it was a significant (α=.05) predictor in the model. Statistical analysis was performed using SAS, v. 9.1. For transendothelial migration assay results, an unpaired two-tailed Ttest was used to compare the means between groups. IL-1 and IL-6 systemic cytokine production was analyzed using an unpaired single-tailed Ttest to compare the means between groups.
Results
GRK6−/− and GRK2+/− mice have more severe arthritis early in the K/BxN model compared to controls
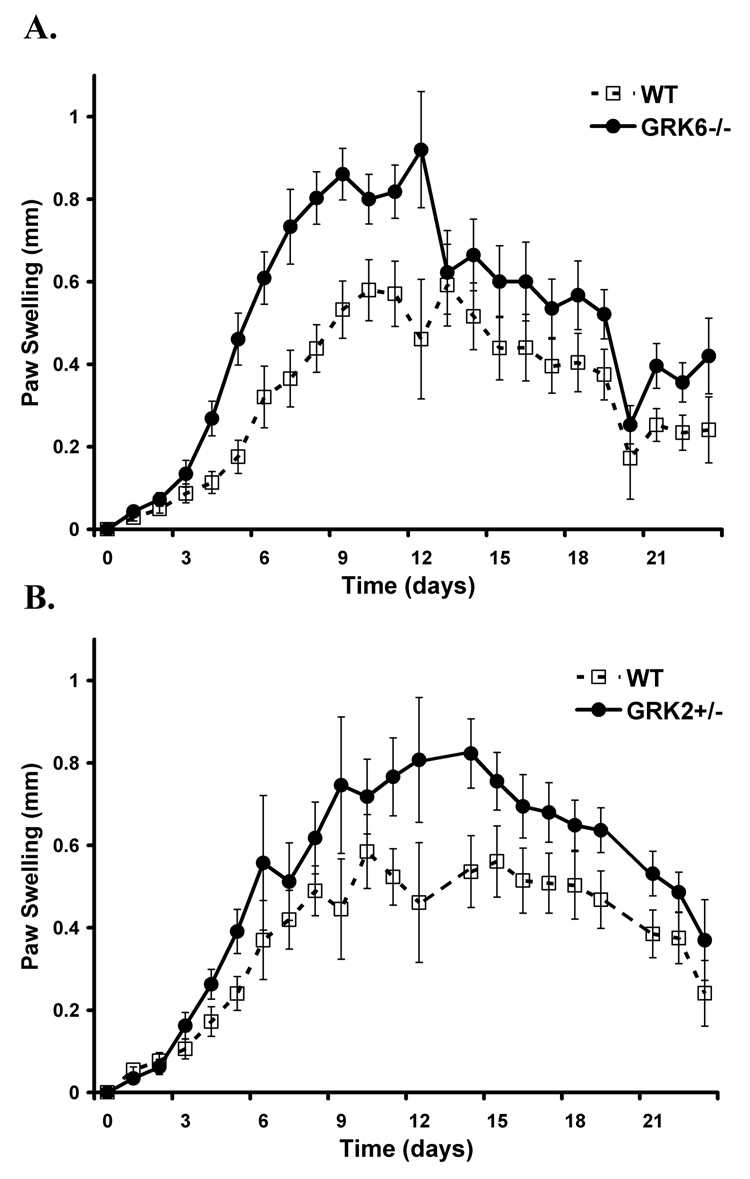
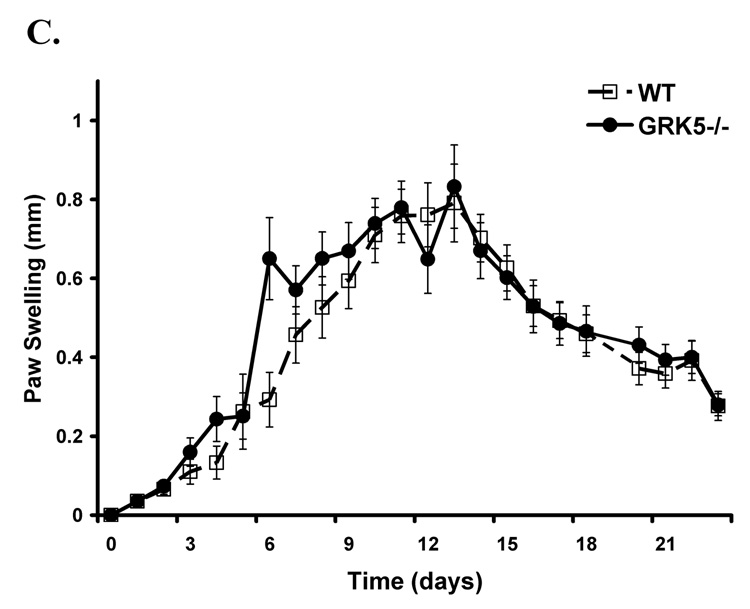
To investigate whether or not the reduction in GRK expression previously described in rheumatoid arthritis patients [11] directly contributes to the pathophysiology of inflammatory arthritis in our animal model, we examined GRK6−/−, GRK2+/−, GRK5−/−, and wildtype mice in the K/BxN serum transfer model. We found that animals with a targeted genetic deletion of GRK6 had the most pronounced enhanced arthritis early in their disease compared to their wildtype littermate controls by two independent measures of paw swelling (p<0.0001) and clinical disease severity index (p<0.0003) (Figure 1A, Figure 2A). Although GRK2+/− mice also showed enhanced disease compared to controls by paw swelling (p<0.0001) and clinical disease index (p=0.0003) (Figure 1B, Figure 2B), it was not as severe as GRK6−/− animals. In contrast, GRK5−/− mice had similar disease indices when compared to wildtypes (Figure 1C, Figure 2C), suggesting that the observed effects were specific and differing between the GRK subtype.
