Abstract
The macrophage proinflammatory response to Francisella tularensis (Ft) LVS was shown previously to be TLR2-dependent. The observation that intracellular Ft LVS co-localizes with TLR2 and MyD88 inside macrophages suggested that Ft LVS might signal from within the phagosome. Macrophages infected with LVSΔiglC, a Ft LVS mutant that fails to escape from the phagosome, displayed greatly increased expression of a subset of TLR2-dependent, proinflammatory genes (e.g., Tnf) but decreased expression of others (e.g., Ifnb1). This latter subset was similarly mitigated in IFN-β−/− macrophages indicating that while Ft LVS-induced TLR2 signaling is necessary, cytosolic sensing of Ft to induce IFN-β is required for full induction of the macrophage proinflammatory response. While LVSΔiglC greatly increased IL-1β mRNA in wild-type macrophages, protein secretion was not observed. IL-1β secretion was also diminished in Ft LVS-infected IFN-β−/− macrophages. rIFN-β failed to restore IL-1β secretion in LVSΔiglC-infected macrophages, suggesting that signals in addition to IFN-β are required for assembly of the inflammasome and activation of caspase-1. IFN-β plays a central role in controlling the macrophage bacterial burden: bacterial recovery was greater in IFN-β−/− than in wild-type macrophages and treatment of Ft LVS-infected macrophages with rIFN-β or 5,6-dimethylxanthenone-4-acetic acid (DMXAA), a potent IFN-β inducer, greatly decreased the intracellular Ft LVS burden. In toto, these observations support the hypothesis that the host inflammatory response to Ft LVS is complex and requires engagement of multiple signaling pathways downstream of TLR2 including production of IFN-β via an unknown cytosolic sensor and activation of the inflammasome.
Introduction
Francisella tularensis (Ft) is a intracellular Gram-negative coccobacillus and the etiologic agent of tularemia, a potentially fatal infectious disease (reviewed in refs. (1–3)). Humans can be infected by F. tularensis from insect bites, handling infected animals, ingestion, or inhalation. Symptoms usually appear 3 to 5 days after infection; however, the size of the infectious dose, symptoms, and the severity of illness, are highly dependent upon the route of inoculation (3).
There has been increased attention paid to Ft in recent years due to its potential use as a biological weapon. Ft is classified as a Category A agent as it can be disseminated by the aerosol route, has an extremely low infectious dose, and can potentially cause severe morbidity and mortality (4). The use of Ft as a biological weapon is not without precedent. During the World War II occupation of Manchuria, the Japanese biological warfare program exposed men, women and children to F. tularensis to determine its lethal dose (5, 6). Furthermore, during the Cold War, both the United States and the former Soviet Union stockpiled Ft for use as a potential biological weapon (3, 7). Because untreated tularemia can have a mortality rate of >30% (1–3, 8, 9), most of the research into the pathogenesis of Ft has employed the “Live Vaccine Strain” (LVS) (reviewed in refs. (1, 10)) which, while attenuated for humans, is virulent in mice and causes an infection that resembles human tularemia (10).
Host defense against pathogens involves both innate and adaptive immunity. The innate immune response provides pathogen-specific recognition that ultimately shapes the adaptive immune response. Germline-encoded pattern-recognition receptors (PRRs) recognize evolutionarily conserved structures (pathogen-associated molecular patterns; PAMPs) that are present in microbes, but not in the host (11). PRR recognition of PAMPs triggers a coordinated inflammatory response aimed at eliminating invading pathogens (11). Several structurally related “families” of PRRs have been described, e.g., the membrane-anchored Toll-like receptors (TLRs), the cytosolic nucleotide-binding domain, leucine rich repeat containing family (or Nod-like Receptors, NLRs), and the cytosolic RIGI-like receptors (RLRs) (reviewed in refs. (12, 13)). Because Ft initially resides inside a membrane-bound phagosome before escaping into the cytosol (14, 15), it has the potential to engage both membrane-associated and cytosolic PRRs. Indeed, both TLR2 (16–18) and an unknown cytosolic sensor (19, 20) have been implicated in the response to Ft.
We previously showed that Ft LVS-induced macrophage proinflammatory gene expression was entirely TLR2-dependent and that cytokines encoded by these genes were expressed in a highly reproducible temporal pattern (17). Using LVSΔiglC, a mutant strain of Ft LVS that is retained within the phagosome, we now demonstrate that Ft LVS is capable of prolonged TLR2-dependent signaling from within the phagosome. We also show that TLR2-dependent expression of IFN-β, IFN-β-stimulated genes, and IL-1β secretion requires bacterial escape from the phagosome. Lastly, we delineated important roles for both endogenous and inducible IFN-β in the control of Ft LVS intracellular survival within macrophages. In IFN-β−/− macrophages, intracellular Ft LVS displayed enhanced survival, while treatment of Ft LVS-infected macrophages with either rIFN-β or DMXAA, a potent inducer of IFN-β (21), led to a significant decrease in intracellular bacterial burden. In conclusion, the coordinated engagement of multiple PRRs including TLR2, unknown cytosolic sensor(s), and inflammasome activation by Ft LVS is required to elicit the host inflammatory response to this pathogen.
Materials and Methods
Mice
Wild-type (WT) C57BL/6J and TLR2−/− (B6.129-Tlr2<tm1Kir>/J) mice were purchased from the Jackson Laboratory. IFN-β−/− mice (N8 on a C57BL/6 background (22)) were bred homozygously at UMB. Peritoneal macrophages were isolated from mice 4 days after intraperitoneal (i.p.) injection of sterile 3% thioglycollate and cultured as described (16). Macrophages were plated in 6-well (4 × 106 cells/well) or 12-well (2 × 106 cells/well) tissue culture plates (Corning, Inc.). After overnight incubation, cells were washed with PBS to remove non-adherent cells. Cells were cultured in antibiotic-free media (RPMI 1640 containing 2% FBS and 2 mM L-glutamine) for 24 h prior to and during all experiments. Treatments were carried out in duplicate. Cytokine concentrations in culture supernatants were measured by ELISA by the Cytokine Core Laboratory (UMB). All animal experiments were conducted with Institutional Animal Care and Use Committee approval.
Reagents
Mouse rIFN-β (R&D Systems) was used at a final concentration of 100 U/ml. DMXAA (Sigma-Aldrich) was dissolved in sterile 7.5% sodium bicarbonate and used at a final concentration of 100 μg/ml.
