Abstract
Francisella tularensis(Ft), the causative agent of tularemia, elicits a potent inflammatory response early in infection, yet persists within host macrophages and can be lethal if left unchecked. We report herein that Ft live vaccine strain (LVS) infection of murine macrophages induced TLR2-dependent expression of “alternative activation” markers that followed the appearance of “classically activated” markers. Intraperitoneal infection with Ft LVS also resulted in induction of alternatively activated macrophages (AA-Mφ). Induction of AA-Mφ by treatment of cells with rIL-4 or by infection with Ft LVS promoted replication of intracellular Ft, in contrast to classically activated (IFN-γ + LPS) macrophages that promoted intracellular killing of Ft LVS. Ft LVS failed to induce alternative activation in IL-4Rα−/− or STAT6−/− macrophages and prolonged the classical inflammatory response in these cells, resulting in intracellular killing of Ft. Treatment of macrophages with anti-IL-4 and anti-IL-13 antibody blunted Ft-induced AA-Mφ differentiation and resulted in increased expression of IL-12 p70 and decreased bacterial replication. In vivo, Ft-infected IL-4Rα−/− mice exhibited increased survival compared to WT mice. Thus, redirection of macrophage differentiation by Ft LVS from a classical to an alternative activation state enables the organism to survive at the expense of the host.
Keywords: Monocytes/macrophages, Bacterial, Tularemia, Innate Immunity, Alternative Activation
Introduction
Francisella tularensis (Ft) is an intracellular, Gram-negative coccobacillus that is the etiological agent of tularemia (reviewed in refs. 1–4). Ft has been classified as a Category A agent by the Center for Disease Control and Prevention because it can be spread via an airborne route, is infectious at very low doses, and causes severe disease that may be fatal unless treated rapidly (1). Most basic research studying the pathogenesis of Ft have utilized a live vaccine strain (LVS) (1,4,5) because it is attenuated in humans, yet causes a disease resembling human tularemia in mice when administered by some routes of infection (4). Infection of mice with Ft LVS results in a pronounced inflammatory response, yet the organism is able to persist within host cells (6,7).
Macrophages differentiate into functionally distinct immunological populations depending on the cytokine environment. Macrophages exposed to IFN-γ and LPS become “classically activated” (CA-Mφ) and function predominantly in inflammation, tissue damage, killing of intracellular microbes, and increased tumoricidal activity (rev. in 8). Conversely, certain cytokines, specifically IL-4 and IL-13, induce an “alternatively activated” phenotype in macrophages. AA-Mφ are principally associated with allergic and parasitic immune responses, tissue remodeling, angiogenesis, tumor promotion, and humoral immunity (rev. in 8), and more recently, have been associated with Alzheimer’s Disease (9,10). Some organisms, such as Mycobacterium tuberculosis, induce alternative activation as a means of survival; however, the organism remains in a persistent, non-replicating state within granulomas (11). A third macrophage differentiation state has been referred to as “MII” or “Mφ-II” and is induced by co-stimulation of macrophages through Fcγ receptor ligation and TLR signaling (12,13). Although the MII phenotype was first associated with increased IL-10 and decreased IL-12 production (14), recent evidence suggests that the inhibition of proinflammatory gene expression in MII macrophages extends to iNOS and other inflammatory gene products regulated by Interferon Regulatory Factor-8 (13). AA-Mφ markers are not expressed by MII macrophages (12). Mφ-II cells may play a role in the exacerbation of infectious diseases where the presence of immune complexes can induce the production of IL-10 from macrophages, allowing for disease progression.
Therefore, we hypothesized that Ft may avoid macrophage-mediated killing by altering the macrophage differentiation state from one that is classically activated to one that is alternatively activated. We observed that after an initial, robust proinflammatory response, Ft LVS infection induces expression of markers associated with AA-Mφ both in vitro and in vivo. This “reprogramming” of macrophage differentiation promotes the survival and replication of the bacterium while mitigating the pro-inflammatory response. The failure of Ft LVS infection to induce AA-Mφ in IL-4Rα−/− or STAT6−/− macrophages results in a sustained CA-Mφ phenotype and clearance of the bacterium. In vivo, IL-4Rα−/− mice exhibited increased survival compared to WT mice. Antibody-mediated neutralization of IL-4 and IL-13 also reversed the AA-Mφ phenotype in wild-type (WT) macrophages, blocked production of IL-4 and IL-13 by macrophages, increased IL-12 p70 secretion, and curtailed intracellular replication. Our data support the notion that macrophage differentiation is malleable, allowing for rapid responses to environmental conditions. This study provides new insights into the innate immune response to Ft LVS infection and the mechanism by which Ft LVS evades the host innate immune response.