Figure 1. Paw swelling is increased in animals deficient in GRK 6 and -2, but not -5,compared to wildtype controls.
Shown is the mean difference in mm swelling from baseline thickness of the ankles of wildtype and GRK-deficient animals (± SEM) over time in days. The data are combined from 3 (GRK6−/−) and 2 (GRK2+/− and GRK5−/−) separate experiments. A. GRK6−/− mice have the most disease severity that is more pronounced earlier in the disease course (LRT=56, 3 df, p<0.0001; GRK6−/− n=14, WT n=13). B. GRK2 +/− mice also show enhanced disease (LRT=32.4, 3 df, p<0.0001; GRK2+/− n=12, WT n=13). C. GRK5−/− mice do not differ from wildtype controls (LRT=1.3, df=2, p=0.522; GRK5−/− n=11, WT n=13).
Figure 2. Clinical index of disease activity is increased in animals deficient in GRK6 and -2, but not -5, compared to wildtype controls.
Shown is the mean clinical index ± SEM over time in days as determined by the summation of each paw scored on a scale of 0–3 and averaged for the group. The data are combined from 3 (GRK6−/−) and 2 (GRK2+/− and GRK5−/−) separate experiments. A. GRK6−/− mice have the most disease severity that is more pronounced earlier in the disease course (LRT=19, 3 df, p<0.0003; GRK6−/− n=14, WT n=13). B. GRK2 +/− mice also show enhanced disease (LRT=18.9, 3 df, p=0.0003; GRK2+/− n=12, WT n=13). C. GRK5−/− mice do not differ from wildtype controls (LRT=6.1,df=3, p=0.1068; GRK5−/− n=11, WT n=13).
To investigate whether or not the earlier disease enhancement observed in the GRK6−/− and GRK2+/− mice translated into chronic disease differences, we examined histopathology of the hindlimbs at experiment termination (day 22 or 23) by hematoxylin and eosin staining. There were no substantial differences between the different GRK genotypes and the wildtype controls with respect to the severity of inflammation or bony erosions at the later stage of disease (Figure 3).
Figure 3. Late stage inflammatory disease and erosions do not differ between the GRK genotypes and wildtype controls in the K/BxN model.
Shown is representative H&E histology at experiment termination (Day 22 or 23) of A. wildtype, B. GRK6−/−, C. GRK2 +/−, and D. GRK5−/− mice. The severity of inflammatory infiltrate and erosions did not differ between groups;↑ localize the erosions on hematoxylin and eosin stained sections.
Granulocytes from GRK6−/− mice have enhanced chemotaxis to LTB4 and C5a, but not to IL-8, in vitro
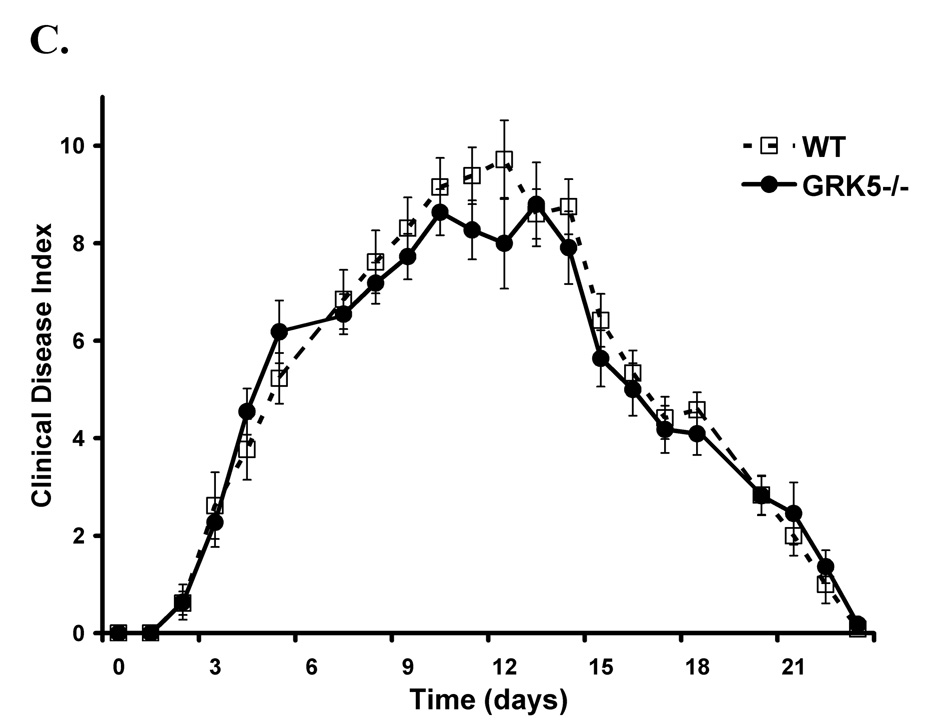
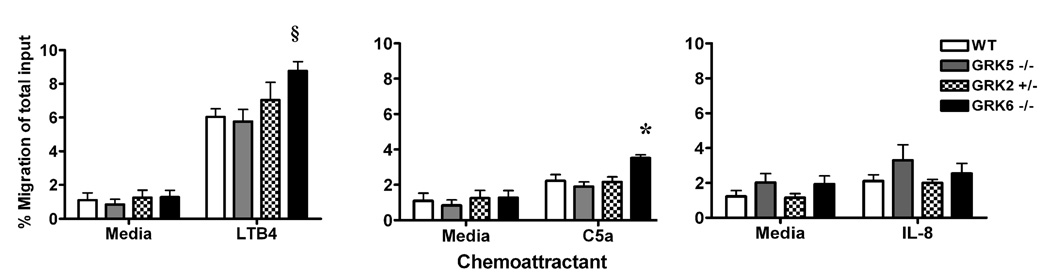
LTB4 and C5a are potent chemoattractants for granulocytes and signal through their respective GPCRs (BLT and C5aR). Disease expression in the K/BxN serum transfer model is critically dependent on granulocytes [21] as well as LTB4 [22] and C5a [23]. Therefore, we hypothesized that the enhanced disease seen in the GRK6 and -2 deficient mice may be mediated through interactions with the BLT and C5a receptors on granulocytes. In agreement with a previous report [14], we found that GRK6−/− granulocytes have enhanced chemotaxis to LTB4 in vitro (p=0.0042) that is approximately 30% more compared to wildtype cells (Figure 4). We also show that GRK6−/− granulocytes have 30% more chemotaxis toward C5a (p=0.0074) (Figure 4). Interleukin-8 (IL-8 or CXCL8) is also a potent chemoattractant of granulocytes and signals through the GPCR, CXCR1. However, migration to IL-8 was not different in any of the GRK-deficient subtypes compared to wildtype controls (Figure 4).