Bacteria
Frozen aliquots of Ft LVS (ATCC 29684) were prepared as described (23). All Ft LVS were grown in Mueller Hinton Broth (MHB) (Becton Dickinson Microbiology Systems) supplemented with 1% IsoVitaleX (Becton Dickinson), 0.1% glucose and Ferric PPi (Sigma-Aldrich) Mueller Hinton agar (MHA) was used as solid culture media.
The Ft gene, iglC, was previously shown to be essential for Ft escape from the phagosome and replication in the cytosol (24–28). We constructed LVSΔiglC, a Ft LVS mutant containing deletions in both copies of iglC, by allelic exchange using the suicide plasmid, pFT725. Briefly, 1,470 and 1,495 bp flanking regions, upstream and downstream, respectively, of iglC were amplified from Ft LVS and cloned into pSacB, a pUC19 derivative plasmid containing the sacB gene, to result in pFT634. The following primers were used for amplification:
iglC 5′ sense (P557)
TCCCTAAGGATCCGATCTACAGAAGTTGATAGTGTACTC
iglC 5′ reverse (P556)
TCCCTAAGCTAGCGTCGACCCCGGGTTAGTTATTATTTGTACCGAATAATTCTG
iglC 3′ sense (P555)
TCCCTAAGTCGACCCCGGGTAAGATCGGAGTTGATTCTAATGTTTC
iglC 3′ reverse (P554)
TCCCTAAGCATGCCTGCAGCATGATAAAGAAGAATCTCCACCAGA
Plasmid pFT634 was further modified by the addition of a kanamycin (km) resistance cassette driven by the Ft guaB promoter resulting in pFT725.
Electrocompetent Ft LVS were prepared from two confluent plates of bacteria washed 4 times in sucrose wash buffer (0.5 M sucrose). The final pellet was suspended in 300 μl sucrose wash buffer plus the pFT725 suicide plasmid. Following electroporation, the bacteria were incubated with shaking for 3 h at 37°C, and plated on MHA with 10 μg ml−1 of km. Isolated colonies were analyzed by PCR to evaluate the suicide plasmid integration in the Ft genome. A positive cointegrant colony was selected and grown on MHB with 10% sucrose until the optical density was ~0.4 at 600 nm. The bacteria were spread on MHA plates and resulting colonies were screened for km sensitivity. Isolated km-sensitive colonies were evaluated by PCR for the iglC deletion. A deletion mutant was selected and the second iglC locus was deleted using the same procedure. The deletion of both copies of iglC in the LVSΔiglC mutant was confirmed by PCR and by Southern blot.
Uptake and intracellular replication of LVSΔiglC was evaluated in J774A.1 macrophages (American Type Culture Collection). Ft infection was performed in duplicate in 12-well plates (Costar, Corning, NY). Wells containing 3 × 105 cells per well were infected at a multiplicity of infection (MOI) of 150 for 2 h and maintained at 37°C in humidified air containing 5% CO2. Cells were then washed 3 times with PBS, and incubated in DMEM medium with 50 μg/ml of gentamicin for 1 h. The cells were washed and incubated in DMEM with 2 μg/ml of gentamicin. Ft replication in macrophages was evaluated at 0–72 h post 50 μg/ml gentamicin treatment by lysis of cells with 0.02% SDS-PBS solution and plating 10-fold dilutions on MHA plates. While both WT Ft LVS and LVSΔiglC were taken up comparably by J774A.1 macrophages, the LVSΔiglC mutant was impaired in its ability to replicate: there was a >3-log difference between WT and LVSΔiglC recovery at both 48 and 72 h (Table I). The differences in bacterial recovery were not due to a general growth defect as the growth of an iglC mutant was previously shown to be equivalent to WT Ft LVS in Chamberlain medium (26) and Tryptic soy broth (data not shown).
Peritoneal Macrophage Intracellular Bacteria Assay
Intracellular survival of Ft LVS was evaluated in thioglycollate-elicited peritoneal macrophages. Cells were infected with Ft LVS at an MOI of 10–20 for 2 h. After washing twice with PBS, infected cells were incubated for 1 h in media containing 50 μg/ml gentamicin to kill extracellular bacteria. Cells were washed twice with PBS and then incubated with medium only, or with medium supplemented with DMXAA (100 μg/ml) or rIFN-β (100 U/ml). This addition of medium was defined as the zero time point. At the indicated time points, cells were washed twice with PBS prior to being lysed in 1 ml of ice-cold 0.02% SDS (Teknova) in PBS. For experiments of 48 h or more, media was replaced every 24 h. Lysates were serially diluted and plated on MHA plates.
Real-Time PCR
Total RNA extraction from macrophage cultures, as well as real-time PCR, was carried out as described previously (16).
Statistics
Data analysis was performed using SigmaStat program for Windows (Systat Software, Inc. Richmond, CA).