Materials and Methods
Reagents
Murine rIL-4 and rIFN-γ were purchased from R&D Systems, Inc. Escherichia coli K235 LPS was prepared as previously described (15). Rabbit anti-mouse FIZZ1 antibody was isolated from serum (kindly provided by Dr. Steven Kunkel, Univ. of Michigan) using an ImmunoPure (A) IgG Purification kit (Pierce Endogen) per the manufacturer’s protocol. The following antibodies were purchased: anti-murine arginase-1 MAb (IgG1) (BD Biosciences); polyclonal goat anti-mouse IL-12 p70, anti-IL-4 MAb, anti-IL-13 MAb (R&D Systems, Inc.); anti-mouse CD206 (mannose receptor):FITC (IgG2a) (AbD Serotec); anti-murine F4/80 MAb (IgG2b) (Abcam); mouse IgG2a and IgG1, rat IgG2b, goat IgG, and rabbit IgG isotype control Abs (Sigma); Cy2-conjugated donkey anti-rabbit IgG, Cy2-conjugated donkey anti-mouse IgG, and Cy3-conjugated donkey anti-goat IgG (Jackson ImmunoResearch Labs).
Frozen aliquots of Ft LVS (ATCC 29684; American Type Culture Collection were kindly provided by Dr. Karen Elkins (FDA, Rockville, MD) and stored as previously described (16).
Macrophage cell culture
Six to 8-week old C57BL/6J, TLR2−/− (B6.129-Tlr2<tm1Kir>/J), and BALB/cByJ mice were purchased from the Jackson Laboratory. Breeding pairs of IL-4Rα−/− and STAT6−/− mice on a BALB/c background were obtained from Dr. Nancy Noben-Trauth (George Washington University Medical Center, Washington, DC) and Dr. William E. Paul (Laboratory of Immunology, NIAID, NIH, Bethesda, MD), respectively, and bred within UMB’s accredited facility. Peritoneal macrophages isolated from mice 4 days after i.p. injection of sterile 3% thioglycollate were cultured as described previously (17). Macrophages were plated in 6-well (4 × 106 cells/well) or 12-well (2 × 106 cells/well) tissue culture plates (Corning, Inc.). After overnight incubation, cells were washed with PBS to remove non-adherent cells and were cultured in antibiotic-free media for 24 h prior to treatment. All animal experiments were conducted with institutional approval.
Thioglycollate-elicited peritoneal macrophages [99.5% positive for F4/80] were plated in 6- (4 × 106) or 12-well (2 × 106 cells) plates and stimulated with medium alone, rIL-4 (40 ng/ml), or rIFN-γ (20 ng/ml) plus LPS (10 ng/ml) for the indicated times as positive controls for AA-Mφ and CA-Mφ, respectively. Macrophages were infected with Ft LVS (MOI = 5; confirmed by colony count) and incubated at 37° C. In initial experiments (e.g., Fig. 1A), macrophages were not washed to remove extracellular bacteria. For all other experiments, cells were washed with PBS 1 h post-infection and placed in RPMI with gentamicin (50 μg/ml) for 1 h to kill extracellular bacteria. Cells were washed again to remove gentamicin and then lysed at the indicated time for preparation of RNA or measuring intracellular replication of Ft LVS. Ft LVS in cell lysates were determined by colony count. Ft LVS 16S rRNA was also quantified by real-time PCR to confirm the results of colony counts (18).