Figure 4. GRK6 deficient mice have increased granulocyte migration to LTB4 and C5a, but not IL-8, in vitro compared to wildtype controls.
The migration of granulocytes in response to 10 nM LTB4,100 nM C5a, or 100 ng/ml IL-8 were determined by anti-Gr-1 staining and flow cytometry of migrated primary bone marrow cells of the different murine GRK genotypes compared to wildtype. Data are represented as % migration of the total input (i.e. total number of anti-Gr-1 positive cells harvested from the lower chamber divided by the total number of anti-Gr-1 positive cells in the upper chamber) ± SEM, n=6. Only GRK6−/− granulocytes show statistically increased migration to LTB4 (§, p≤0.01) and C5a (*, p≤0.01). There was no difference between migration of the different GRK genotypes compared to wildtypes in response to IL-8.
While statistical significance was not reached (p=0.4074), GRK2+/− granulocytes had a trend towards increased migration to LTB4 with approximately 15% enhanced migration compared to wildtype cells. The migration of GRK2+/− granulocytes toward C5a (p=0.8753) was similar to controls (Figure 4). These data suggest that the absence of GRK6, leading to increased granulocyte migration, may enhance inflammation through a failure to desensitize signaling of the LTB4 and C5a GPCRs (BLT1&2 and C5aR respectively), and partial GRK2 deficiency may also play a role in BLT1&2 receptor signaling.
GRK6−/− mice have enhanced systemic effects in the K/BxN model
In the K/BxN model, wildtype mice typically experience a reversible weight loss approximating <5% of total body weight that begins during the acute inflammatory phase and resolves with improved clinical arthritis scores (Figure 5 and unpublished observations). While GRK5−/− mice had a similar level of weight loss compared to wildtypes, GRK6−/− mice had a profound weight loss of >10% of their total body weight compared to that of controls (p<0.0001) (Figure 5A). We hypothesized that this finding may result from increased inflammation leading to enhanced systemic production of proinflammatory cytokines. Indeed, GRK6−/− mice have increased IL-6 detectable in the serum (Figure 6A), which correlates temporally with the nadir weight loss occurring between days 3–10. Systemic production of IL-1β was detected in the serum but was not significantly different between GRK6-deficient animals and controls (Figure 6B). Although GRK2+/− animals had an average 5% more weight loss than wildtypes that was significant (p<0.0001) (Figure 5B), it was less pronounced than GRK6−/− and the systemic cytokine profile did not differ significantly from that of wildtype controls (data not shown). We attempted to measure TNF-α in the serum in addition to the IL-1β and IL-6 of the GRK-deficient and wildtype mice, but it was rarely measured above the limit of detection in the majority of samples for an analysis to be reliable (data not shown).