Results
Retention of Ft LVS in the phagosome differentially affects TLR2-dependent gene and protein expression in murine macrophages
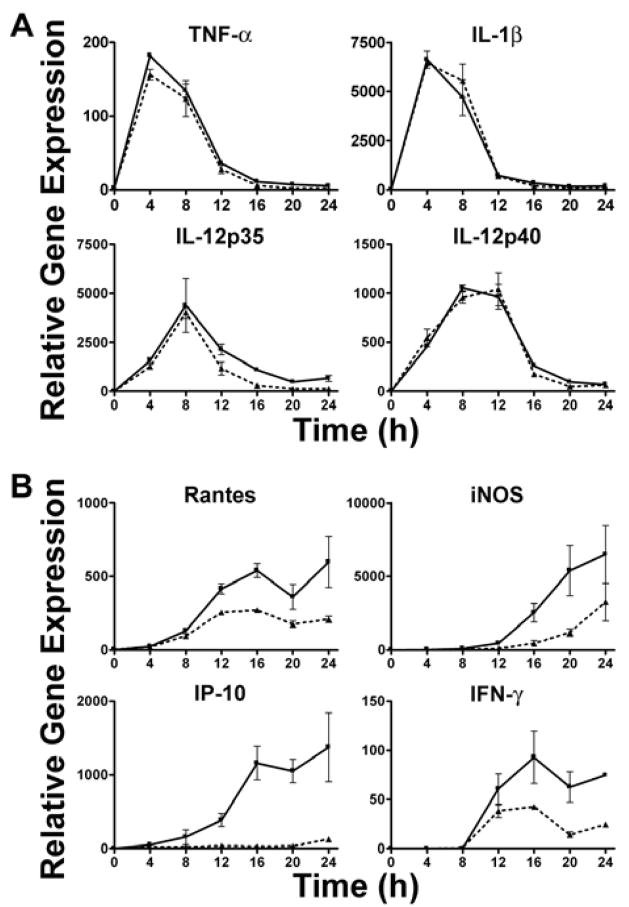
All of the genes examined in Figure 1 were previously shown to be completely TLR2-dependent in Ft LVS-stimulated macrophages as evidenced by a failure to be expressed in Ft LVS infected TLR2−/− macrophages (17). In this study, macrophages derived from WT mice were infected with either Ft LVS or LVSΔiglC (MOI = 5) for up to 24 h and proinflammatory gene and protein expression was measured. When compared to Ft LVS-induced gene expression, levels of TNF-α, IL-1β, KC, Cox-2, IL-12 p35, IL-12 p40, MCP-1, and TLR2 mRNA were greatly enhanced in response to LVSΔiglC (Figure 1A), while expression of IFN-β, IFN-γ, IP-10 and iNOS mRNA was sharply reduced (Figure 1B). The strong enhancement in expression of a subset of proinflammatory genes strongly supports the hypothesis that prolonging the interaction of Ft and TLR2 within the phagosome enhances expression of genes that are solely TLR2-dependent. Conversely, the subset of genes that were not induced at all or poorly induced indicates that in addition to TLR2 signaling, Ft LVS must escape from the phagosome to engage a cytosolic sensor to stimulate their expression.
Figure 1.
Bacterial retention in the phagosome alters macrophage proinflammatory gene expression. Macrophages from WT C57BL/6J were exposed to either WT Ft LVS (
 ) or LVSΔiglC (
) or LVSΔiglC (
 ) (MOI = 5) for 0–24 h. At the indicated time points, total RNA was extracted from the macrophage cultures and analyzed by real-time PCR. Gene expression is reported as relative gene expression compared to macrophages exposed medium only. Displayed are genes whose expression was enhanced (A) or diminished (B) by retention of the bacteria within the phagosome. Data is presented as mean ± S.E.M. Data is of a single representative experiment (n = 3).
) (MOI = 5) for 0–24 h. At the indicated time points, total RNA was extracted from the macrophage cultures and analyzed by real-time PCR. Gene expression is reported as relative gene expression compared to macrophages exposed medium only. Displayed are genes whose expression was enhanced (A) or diminished (B) by retention of the bacteria within the phagosome. Data is presented as mean ± S.E.M. Data is of a single representative experiment (n = 3).
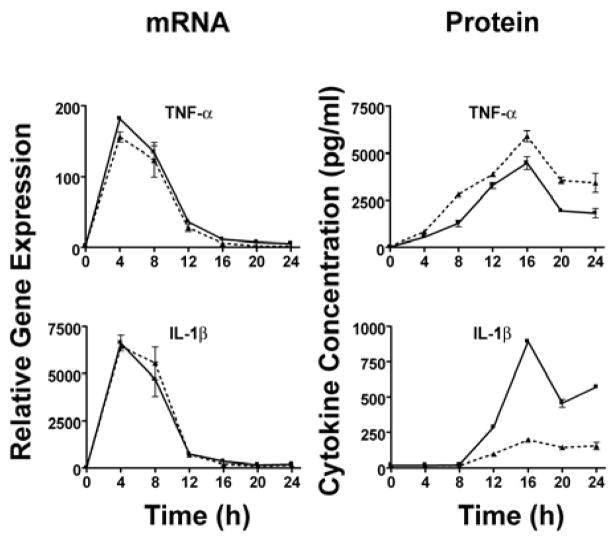
We also measured cytokine protein in culture supernatants to determine if the levels of mRNA correlated with protein concentrations. Consistent with our observations at the level of mRNA (Figure 1), supernatants of LVSΔiglC-infected macrophages displayed elevated levels of TNF-α (Figure 2) and depressed levels of IP-10 (Figure 2) in comparison to the Ft LVS-infected controls. In fact, the only observed discordance between mRNA and protein synthesis was observed for IL-1β expression. While IL-1β mRNA expression was ~10-fold greater in LVSΔiglC-infected cells in comparison to Ft LVS-infected cells 4 h post-infection, there was no detectable IL-1β protein present at any time point examined. This suggests that while Ft-induced IL-1β mRNA expression is fully TLR2-dependent (17), and significantly enhanced when the organism is retained in the phagosome (Figure 1), phagosomal escape of the bacteria is also required for secretion of the active IL-1β (Figure 2).
Figure 2.
Secretion of IL-1β is dependent upon escape from the phagosome. Macrophages from WT C57BL/6J were exposed to either WT Ft LVS (
 ) or LVSΔiglC (
) or LVSΔiglC (
 ) (MOI = 5) for 0–24 h. At the indicated time points, supernatants were collected and total RNA extracted from the macrophage cultures. Gene expression, analyzed by real-time PCR, is reported as relative gene expression compared to macrophages exposed medium only. Supernatants were analyzed by ELISA for the presence of TNF-α, IL-1β, and IP-10. Data is presented as mean ± S.E.M. Data is derived from a single representative experiment (n = 2).
) (MOI = 5) for 0–24 h. At the indicated time points, supernatants were collected and total RNA extracted from the macrophage cultures. Gene expression, analyzed by real-time PCR, is reported as relative gene expression compared to macrophages exposed medium only. Supernatants were analyzed by ELISA for the presence of TNF-α, IL-1β, and IP-10. Data is presented as mean ± S.E.M. Data is derived from a single representative experiment (n = 2).