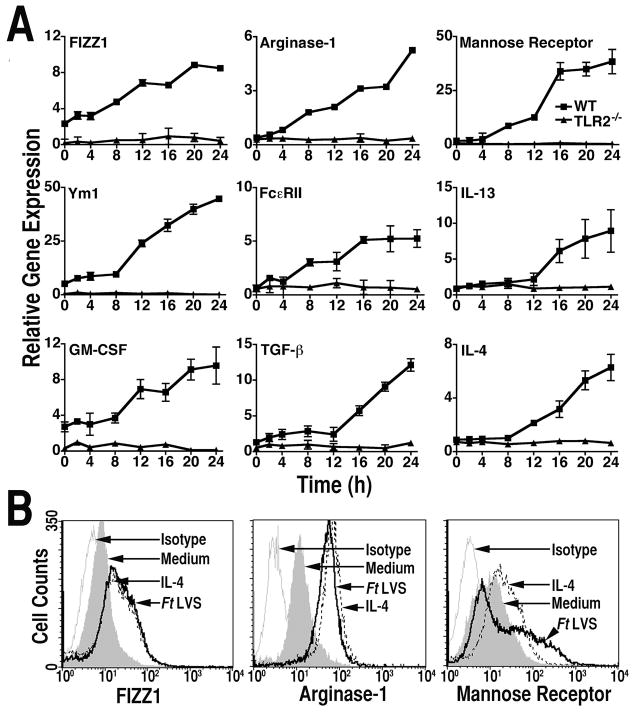
Figure 1.
Induction of AA-Mø by Ft LVS. (A) Peritoneal macrophages from C57BL/6 WT or TLR2−/− mice were infected with Ft LVS (MOI = 5). Total RNA was extracted from the macrophage cultures analyzed by real-time PCR for genes associated with AA-Mø. Gene expression is reported as relative gene expression compared to peritoneal macrophages exposed to medium only. All treatments were performed in triplicate, and data are presented as means ± SEM. Data are represented as a single experiment (n = 2). (B) Peritoneal macrophages from C57BL/6 mice were treated, in triplicate, with medium only, rIL-4 (40 ng/ml), or exposed to Ft LVS (MOI = 5) for 48 h. Cells were harvested and stained for protein expression of FIZZ1, arginase-1 or mannose receptor by FACS analysis. Data presented are histograms from a single representative experiment (n = 4).
Real-time PCR
Total RNA was isolated from macrophage cultures using Trizol (Invitrogen) according to manufacturer’s protocol. Real-time PCR was performed with an ABI 7900HT (Applied Biosystems) sequence detection system and software as previously described (19). Relative mRNA levels for specific genes are reported as relative gene expression normalized to untreated control samples. The sequences for IL-4, IL-12 p35, IL-12 p40, HPRT, iNOS, and 16S Ft LVS rRNA have been reported previously (18). New primers were designed using the Primer Express program (Applied Biosystems) in conjuction with GenBank sequences using the Blastn program and include the following:
Arginase-1, sense (5′-CAGAAGAATGGAAGAGTCAG-3′) and antisense (5′-CAGATATGCAGGCAGGGAGTCACC-3′);
FIZZ1, sense (5′-GGTCCCAGTGCATATGGATGAGACCATAGA-3′) and antisense (5′-CACCTCTTCACTCGAGGGACAGTTGGCAGC-3′);
Ym1, sense (5′-CATGAGCAAGACTTGCGTGAC-3′) and antisense (5′-GGTCCAAACTTCCATCCTCCA-3′);
IL-13, sense (5′-GGAGCTGAGCAACATCACACAA-3′) and antisense (5′-GAATCCAGGGCTACACAGAACC-3′);
Mannose Receptor, sense (5′-GATATGAAGCCATGTACTCCTTACTGG-3′) and antisense (5′-GGCAGAGGTGCAGTCTGCAT-3′);
TGF-β, sense (5′-GCAACATGTGGAACTCTACCAGAA-3′) and antisense (5′-GACGTCAAAAGACAGCCACTCA-3′);
GM-CSF, sense (5′-TTGAATGAAGAGGTAGAAGTCGTCTC-3′) and antisense (5′-AATTGCCCCGTAGACCCTG-3′);
Fcε Receptor II (FcεRII), sense (5′-CAGCTGGGAGACACTGCAATT-3′) and antisense (5′-ATCTGAACAACCTGGGACTTCTG-3′)
All primers were synthesized at the Biopolymer and Genetics Core Facility (UMB).