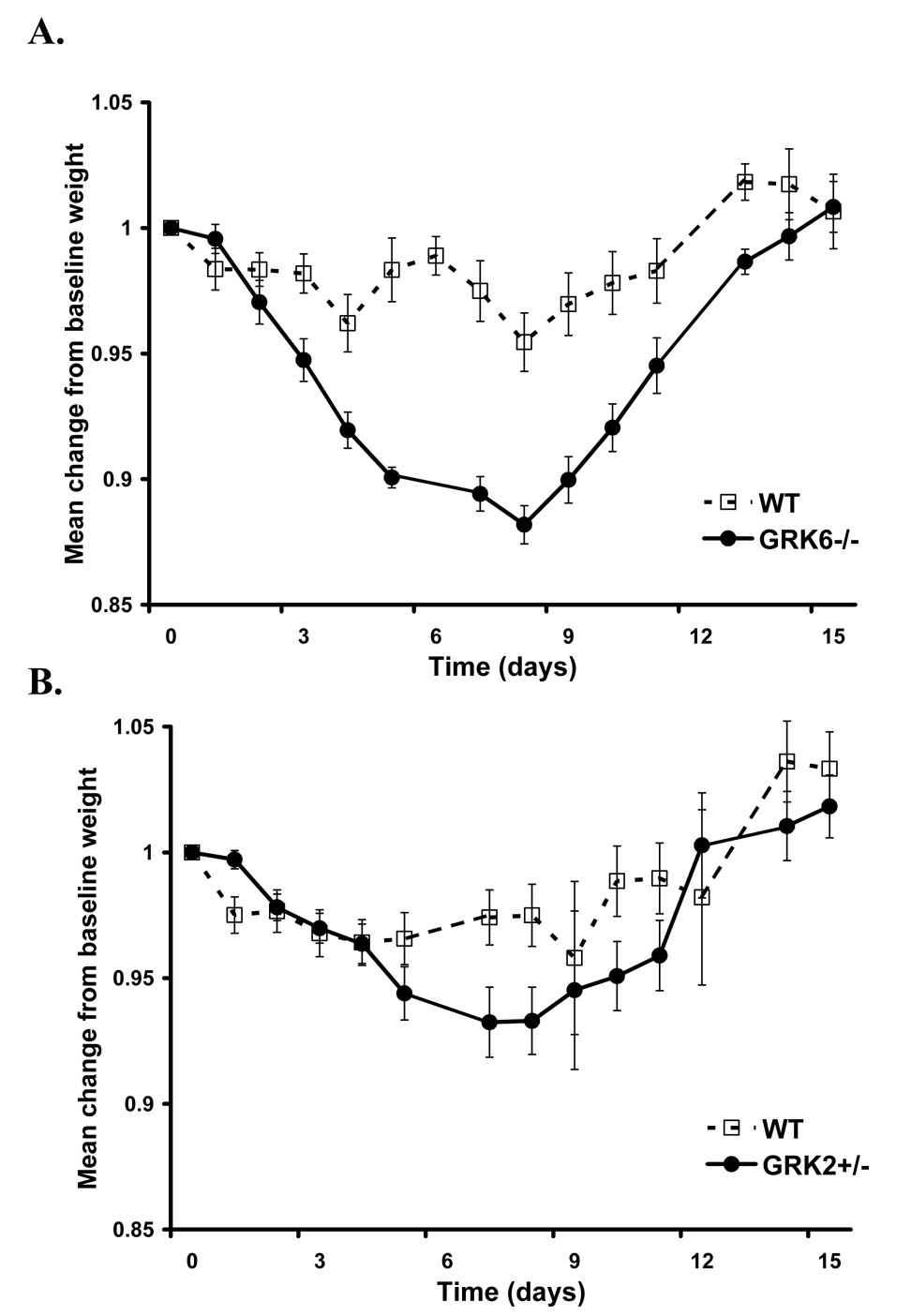
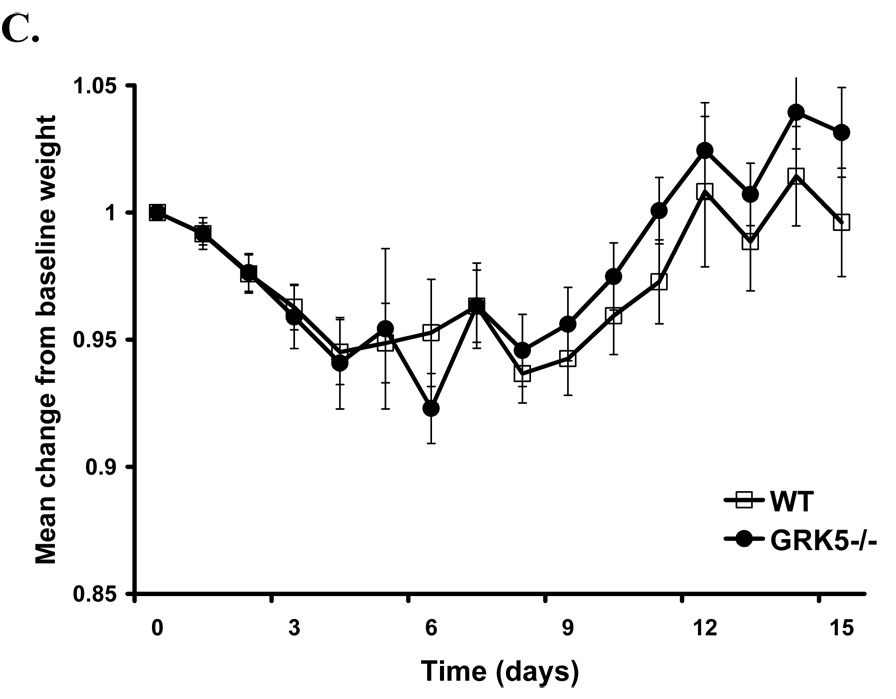
Figure 5. GRK6-deficient animals demonstrate maximum acute weight loss in the K/BxN model that reverses with disease improvement.