Role of IFN-β and caspase-1 in Ft LVS macrophage proinflammatory response
Although all of the Ft LVS-induced proinflammatory genes examined in Figure 1 were shown previously to be TLR2-dependent (17, 18), as evidenced by their failure to be upregulated in TLR2−/− macrophages, recent work by Monack and colleagues demonstrated F. novicida induced production of active IL-1β protein is dependent upon activation of the cytosolic inflammasome (19, 20). Inflammasomes are multiprotein molecular platforms that recruit and activate inflammatory caspases, such as caspase-1, in response to stimuli. Once activated, caspase-1 converts inactive pro-IL-1β to its fully active, secreted form. Macrophages derived from caspase-1−/− mice are unable to convert the biologically inactive pro-IL-1β to the active, secreted cytokine (29, 30). Thus, our observation that LVSΔiglC induced IL-1β mRNA, but failed to induce secreted protein, is consistent with the hypothesis that Ft LVS-induced IL-1β depends both on TLR2 signaling and inflammasome activation. Therefore, macrophages derived from WT and caspase1−/− mice were infected with live Ft LVS. Caspase-1−/− macrophages infected with Ft LVS produced levels of TNF-α, IL-1β, KC, Cox-2, IL-12 p35, IL-12 p40, MCP-1, and TLR2 mRNA, as well as IFN-β, IFN-γ, IP-10 and iNOS mRNA that were equal to or greater than WT levels (data not shown). This suggests that the reduced expression of IFN-γ, iNOS, and IP-10 mRNA observed in cells infected with LVSΔiglC did not result from a lack of active caspase-1 or caspase-1-dependent cytokines (e.g., IL-1β, IL-18).
Toshchakov et al. previously showed that TLR2 stimulation of macrophages fails to engage the MyD88-independent pathway and, therefore, fails to elicit not only IFN-β, but also IFN-β-dependent genes including IP-10 and iNOS (31). Since LVSΔiglC infection of macrophages did not induce IFN-β mRNA, and induced decreased levels of iNOS, IP-10, and RANTES mRNA when compared to levels induced by Ft LVS, we tested the hypothesis that IFN-β was required for expression of those genes whose induction had been mitigated by retention of the Ft LVS in the phagosome. Accordingly, macrophages from WT or IFN-β−/− mice were stimulated with Ft LVS (MOI = 5) for 0 to 24 h and proinflammatory gene expression and cytokine production measured. While WT and IFN-β−/− macrophages displayed similar levels of TNF-α, IL-1β, IL-12 p35, and IL-12 p40 mRNA (Figure 3A), there was a sharp reduction in mRNA levels of IP-10, iNOS, IFN-γ and RANTES mRNA in IFN-β−/−-infected macrophages (Figure 3B). This observation confirms that the reduced levels of IP-10, iNOS, IFN-γ (Figure 1B) and RANTES (data not shown) seen after LVSΔiglC infection of macrophages are secondary to diminished IFN-β production and its subsequent autocrine and paracrine utilization by macrophages.
Figure 3.
Genes that require escape of Ft LVS from the phagosome also require expression of IFN-β for normal levels of Ft LVS-induced mRNA. Macrophages from WT C57BL/6J (
 ) or IFN-β−/− (
) or IFN-β−/− (
 ) mice were exposed to Ft LVS (MOI = 5) for 0–24 h. At the indicated time points, supernatants were collected and total RNA extracted from the macrophage cultures. Gene expression, analyzed by real-time PCR, is reported as relative gene expression compared to macrophages exposed medium only. Displayed are genes whose expression was unaffected (A) or diminished (B) by the lack of IFN-β. Data is presented as mean ± S.E.M. Data is derived from a single representative experiment (n = 2).
) mice were exposed to Ft LVS (MOI = 5) for 0–24 h. At the indicated time points, supernatants were collected and total RNA extracted from the macrophage cultures. Gene expression, analyzed by real-time PCR, is reported as relative gene expression compared to macrophages exposed medium only. Displayed are genes whose expression was unaffected (A) or diminished (B) by the lack of IFN-β. Data is presented as mean ± S.E.M. Data is derived from a single representative experiment (n = 2).
The levels of various cytokines in the supernatants of Ft LVS-infected WT- and IFN-β−/−-infected macrophages were also measured. Figure 4 shows that while the level of TNF-α protein and mRNA detected in Ft LVS-infected WT and IFN-β−/− macrophages were nearly equivalent, there was significantly less IL-1β protein detected in the supernatants of IFN-β−/− macrophages in comparison to WT macrophages, despite similar mRNA levels. This suggests that IL-1β secretion from macrophages requires both IFN-β and bacterial escape from the phagosome to facilitate production of mature protein.
Figure 4.
Secretion of IL-1β is dependent upon IFN-β. Macrophages from WT C57BL/6J (
 ) or IFN-β−/− (
) or IFN-β−/− (
 ) mice were exposed to Ft LVS (MOI = 5) for 0–24 h. At the indicated time points, supernatants were collected and total RNA extracted from the macrophage cultures. Gene expression, analyzed by real-time PCR, is reported as relative gene expression compared to peritoneal macrophages exposed medium only. Supernatants were analyzed by ELISA for the presence of TNF-α and IL-1β. Data is presented as mean ± S.E.M. Data is derived from a single representative experiment (n = 2).
) mice were exposed to Ft LVS (MOI = 5) for 0–24 h. At the indicated time points, supernatants were collected and total RNA extracted from the macrophage cultures. Gene expression, analyzed by real-time PCR, is reported as relative gene expression compared to peritoneal macrophages exposed medium only. Supernatants were analyzed by ELISA for the presence of TNF-α and IL-1β. Data is presented as mean ± S.E.M. Data is derived from a single representative experiment (n = 2).
Since IFN-β mRNA induction and IL-1β secretion were both dependent upon Ft LVS escape into the cytosol, we hypothesized that if escape from the phagosome were merely providing IFN-β for the local activation of macrophages, then addition of exogenous rIFN-β to LVSΔiglC-infected macrophages would be sufficient to induce IL-1β secretion. After infection with either LVSΔiglC or Ft LVS, WT macrophages were treated with rIFN-β (100 U/ml). However, rIFN-β treatment failed to restore IL-β secretion in the LVSΔiglC infected macrophages (data not shown). This suggests that although IFN-β has been shown to be required for cleavage of caspase-1 from its zymogen form to an active enzyme (32), IFN-β alone is not sufficient to induce assembly and activation of the caspase-1-containing inflammasome.