Flow cytometry analysis
To preclude the need for scraping cells for flow cytometric studies, macrophages from C57BL/6J, BALB/cByJ, IL-4Rα−/−, or STAT6−/− mice were cultured on 6-well low cluster and low adhesion plates (Corning, Inc.). Washes were carried out in centrifuge tubes and the cells were re-plated and treated with medium alone, rIL-4 (40 ng/ml), IFN-γ (20 ng/ml) plus LPS (10 ng/ml), or infected with Ft LVS (MOI = 5) for 24 or 48 h. Cells were harvested for analysis by gentle shaking, washed with PBS, and then fixed with 4% p-formaldehyde (PFA) for FIZZ1 and arginase-1 or with 70% methanol for mannose receptor for 10 min at room temperature. Cells were blocked and permeabilized for 30 min with PBST (PBS, 1% BSA, 1% normal donkey serum, 0.3 % Triton X-100) at room temperature. FIZZ1 and IL-12 p70 were detected using polyclonal antibodies directed against the proteins, followed by Cy2-conjugated donkey anti-rabbit IgG, or Cy3-conjugated donkey anti-goat IgG, respectively. Arginase-1 was detected by staining the cells with a MAb, followed by a secondary Cy2-conjugated donkey anti-mouse IgG2a antibody and mannose receptor expression was determined by using a primary FITC-conjugated monoclonal antibody. Cells were washed in PBST and suspended in PBS for immediate analysis using a FACSCalibur. Analytic gates were set to exclude cellular debris and aggregates. CELLQuest software (Becton Dickinson) was used to analyze the data.
To assess whether in vivo infection with Ft LVS also resulted in AA-Mφ, C57BL/6 mice were injected i.p. with saline or Ft LVS (10,000 CFU) for 3 days. The mice were sacrificed and peritoneal cells were harvested by lavage. After fixation with 4% PFA, cells were simultaneously stained for FIZZ1 (an AA-Mφ marker) and F4/80, a macrophage marker. FIZZ1 was detected as described above and F4/80 was detected by staining the cells with a MAb, followed by a secondary Cy3-conjugated donkey anti-rat IgG antibody. Cells were washed in PBST and suspended in PBS for FACS analysis. Macrophages were identified as F4/80 positive cells with high forward and side scatter properties. A gate was placed on the F4/80 positive cells to exclude any non-macrophage cells and cell debris and analyzed for FIZZ1 expression. Experimental and control groups consisted of six animals each.
Antibody neutralization assays
Peritoneal macrophages were treated with medium alone, rIL-4 (40 ng/ml), rIL-13 (40 ng/ml), or infected with Ft LVS. Parallel sets of cells were additionally treated with either isotype control IgG, anti-IL-4 (100 μg), anti-IL-13 (100 μg), or both anti-IL-4 and anti-IL-13 antibodies. After 48 h, total RNA was isolated and FIZZ1, IL-12 p40, and IL-10 mRNA was analyzed by real-time PCR, or protein expression for FIZZ1 and IL-12 p70 determined by FACS analysis. To assess the effect of anti-IL-4 and/or anti-IL-13 antibody treatment on Ft LVS survival, macrophages were treated as described for mRNA or FACS analysis and, after 48 h, the number of Ft LVS in cell lysates was determined by colony counts.
In vivo infection with Ft LVS
To assess the survival rate of mice infected with Ft LVS, BALB/cByJ WT and IL-4Rα−/− mice were inoculated i.p. with either saline or Ft LVS (10,000 CFU); 4–5 mice per treatment in 4 separate experiments). Survival was monitored for up to 10 days post-infection.
Cytokine measurements
Cytokine concentrations in cell culture supernatants were measured by ELISA by the Cytokine Core Laboratory (UMB).
Statistics
Statistical differences between two groups were determined using an unpaired, two-tailed Student’s t test with significance set at p <0.05. For comparisons between three or more groups, analysis was done by one-way ANOVA followed by a Tukey’s multiple comparison test with significance determined at p < 0.05. All statistical analyses were performed using Prism Graph Pad software.