Data is represented as a mean of the change in % weight from an individual animal’s baseline = 1.0 of wildtype and GRK-deficient animals (± SEM) over time in days. The data are combined from 3 (GRK6−/−) and 2 (GRK2+/− and GRK5−/−) separate experiments. A. There is a significant group effect indicating more weight loss in the GRK6−/− mice (LRT=102; df=3; p-value<0.0001; GRK6−/− n=14, WT n=13). B. There is also a significant group effect in the GRK2+/− mice (LRT=35.2; df=4; p-value<0.0001; GRK2+/− n=12, and WT n=13). C. There is no significant difference between GRK5−/− animals and controls (LRT=2.7; df=2; p value= 0.2592; GRK5−/− n=11, and WT n=13).
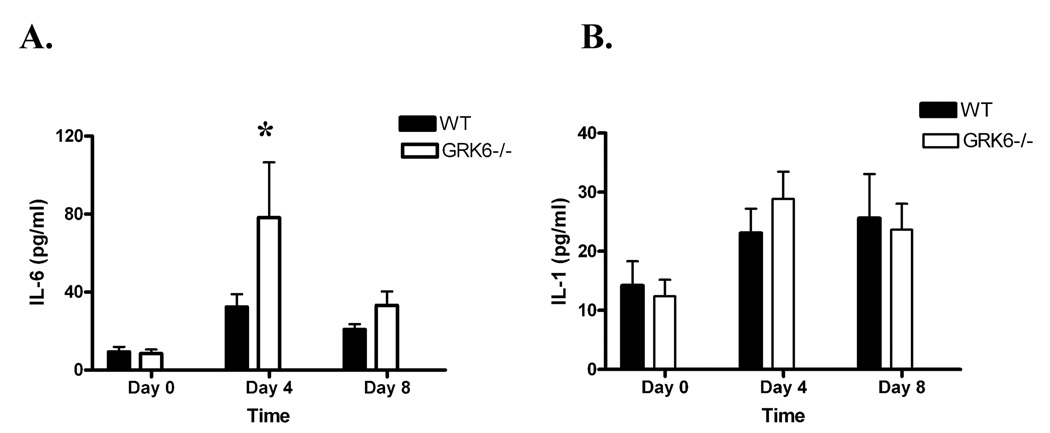
Figure 6. GRK6-deficient animals produce higher systemic levels of IL-6 compared to wildtype controls in the K/BxN model of inflammatory arthritis.
Data represent the mean serum cytokine production + SEM in pg/ml of A. IL-6, and B. IL-1β on Days 0, 4, and 8 after i.p. injection with K/BxN serum. Results presented are from 2 separate experiments; GRK6−/− n=8, WT n=9. Animals were bled by tail vein nick, and serum cytokine analysis was performed using an Upstate BeadLyte assay and interpreted on a Luminex 100 instrument. GRK6−/− had statistically increased levels of systemic IL-6 on Day 4 (* p=0.05) compared to wildtype animals. Limit of detection of the assay was 18.7 pg/ml for IL-6 and 4.8 pg/ml for IL-1β.
Discussion
GPCR-mediated leukocyte trafficking is critical in inflammatory disease states, and the GRK regulatory system has been implicated in animal models of autoimmune disease. Recently, GRK6-deficient animals have been shown to have increased neutrophil migration to the gut and impaired regulatory T cell responses in a DSS-induced colitis model of inflammatory bowel disease [15]. In the EAE model of multiple sclerosis, GRK2 heterozygote animals exhibit earlier inflammation that correlates with T cell and macrophage infiltration into the CNS, but then subsequently have less disease and inflammatory cell infiltrate than wildtype controls at later time points [17]. Given that GRK6 and -2 levels are reduced in disease flares of adjuvant induced arthritis [12] and in the PBMC of rheumatoid arthritis patients [11], we investigated how and which GRK subtypes play a role in leukocyte migration to the joint in an acute model of inflammatory arthritis, the K/BxN serum transfer model. This model system closely mirrors the acute flares of rheumatoid arthritis and allowed us to evaluate mononuclear cell contribution toward disease independent of the lymphocytic response.