Role of IFN-β in the control of the macrophage bacterial burden
IFN-β was previously shown to inhibit replication of intracellular bacteria (33). Therefore, we sought to address the role of IFN-β in the control of macrophage Ft LVS bacterial burden. Both IFN-β−/− and WT macrophages phagocytosed comparable numbers of Ft LVS as evidenced by similar bacterial burdens early after infection. However by 24 h, there was a reduction in bacterial burden in the WT macrophages, while the Ft LVS burden increased in the infected IFN-β−/− macrophages (Figure 5). This suggests that endogenous IFN-β plays a key role in controlling the intracellular replication or killing of Ft LVS within macrophages. This finding also suggested the possibility that treatment of macrophages with exogenous IFN-β might be used to control intracellular survival of Ft LVS in macrophages. We tested this hypothesis by treating WT macrophages with rIFN-β after infection with Ft LVS. Cells were lysed at various times, 6–48 h after treatment, to determine the intracellular bacterial burden (Figure 6). By only 12 h post-treatment, the rIFN-β-treated macrophages exhibited a lower bacterial burden than infected macrophages treated with media alone. By 24 and 48 h, there was ~10-fold and nearly 100-fold difference, respectively, in the recovery of bacteria from media- and rIFN-β-treated macrophages (Figure 6). The efficacy of a potent IFN-β inducer, DMXAA (21), to reduce Ft LVS bacterial burden in macrophages was also tested. Treatment of Ft LVS-infected macrophages with DMXAA reduced bacterial burden ~10-fold within 24 h and ~100-fold by 48 h (Figure 6), consistent with the efficacy of exogenous rIFN-β. The effects of both DMXAA and rIFN-β on Ft LVS recovery at 24 and 48 hours were statistically significant (p = 0.001; Kruskal-Wallis One Way Analysis of Variance on Ranks). The bactericidal impact of DMXAA was mediated through DMXAA’s effect on macrophages as DMXAA had no effect on Ft LVS replication in culture over a 24 h period (data not shown). Thus, rIFN-β and DMXAA augmented the capacity of WT macrophages to control the intracellular survival of Ft LVS.
Figure 5.
Lack of IFN-β increases intracellular Ft LVS survival. Peritoneal macrophages from WT C57BL/6J (
 ) or IFN-β−/− (
) or IFN-β−/− (
 ) mice were exposed to Ft LVS (MOI = 20) for 2 h. After a subsequent 1 h incubation in medium containing 50 μg/ml gentamicin, infected cells were washed 2× with PBS and then incubated with media alone. Cells were lysed and the indicated times and lysates were serially diluted and plated on MHA plates. Data is presented as mean ± S.E.M. (n=2).
) mice were exposed to Ft LVS (MOI = 20) for 2 h. After a subsequent 1 h incubation in medium containing 50 μg/ml gentamicin, infected cells were washed 2× with PBS and then incubated with media alone. Cells were lysed and the indicated times and lysates were serially diluted and plated on MHA plates. Data is presented as mean ± S.E.M. (n=2).
Figure 6.
Addition of rIFN-β or treatment with DMXAA decreases intracellular Ft LVS survival in WT macrophages. Peritoneal macrophages from WT C57BL/6J mice were exposed to Ft LVS (MOI = 20) for 2 h. After a subsequent 1 h incubation in 50 μg/ml gentamicin, infected cells were washed 2× with PBS and then incubated with either media alone (
 ) or media supplemented with DMXAA (100 μg/ml) (
) or media supplemented with DMXAA (100 μg/ml) (
 ), rIFN-β (100 U/ml) (
), rIFN-β (100 U/ml) (
 ). Cells were lysed at the indicated times and lysates were serially diluted and plated on MHA plates. Data is presented as mean ± S.E.M. Data is of a single representative experiment (n = 4)
). Cells were lysed at the indicated times and lysates were serially diluted and plated on MHA plates. Data is presented as mean ± S.E.M. Data is of a single representative experiment (n = 4)
Discussion
Ft is an intracellular pathogen that can inhibit phagosome/lysosome fusion prior to escaping into the cytoplasm where it replicates (15, 28). Previously, we demonstrated that Ft LVS specifically activates a NF-κB luciferase reporter in HEK293T cells transfected with a vector encoding human TLR2, but not with other TLR expression vectors (16), and that murine macrophage cytokine gene expression and secretion in response to Ft LVS is overwhelmingly TLR2-dependent (16–18). Furthermore confocal microscopy revealed that Ft LVS co-localized with both TLR2 and a key adapter protein, MyD88, within macrophages (17). Taken together, this suggests that Ft LVS signals through TLR2 both at the cell surface and within the phagosome as has been demonstrated for other organisms that signal through TLR2 (34–36). We hypothesized that if intra-phagosomal signaling occurred, retention of Ft within the phagosome would enhance the TLR2-mediated proinflammatory response by prolonging the interaction between Ft and TLR2. To test this hypothesis, macrophages were infected with LVSΔiglC, an isogenic mutant strain of Ft LVS that is retained within the phagosome (24–28). Bacterial retention within the phagosome greatly enhanced the mRNA expression of a large subset of proinflammatory genes (Figure 1A), while expression of IFN-β, IFN-γ, IP-10, and iNOS mRNA was markedly reduced (Figure 1B). Interestingly, genes whose expression was enhanced represent those that are induced earliest after macrophage infection, while those with reduced expression represent genes whose expression peaked at the end of the 24 h time course (17).
Failure of LVSΔiglC to induce IFN-β mRNA in WT macrophages supports the previous result that, a related bacterium, F. novicida must escape from the phagosome to induce IFN-β mRNA (19). We previously reported that infection of macrophages with the LVS ΔguaA strain of Ft LVS, a replication-deficient guanine auxotroph, led to TNF-α mRNA expression that was independent of bacterial replication (17). More recently, we observed that the addition of guanine to LVS ΔguaA infected macrophages greatly enhanced IFN-β expression (data not shown). As Ft LVS replicates in the cytosol, this further supports the notion that bacterial escape from the phagosome into the cytosol is required for the induction of IFN-β. Medzhitov and colleagues (37) recently reported that DNA from intracellular bacterial pathogens can induce IFN-β production through a not yet identified IFN regulatory factor 3 (IRF-3)- and Tank Binding Kinase 1 (TBK-1)-dependent, but TLR- and NOD protein-independent cytosolic sensor (38, 39). Experiments are in progress to determine if Ft DNA also activates this same cytosolic sensor. While two Ft LVS lipoproteins, TUL4 and FTT1103, were recently identified as agonist for the TLR2/1 heterodimer (40) and other yet unidentified proteinase K sensitive bacterial products signal though the TLR2/6 heterodimer (40), we are unaware of any reports identifying a cytosolic Ft PAMP.