Results
Ft LVS infection induces macrophage “alternative activation” markers
Ft LVS induces a robust inflammatory response in mice and murine macrophages (20–24). While this array of proinflammatory cytokines and mediators would be expected to control the intracellular bacterial burden, this potent, early inflammatory response fails to control bacterial replication and mice succumb to i.p. or i.v. infection. Therefore, we hypothesized that Ft LVS might evade destruction by reprogramming the differentiation state of infected macrophages, leading to a conversion of naïve or CA-Mφ to an AA-Mφ phenotype. To test this hypothesis, homogenous populations of macophages were prepared from thioglycollate treated WT C57BL/6J and TLR2−/− mice and infected with Ft LVS (MOI = 5) for up to 24 h; gene expression for markers associated with AA-Mφ were measured by quantitative real-time PCR. Ft LVS infection of WT macrophages resulted in an up-regulation of mRNA for all AA-Mφ markers examined: FIZZ1 and Ym1 (25–27), arginase-1, the macrophage mannose receptor, and anti-inflammatory cytokines IL-4, IL-13, and TGF-β, as well as GM-CSF, known to induce myeloid cell differentiation (28). In contrast, Ft LVS infection failed to stimulate macrophages from TLR2−/− mice to up-regulate mRNA expression of any of these AA-Mφ markers (Fig. 1A). This finding indicates that TLR2 is required for AA-Mφ gene induction as well as pro-inflammatory gene expression (20). These findings were confirmed at the protein level: flow cytometric analysis of primary murine macrophages infected with Ft LVS revealed a significant (p < 0.01) up-regulation of FIZZ1 protein (Fig. 1B) that was comparable in magnitude to that observed by stimulation of macrophages with rIL-4, a known inducer of AA-Mφ. Increased protein expression in macrophages infected with Ft LVS was also observed for arginase-1 and the mannose receptor (Fig. 1B).
Ft LVS induces alternative activation of macrophages for survival in the host
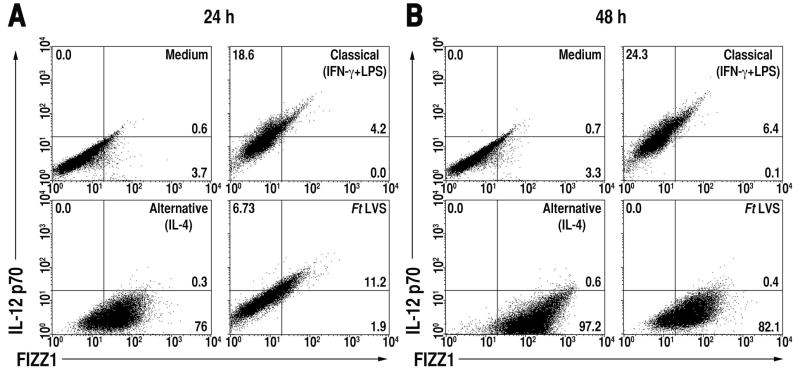
CA-Mφ and AA-Mφ exhibit differential regulation of cytokines, chemokines, and surface receptors. CA-Mφ produce high levels of IL-12 p70, TNF-α, IL-1β, but low levels of IL-4, IL-13, and TGF-β (29,30). While our previous reports (18,20) showed that Ft LVS induces a strong pro-inflammatory response in vivo and in vitro, our current data strongly suggests that after an initial pro-inflammatory or classical activation response, Ft LVS infected macrophages are directed to differentiate into anti-inflammatory AA-Mφ. To determine the proportion of cells positive for either CA-Mφ or AA-Mφ markers, 2-color FACS analysis was performed for concurrent detection of the CA-Mφ marker, IL-12 p70, and the AA-Mφ marker, FIZZ1. Primary murine macrophages were treated with medium alone, rIFN-γ and LPS (to induce CA-Mφ), rIL-4 only (to induce AA-Mφ), or infected with Ft LVS for 24 or 48 h. Cells were then fixed and stained concurrently for IL-12 p70 and FIZZ1 and analyzed by flow cytometry. Untreated macrophages expressed essentially no IL-12 p70 or FIZZ1 at 24 and 48 h (Fig. 2A and 2B, upper left panels). Consistent with CA-Mφ polarization, stimulation of macrophages by rIFN-γ and LPS induced expression of IL-12 p70 (18.6% and 24.3% positive at 24 and 48 h, respectively), but not FIZZ1 (Fig. 2A and 2B, upper right panels), whereas alternative activation of macrophages by rIL-4 led to high levels of FIZZ1 (76% and 97.2% positive at 24 and 48 h, respectively), but little IL-12 p70 (Fig. 2A and 2B, lower left panels). Macrophages infected with Ft LVS for 24 h show a similar phenotype to CA-Mφ, with relatively higher expression IL-12 p70 and lower FIZZ1 expression (Fig. 2A, lower right panel), but by 48 h post-infection, 82% (p < 0.001) of the macrophage population became positive for FIZZ1 (Fig. 2B, lower right panel), indicating that the majority of macrophages acquire an AA-Mφ phenotype late in infection.