The in vitro studies further support a role for GRK6 and -2 in granulocyte-mediated chemotaxis to LTB4 and C5a (Fig 3). LTB4 is a potent neutrophil chemoattractant [24] and critical for disease in the K/BxN model [22]. One of its high affinity receptors (BLT1) is a GPCR that has been shown to have GRK6 and -2 mediated receptor internalization [25] and desensitization [26] respectively. Previous work by Kavelaars et al. has demonstrated enhanced chemotaxis in GRK6 deficient granulocytes in vitro to LTB4 [14], and our data here support this finding. We also show that GRK6 deficient granulocytes have enhanced chemotaxis to C5a compared to controls. The C5a anaphylatoxin receptor (C5aR) is a GPCR important in the chemotaxis and activation of myeloid cells [27] and necessary for K/BxN disease expression [23]. Over expression of wildtype or catalytically inactive forms of GRK6 and -2 in COS-7 cells does not change agonist-mediated phosphorylation of C5aR [28]. However, Milcent et. al. noted that transfection of COS-7 cells with wildtype GRK6 consistently increased expression of C5aR by two fold and that GRK6 appeared to be autophosphorylated [28], suggesting a biologic relevance not apparent in this in vitro model system. Our data suggests that GRK6 may have receptor-kinase interactions mediated through the BLT and C5aR receptors on granulocytes that are physiologically relevant as it pertains to inflammatory disease.
Interleukin-8 (IL-8 or CXCL8) is also a known chemoattractant of granulocytes and is thought to have pathophysiologic significance in rheumatoid arthritis through its chemoattractant [29] and angiogenic [30] properties. However, we did not observe differences in granulocyte migration to IL-8 in the animals with gene defects in GRK2, -5, or -6 compared to controls (Figure 4). The role of IL-8 in the K/BxN model in specific is largely unknown, whereas LTB4 and C5a in this model have been found to be of critical importance to disease pathogenesis [22; 23].
Our data also suggest that the enhanced inflammatory disease seen in GRK2 heterozygote mice may be mediated through one or both of the BLT GPCRs. GRK2+/− granulocytes have slightly increased chemotaxis to LTB4 in vitro, albeit our results did not achieve statistical significance. This could be partially explained by the heterozygous expression of GRK2 that may have reduced this phenotype. Further support that GRK2 plays an important role in BLT1 signaling has been suggested in transfection model systems [25]. LTB4-induced internalization of BLT1 can be blocked by dominant negative GRK2 co-expression, and GRK2 co-localizes with the BLT1 receptor through the C terminus [25].
We also show that in addition to increased organ-specific inflammation, GRK6-deficient animals have enhanced systemic effects in the K/BxN model. Weight loss is observed in inflammatory disease states such as rheumatoid arthritis and has been linked to elevated cytokine production, particularly TNF-α [31]. We were unable to reliably detect TNF-α in the serum of our arthritic animals to investigate its role in the cachexia, but we do show that GRK6−/− animals have elevated systemic levels of IL-6 that coincide with the observed weight loss. IL-6 can be elevated in rheumatoid arthritis patients and may correlate with disease activity [32]. In addition, IL-6 has been associated with profound cachectic states such as malignancy [33; 34]. Importantly, IL-6 is not elevated chronically in the K/BxN model in either wildtypes or GRK6-deficient animals (Figure 6A and unpublished observations), and the presence of malignancy has not been observed in the K/BxN model in either controls or GRK-deficient mice.
Unlike the DSS-colitis model studied by Kavelaars et al., we did not observe significant differences in systemic production of IL-1β between groups [15]. This could be explained by the different types of inflammatory responses being observed (i.e. DSS, chronic v. K/BxN, acute) or by different pathophysiologic mechanisms regulating the two disease models. Although we did observe increased weight loss in the GRK2+/− mice, we did not detect any significant differences in systemic cytokine production, possibly because their overall weight loss and disease was less severe.
In conclusion, the GRK system is an important regulatory pathway in the K/BxN serum transfer model of arthritis, particularly as it pertains to the early infiltration of granulocyte-mediated acute inflammatory responses. Granulocytes, which are critical to disease expression in the K/BxN model, have increased chemotaxis to LTB4 and C5a, but not IL-8, and GRK6−/− mice have more arthritis, weight loss, and IL-6 production. These data highlight the unique and subtype-specific effects of the GRKs on leukocyte trafficking and inflammatory disease and underscore the important regulatory role that GRK molecules may play in the acute versus chronic phases of autoimmunity. Although specific gene polymorphisms in the GRKs have not been described in the human rheumatoid arthritis population, an increased understanding of these regulators as they pertain to acute versus chronic inflammatory cell trafficking may lead to targeted therapies or diagnostics for patients with rheumatoid arthritis or other autoinflammatory diseases.