To test the hypothesis that the reduced levels of IFN-γ, iNOS, and IP-10 in LVSΔiglC-infected macrophages might be attributable, in part, to the lack of IFN-β induction, we compared expression of these genes in Ft LVS-infected WT and IFN-β−/− macrophages. Expression of IFN-γ, iNOS, IP-10, and RANTES mRNA in WT and IFN-β−/− macrophages after infection with Ft LVS mirrored the responses observed in WT macrophages infected with Ft LVS and LVSΔiglC, respectively (Figure 3). In contrast, Ft LVS-induced mRNA expression of TNF-α, IL-1β, IL-12 p35, and IL-12 p40 was equivalent in the WT and IFN-β−/− macrophages (Figure 3). These findings led to the conclusion that the macrophage response to Ft LVS infection can be divided into two major subsets: genes that are strictly TLR2-dependent and whose expression is increased by Ft retention in the phagosome and genes that are both TLR2- and IFN-β-dependent and whose maximal expression requires bacterial escape from the phagosome.
After infection of macrophages with LVSΔiglC or WT Ft LVS, supernatant concentrations of all cytokines examined, except IL-1β, were observed to correspond with their relative levels of mRNA. In contrast, while infection with LVSΔiglC greatly enhanced IL-1β mRNA expression, IL-1β protein was negligible (Figure 2). We have previously demonstrated that IL-1β gene expression and protein secretion are wholly dependent upon TLR2, as TLR2−/− macrophages infected with Ft LVS produced neither IL-1β mRNA nor protein (17). IL-1β is synthesized as a biologically inactive 31 kDa pro-protein that is activated via cleavage by the cysteine protease caspase-1, resulting in the generation of the mature, 17 kDa, biologically active cytokine. Joshi et al. (32) recently reported that TLR4-mediated secretion of IL-1β requires both the MyD88-dependent induction of IL-1β pro-protein, as well as IFN-β production through an MyD88-independent pathway. The authors found that autocrine utilization of IFN-β by macrophages leads to STAT1-mediated processing of pro-caspase-1 that, in turn, generates active caspase-1 that cleaves IL-1β pro-protein into a secreted cytokine. Since production of IL-1β by Ft LVS infection of macrophages appeared to exhibit similar signaling requirements, we next investigated the role of IFN-β in the secretion of active IL-1β.
We found that while the induction of TNF-α and IL-1β mRNA by Ft LVS in WT and IFN-β−/− macrophages were nearly equivalent, significantly less IL-1β protein was released into the supernatants of IFN-β−/− macrophages. These findings extend those of Henry et al. who showed that F. novicida infection of macrophages derived from IFN-α/β receptor-deficient mice did not induce IL-1β secretion (19). Together, these results suggest that while IL-1β gene and pro-protein expression are wholly TLR2-dependent and independent of bacterial escape from the phagosome, Ft LVS-induced IL-1β secretion requires both escape-induced IFN-β expression as well as subsequent autocrine IFN-β-mediated signaling. The failure of rIFN-β treatment to compensate in LVSΔiglC-infected macrophages suggests that Ft LVS must also directly interact with components of the inflammasome to enable recruitment of caspase-1 and facilitate its subsequent activation. Although the specific Nod-like receptors used by F. tularensis LVS has not yet been identified, the Nod-like receptor adapter, ASC, has been shown previously to be essential for F. novicida induced caspase-1 activation and IL-1β secretion in macrophages (20).
That Ft LVS-induced production of IL-1β is regulated at multiple levels suggests that IL-1β may play an important role in tularemia. In a recent study, mice were challenged intranasally with Ft LVS and cytokine levels were measured in the lungs and spleens. Mice that were moribund had significantly higher levels of MIP-2, MCP-1, and IL-6 in both their lungs and spleens compared to mice that survived infection (41). The levels of these immune mediators in the survivors were similar to those observed in uninfected control animals (41). In contrast, at 7 days post infection, mice that were going to survive Ft LVS challenge had significantly higher concentrations of IL-1β in both their lungs and spleens than mice that were moribund (41). Further, Asc−/− and Casp1−/− mice, which fail to produce IL-1β in response to F. novicida infection, succumb more quickly and had a higher bacterial organ burden one day after F. novicida challenge than WT mice (20). These data suggest that IL-1β plays an important role in murine survival of tularemia-like infection.
Finally, the potential role of IFN-β in survival of Ft LVS within macrophages was examined. IFN-β has been shown previously to inhibit replication of other intracellular bacteria (33). While WT and IFN-β−/− macrophages were equally susceptible to infection with Ft LVS, the bacterial burden was greater in the IFN-β−/− macrophages than WT macrophages (Figure 5). This suggested that IFN-β contributes to control of the replication and/or killing of Ft LVS within macrophages. Treatment of Ft LVS-infected macrophages with either rIFN-β or a potent inducer of IFN-β greatly reduced the intracellular survival of Ft LVS, suggesting a potential therapeutic approach to control of Ft infection. Although previous studies have shown that macrophages can be activated by IFN-γ to become microbicidal for Ft LVS (42–45), this is the first report to suggest that endogenous IFN-β, as well as exogenous or inducible IFN-β, produced by macrophages, figure centrally in this process.
In conclusion, these data support a model in which Ft LVS initiates signaling via an interaction with TLR2, either at the cell surface or after phagocytosis. However, escape of Ft LVS from the phagosome into the cytosol is necessary for the induction of IFN-β and IFN-β-inducible genes (e.g., IP-10, iNOS) and we postulate that this occurs through the interaction of Ft with the cytosolic sensor that has been shown to produce IFN-β in response to bacterial DNA (37). Cytosolic localization of Ft as well as IFN-β are required for the activation of the inflammasome that, in turn, recruits and activates caspase-1. Activated caspase-1 mediates the processing of pro-IL-1β to its secreted form. Finally, although autocrine signaling induced by secreted IFN-β controls the survival of Ft LVS, exogenous treatment of macrophages with IFN-β or DMXAA greatly enhanced this effect.
Footnotes
This work was supported in part by NIH NIAID AI-18797 (SNV), NIH NIAID Mid-Atlantic Regional Center of Excellence grant U54 AI-157168 (ASC, EB, and SNV) and an NIAID/NIH Interagency Agreement (KLE).