Acknowledgements
The authors would like to thank James Ellinger for technical assistance in the arthritis disease models, Dr. M.G. Caron for providing the GRK2 heterozygote animals for these studies, and Dr. David Siderovski for critical review of the manuscript. These experiments were generously supported by the Thurston Arthritis Research Center at UNC, the American College of Rheumatology Research Education Foundation Physician Scientist Award, and the National Institutes of Health (grants K12 HD01441 and K08 AI070684).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vroon A, Heijnen CJ, Kavelaars A. GRKs and arrestins: regulators of migration and inflammation. J Leukoc Biol. 2006;80:1214–1221. doi: 10.1189/jlb.0606373. [DOI] [PubMed] [Google Scholar]
- 2.Tarrant TK, Patel DD. Chemokines and leukocyte trafficking in rheumatoid arthritis. Pathophysiology. 2006;13:1–14. doi: 10.1016/j.pathophys.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Kohout TA, Lefkowitz RJ. Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol Pharmacol. 2003;63:9–18. doi: 10.1124/mol.63.1.9. [DOI] [PubMed] [Google Scholar]
- 4.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 5.Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- 6.Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- 7.Ribas C, Penela P, Murga C, Salcedo A, Garcia-Hoz C, Jurado-Pueyo M, Aymerich I, Mayor F., Jr. The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta. 2007;1768:913–922. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Chuang TT, Sallese M, Ambrosini G, Parruti G, De Blasi A. High expression of beta-adrenergic receptor kinase in human peripheral blood leukocytes. Isoproterenol and platelet activating factor can induce kinase translocation. J Biol Chem. 1992;267:6886–6892. [PubMed] [Google Scholar]
- 9.De Blasi A, Parruti G, Sallese M. Regulation of G protein-coupled receptor kinase subtypes in activated T lymphocytes. Selective increase of beta-adrenergic receptor kinase 1 and 2. J Clin Invest. 1995;95:203–210. doi: 10.1172/JCI117641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haribabu B, Snyderman R. Identification of additional members of human G-protein-coupled receptor kinase multigene family. Proc Natl Acad Sci U S A. 1993;90:9398–9402. doi: 10.1073/pnas.90.20.9398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lombardi MS, Kavelaars A, Schedlowski M, Bijlsma JW, Okihara KL, Van de Pol M, Ochsmann S, Pawlak C, Schmidt RE, Heijnen CJ. Decreased expression and activity of G-protein-coupled receptor kinases in peripheral blood mononuclear cells of patients with rheumatoid arthritis. Faseb J. 1999;13:715–725. doi: 10.1096/fasebj.13.6.715. [DOI] [PubMed] [Google Scholar]
- 12.Lombardi MS, Kavelaars A, Cobelens PM, Schmidt RE, Schedlowski M, Heijnen CJ. Adjuvant arthritis induces down-regulation of G protein-coupled receptor kinases in the immune system. J Immunol. 2001;166:1635–1640. doi: 10.4049/jimmunol.166.3.1635. [DOI] [PubMed] [Google Scholar]
- 13.Fong AM, Premont RT, Richardson RM, Yu YR, Lefkowitz RJ, Patel DD. Defective lymphocyte chemotaxis in beta-arrestin2-and GRK6-deficient mice. Proc Natl Acad Sci U S A. 2002;99:7478–7483. doi: 10.1073/pnas.112198299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kavelaars A, Vroon A, Raatgever RP, Fong AM, Premont RT, Patel DD, Lefkowitz RJ, Heijnen CJ. Increased acute inflammation, leukotriene B4-induced chemotaxis, and signaling in mice deficient for G protein-coupled receptor kinase 6. J Immunol. 2003;171:6128–6134. doi: 10.4049/jimmunol.171.11.6128. [DOI] [PubMed] [Google Scholar]
- 15.Eijkelkamp N, Heijnen CJ, Lucas A, Premont RT, Elsenbruch S, Schedlowski M, Kavelaars A. G protein-coupled receptor kinase 6 controls chronicity and severity of dextran sodium sulphate-induced colitis in mice. Gut. 2007;56:847–854. doi: 10.1136/gut.2006.107094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaber M, Koch WJ, Rockman H, Smith B, Bond RA, Sulik KK, Ross J, Jr., Lefkowitz RJ, Caron MG, Giros B. Essential role of beta-adrenergic receptor kinase 1 in cardiac development and function. Proc Natl Acad Sci U S A. 1996;93:12974–12979. doi: 10.1073/pnas.93.23.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vroon A, Kavelaars A, Limmroth V, Lombardi MS, Goebel MU, Van Dam AM, Caron MG, Schedlowski M, Heijnen CJ. G protein-coupled receptor kinase 2 in multiple sclerosis and experimental autoimmune encephalomyelitis. J Immunol. 2005;174:4400–4406. doi: 10.4049/jimmunol.174.7.4400. [DOI] [PubMed] [Google Scholar]