Abbreviations used in this paper: Ft, Francisella tularensis; LVS, live vaccine strain; MOI, multiplicity of infection; iglC, intracellular growth locus C; WT, wild-type; DMXAA, 5,6-dimethylxanthenone-4-acetic acid; PRR, pattern-recognition receptor; PAMP, pathogen-associated molecular pattern; km, kanamycin
References
- 1.Tarnvik A. Nature of protective immunity to Francisella tularensis. Rev Infect Dis. 1989;11:440–451. [PubMed] [Google Scholar]
- 2.Ellis J, Oyston PC, Green M, Titball RW. Tularemia. Clin Microbiol Rev. 2002;15:631–646. doi: 10.1128/CMR.15.4.631-646.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Friedlander AM, Hauer J, Layton M, Lillibridge SR, McDade JE, Osterholm MT, O’Toole T, Parker G, Perl TM, Russell PK, Tonat K. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763–2773. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 4.Saslaw S, Eigelsbach HT, Prior JA, Wilson HE, Carhart S. Tularemia vaccine study. II Respiratory challenge. Arch Intern Med. 1961;107:702–714. doi: 10.1001/archinte.1961.03620050068007. [DOI] [PubMed] [Google Scholar]
- 5.Harris S. Japanese biological warfare research on humans: a case study of microbiology and ethics. Ann N Y Acad Sci. 1992;666:21–52. doi: 10.1111/j.1749-6632.1992.tb38021.x. [DOI] [PubMed] [Google Scholar]
- 6.Harris S. Japanese biological warfare experiments and other atrocities in Manchuria, 1932–1945, and the subsequent United States cover up: a preliminary assessment. Crime, Law and Social Change. 1991;15:171–199. [Google Scholar]
- 7.Vogel G. Infectious diseases. An obscure weapon of the cold war edges into the limelight. Science. 2003;302:222–223. doi: 10.1126/science.302.5643.222. [DOI] [PubMed] [Google Scholar]
- 8.Tularemia transmitted by insect bites--Wyoming, 2001–2003. MMWR Morb Mortal Wkly Rep. 2005;54:170–173. [PubMed] [Google Scholar]
- 9.Oyston PC, Sjostedt A, Titball RW. Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat Rev Microbiol. 2004;2:967–978. doi: 10.1038/nrmicro1045. [DOI] [PubMed] [Google Scholar]
- 10.Elkins KL, Cowley SC, Bosio CM. Innate and adaptive immune responses to an intracellular bacterium, Francisella tularensis live vaccine strain. Microbes Infect. 2003;5:135–142. doi: 10.1016/s1286-4579(02)00084-9. [DOI] [PubMed] [Google Scholar]
- 11.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Koyama S, Ishii KJ, Kumar H, Tanimoto T, Coban C, Uematsu S, Kawai T, Akira S. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J Immunol. 2007;179:4711–4720. doi: 10.4049/jimmunol.179.7.4711. [DOI] [PubMed] [Google Scholar]
- 13.Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Anthony LD, Burke RD, Nano FE. Growth of Francisella spp. in rodent macrophages. Infect Immun. 1991;59:3291–3296. doi: 10.1128/iai.59.9.3291-3296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golovliov I, Baranov V, Krocova Z, Kovarova H, Sjostedt A. An attenuated strain of the facultative intracellular bacterium Francisella tularensis can escape the phagosome of monocytic cells. Infect Immun. 2003;71:5940–5950. doi: 10.1128/IAI.71.10.5940-5950.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole LE, Elkins KL, Michalek SM, Qureshi N, Eaton LJ, Rallabhandi P, Cuesta N, Vogel SN. Immunologic Consequences of Francisella tularensis Live Vaccine Strain Infection: Role of the Innate Immune Response in Infection and Immunity. J Immunol. 2006;176:6888–6899. doi: 10.4049/jimmunol.176.11.6888. [DOI] [PubMed] [Google Scholar]
- 17.Cole LE, Shirey KA, Barry E, Santiago A, Rallabhandi P, Elkins KL, Puche AC, Michalek SM, Vogel SN. Toll-like receptor 2-mediated signaling requirements for Francisella tularensis live vaccine strain infection of murine macrophages. Infect Immun. 2007;75:4127–4137. doi: 10.1128/IAI.01868-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz J, Zhang P, Martin M, Vogel SN, Michalek SM. Toll-like receptor 2 is required for inflammatory responses to Francisella tularensis LVS. Infect Immun. 2006;74:2809–2816. doi: 10.1128/IAI.74.5.2809-2816.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henry T, Brotcke A, Weiss DS, Thompson LJ, Monack DM. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J Exp Med. 2007;204:987–994. doi: 10.1084/jem.20062665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med. 2005;202:1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts ZJ, Goutagny N, Perera PY, Kato H, Kumar H, Kawai T, Akira S, Savan R, van Echo D, Fitzgerald KA, Young HA, Ching LM, Vogel SN. The chemotherapeutic agent DMXAA potently and specifically activates the TBK1-IRF-3 signaling axis. J Exp Med. 2007;204:1559–1569. doi: 10.1084/jem.20061845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas KE, Galligan CL, Newman RD, Fish EN, Vogel SN. Contribution of interferon-beta to the murine macrophage response to the toll-like receptor 4 agonist, lipopolysaccharide. J Biol Chem. 2006;281:31119–31130. doi: 10.1074/jbc.M604958200. [DOI] [PubMed] [Google Scholar]
- 23.Elkins KL, Winegar RK, Nacy CA, Fortier AH. Introduction of Francisella tularensis at skin sites induces resistance to infection and generation of protective immunity. Microb Pathog. 1992;13:417–421. doi: 10.1016/0882-4010(92)90085-3. [DOI] [PubMed] [Google Scholar]