- 18.Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. A new mouse model of rheumatoid arthritis: organ-specific disease provoked by systemic autoimmunity. Ryumachi. 1997;37:147. [PubMed] [Google Scholar]
- 19.Ji H, Gauguier D, Ohmura K, Gonzalez A, Duchatelle V, Danoy P, Garchon HJ, Degott C, Lathrop M, Benoist C, Mathis D. Genetic influences on the end-stage effector phase of arthritis. J Exp Med. 2001;194:321–330. doi: 10.1084/jem.194.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto I, Maccioni M, Lee DM, Maurice M, Simmons B, Brenner M, Mathis D, Benoist C. How antibodies to a ubiquitous cytoplasmic enzyme may provoke joint-specific autoimmune disease. Nat Immunol. 2002;3:360–365. doi: 10.1038/ni772. [DOI] [PubMed] [Google Scholar]
- 21.Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J Immunol. 2001;167:1601–1608. doi: 10.4049/jimmunol.167.3.1601. [DOI] [PubMed] [Google Scholar]
- 22.Chen M, Lam BK, Kanaoka Y, Nigrovic PA, Audoly LP, Austen KF, Lee DM. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J Exp Med. 2006;203:837–842. doi: 10.1084/jem.20052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji H, Ohmura K, Mahmood U, Lee DM, Hofhuis FM, Boackle SA, Takahashi K, Holers VM, Walport M, Gerard C, Ezekowitz A, Carroll MC, Brenner M, Weissleder R, Verbeek JS, Duchatelle V, Degott C, Benoist C, Mathis D. Arthritis critically dependent on innate immune system players. Immunity. 2002;16:157–168. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 24.Palmblad J, Malmsten CL, Uden AM, Radmark O, Engstedt L, Samuelsson B. Leukotriene B4 is a potent and stereospecific stimulator of neutrophil chemotaxis and adherence. Blood. 1981;58:658–661. [PubMed] [Google Scholar]
- 25.Chen Z, Gaudreau R, Le Gouill C, Rola-Pleszczynski M, Stankova J. Agonist-induced internalization of leukotriene B(4) receptor 1 requires G-protein-coupled receptor kinase 2 but not arrestins. Mol Pharmacol. 2004;66:377–386. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 26.Gaudreau R, Le Gouill C, Venne MH, Stankova J, Rola-Pleszczynski M. Threonine 308 within a putative casein kinase 2 site of the cytoplasmic tail of leukotriene B(4) receptor (BLT1) is crucial for ligand-induced, G-protein-coupled receptor-specific kinase 6-mediated desensitization. J Biol Chem. 2002;277:31567–31576. doi: 10.1074/jbc.M202723200. [DOI] [PubMed] [Google Scholar]
- 27.Ye RD, Boulay F. Structure and function of leukocyte chemoattractant receptors. Adv Pharmacol. 1997;39:221–289. doi: 10.1016/s1054-3589(08)60073-3. [DOI] [PubMed] [Google Scholar]
- 28.Milcent MD, Christophe T, Rabiet MJ, Tardif M, Boulay F. Overexpression of wild-type and catalytically inactive forms of GRK2 and GRK6 fails to alter the agonist-induced phosphorylation of the C5a receptor (CD88): evidence that GRK6 is autophosphorylated in COS-7 cells. Biochem Biophys Res Commun. 1999;259:224–229. doi: 10.1006/bbrc.1999.0758. [DOI] [PubMed] [Google Scholar]
- 29.Koch AE, Kunkel SL, Burrows JC, Evanoff HL, Haines GK, Pope RM, Strieter RM. Synovial tissue macrophage as a source of the chemotactic cytokine IL-8. J Immunol. 1991;147:2187–2195. [PubMed] [Google Scholar]
- 30.Park CC, Morel JC, Amin MA, Connors MA, Harlow LA, Koch AE. Evidence of IL-18 as a novel angiogenic mediator. J Immunol. 2001;167:1644–1653. doi: 10.4049/jimmunol.167.3.1644. [DOI] [PubMed] [Google Scholar]
- 31.Rall LC, Roubenoff R. Rheumatoid cachexia: metabolic abnormalities, mechanisms and interventions. Rheumatology (Oxford) 2004;43:1219–1223. doi: 10.1093/rheumatology/keh321. [DOI] [PubMed] [Google Scholar]
- 32.Cohick CB, Furst DE, Quagliata S, Corcoran KA, Steere KJ, Yager JG, Lindsley HB. Analysis of elevated serum interleukin-6 levels in rheumatoid arthritis: correlation with erythrocyte sedimentation rate or C-reactive protein. J Lab Clin Med. 1994;123:721–727. [PubMed] [Google Scholar]
- 33.Tanaka K, Urata N, Mikami M, Ogasawara M, Matsunaga T, Terashima N, Suzuki H. Effect of iguratimod and other anti-rheumatic drugs on adenocarcinoma colon 26-induced cachexia in mice. Inflamm Res. 2007;56:17–23. doi: 10.1007/s00011-007-6022-9. [DOI] [PubMed] [Google Scholar]
- 34.Kuroda K, Nakashima J, Kanao K, Kikuchi E, Miyajima A, Horiguchi Y, Nakagawa K, Oya M, Ohigashi T, Murai M. Interleukin 6 is associated with cachexia in patients with prostate cancer. Urology. 2007;69:113–117. doi: 10.1016/j.urology.2006.09.039. [DOI] [PubMed] [Google Scholar]