- 24.Lai XH, Golovliov I, Sjostedt A. Expression of IglC is necessary for intracellular growth and induction of apoptosis in murine macrophages by Francisella tularensis. Microb Pathog. 2004;37:225–230. doi: 10.1016/j.micpath.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Santic M, Molmeret M, Klose KE, Jones S, Kwaik YA. The Francisella tularensis pathogenicity island protein IglC and its regulator MglA are essential for modulating phagosome biogenesis and subsequent bacterial escape into the cytoplasm. Cell Microbiol. 2005;7:969–979. doi: 10.1111/j.1462-5822.2005.00526.x. [DOI] [PubMed] [Google Scholar]
- 26.Golovliov I, Sjostedt A, Mokrievich A, Pavlov V. A method for allelic replacement in Francisella tularensis. FEMS Microbiol Lett. 2003;222:273–280. doi: 10.1016/S0378-1097(03)00313-6. [DOI] [PubMed] [Google Scholar]
- 27.Gray CG, Cowley SC, Cheung KK, Nano FE. The identification of five genetic loci of Francisella novicida associated with intracellular growth. FEMS Microbiol Lett. 2002;215:53–56. doi: 10.1111/j.1574-6968.2002.tb11369.x. [DOI] [PubMed] [Google Scholar]
- 28.Lindgren H, Golovliov I, Baranov V, Ernst RK, Telepnev M, Sjostedt A. Factors affecting the escape of Francisella tularensis from the phagolysosome. J Med Microbiol. 2004;53:953–958. doi: 10.1099/jmm.0.45685-0. [DOI] [PubMed] [Google Scholar]
- 29.Kostura MJ, Tocci MJ, Limjuco G, Chin J, Cameron P, Hillman AG, Chartrain NA, Schmidt JA. Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci U S A. 1989;86:5227–5231. doi: 10.1073/pnas.86.14.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Black RA, Kronheim SR, Sleath PR. Activation of interleukin-1 beta by a co-induced protease. FEBS Lett. 1989;247:386–390. doi: 10.1016/0014-5793(89)81376-6. [DOI] [PubMed] [Google Scholar]
- 31.Toshchakov V, Jones BW, Lentschat A, Silva A, Perera PY, Thomas K, Cody MJ, Zhang S, Williams BR, Major J, Hamilton TA, Fenton MJ, Vogel SN. TLR2 and TLR4 agonists stimulate unique repertoires of host resistance genes in murine macrophages: interferon-beta-dependent signaling in TLR4-mediated responses. J Endotoxin Res. 2003;9:169–175. doi: 10.1179/096805103125001577. [DOI] [PubMed] [Google Scholar]
- 32.Joshi VD, Kalvakolanu DV, Chen W, Zhang L, Kang TJ, Thomas KE, Vogel SN, Cross AS. A role for Stat1 in the regulation of lipopolysaccharide-induced interleukin-1beta expression. J Interferon Cytokine Res. 2006;26:739–747. doi: 10.1089/jir.2006.26.739. [DOI] [PubMed] [Google Scholar]
- 33.Opitz B, Vinzing M, van Laak V, Schmeck B, Heine G, Gunther S, Preissner R, Slevogt H, N’Guessan PD, Eitel J, Goldmann T, Flieger A, Suttorp N, Hippenstiel S. Legionella pneumophila induces IFN-beta in lung epithelial cells via IPS-1 and IRF3, which also control bacterial replication. J Biol Chem. 2006;281:36173–36179. doi: 10.1074/jbc.M604638200. [DOI] [PubMed] [Google Scholar]
- 34.Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 35.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 36.O’Connell CM, Ionova IA, Quayle AJ, Visintin A, Ingalls RR. Localization of TLR2 and MyD88 to Chlamydia trachomatis inclusions. Evidence for signaling by intracellular TLR2 during infection with an obligate intracellular pathogen. J Biol Chem. 2006;281:1652–1659. doi: 10.1074/jbc.M510182200. [DOI] [PubMed] [Google Scholar]
- 37.Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Stockinger S, Reutterer B, Schaljo B, Schellack C, Brunner S, Materna T, Yamamoto M, Akira S, Taniguchi T, Murray PJ, Muller M, Decker T. IFN regulatory factor 3-dependent induction of type I IFNs by intracellular bacteria is mediated by a TLR- and Nod2-independent mechanism. J Immunol. 2004;173:7416–7425. doi: 10.4049/jimmunol.173.12.7416. [DOI] [PubMed] [Google Scholar]
- 39.O’Connell RM, Vaidya SA, Perry AK, Saha SK, Dempsey PW, Cheng G. Immune activation of type I IFNs by Listeria monocytogenes occurs independently of TLR4, TLR2, and receptor interacting protein 2 but involves TNFR-associated NF kappa B kinase-binding kinase 1. J Immunol. 2005;174:1602–1607. doi: 10.4049/jimmunol.174.3.1602. [DOI] [PubMed] [Google Scholar]
- 40.Thakran S, Li H, Lavine CL, Miller MA, Bina JE, Bina XR, Re F. Identification of Francisella tularensis lipoproteins that stimulate the Toll-like receptor (TLR) 2/TLR1 heterodimer. J Biol Chem. 2008;283:3751–3760. doi: 10.1074/jbc.M706854200. [DOI] [PubMed] [Google Scholar]
- 41.Chiavolini D, Alroy J, King CA, Jorth P, Weir S, Madico G, Murphy JR, Wetzler LM. Identification of immunologic and pathologic parameters of death versus survival in respiratory tularemia. Infect Immun. 2008;76:486–496. doi: 10.1128/IAI.00862-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santic M, Molmeret M, Abu Kwaik Y. Modulation of biogenesis of the Francisella tularensis subsp novicida-containing phagosome in quiescent human macrophages and its maturation into a phagolysosome upon activation by IFN-gamma. Cell Microbiol. 2005;7:957–967. doi: 10.1111/j.1462-5822.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- 43.Polsinelli T, Meltzer MS, Fortier AH. Nitric oxide-independent killing of Francisella tularensis by IFN-gamma-stimulated murine alveolar macrophages. J Immunol. 1994;153:1238–1245. [PubMed] [Google Scholar]
- 44.Fortier AH, Polsinelli T, Green SJ, Nacy CA. Activation of macrophages for destruction of Francisella tularensis: identification of cytokines, effector cells, and effector molecules. Infect Immun. 1992;60:817–825. doi: 10.1128/iai.60.3.817-825.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anthony LS, Morrissey PJ, Nano FE. Growth inhibition of Francisella tularensis live vaccine strain by IFN-gamma-activated macrophages is mediated by reactive nitrogen intermediates derived from L-arginine metabolism. J Immunol. 1992;148:1829–1834. [PubMed] [Google Scholar]