Figure 2.
Ft LVS redirects macrophage differentiation from CA-Mø to AA-Mø over time. Peritoneal macrophages from C57BL/6 mice were treated with medium only, IFN-γ (20 ng/ml) plus LPS (10 ng/ml), rIL-4 (40 ng/ml), or infected with Ft LVS (MOI = 5) for either (A) 24 or (B) 48 h. Cells were harvested and simultaneously stained for FIZZ1 and IL-12 p70. Protein expression was determined by FACS analysis. The numbers in the quadrants indicate the percent of cells within that quadrant and have been rounded to the nearest one-tenth of a percent. All treatments were performed in triplicate and data shown are from a single representative experiment (n = 3).
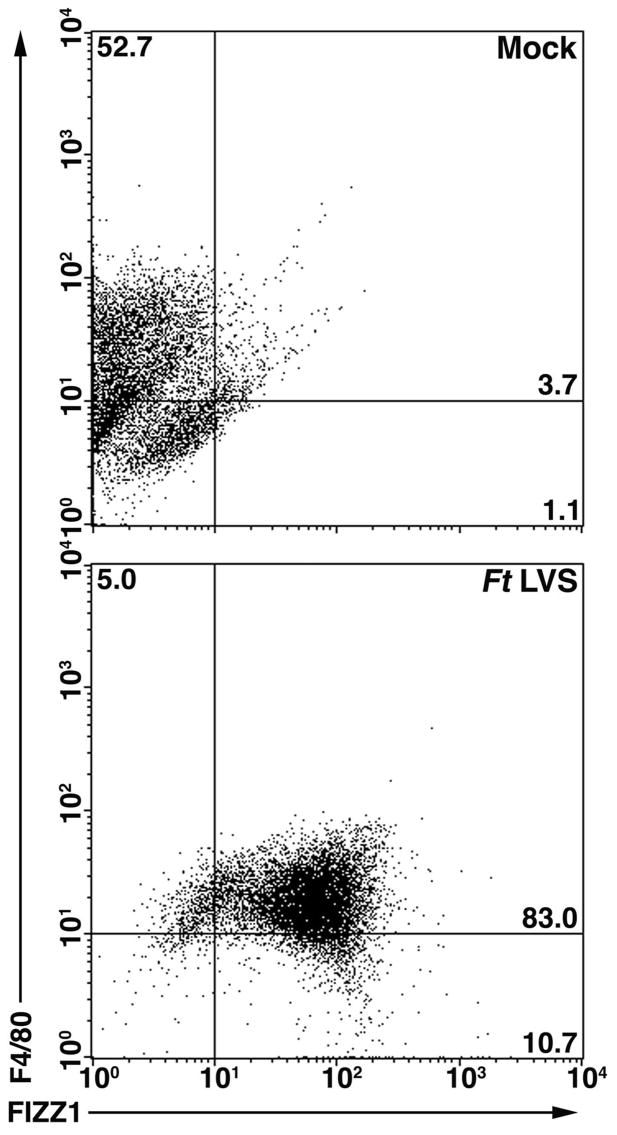
In vivo infection showed a similar trend of AA-Mφ induction in peritoneal macrophages. Mice were injected i.p. with saline (mock) or Ft LVS (10,000 CFU) and sacrificed on day 3 post-infection. Peritoneal cells were harvested and fixed as described above. The cells were stained for FIZZ1 (an AA-Mφ marker) and F4/80 (a pan-macrophage marker) and analyzed by 2-color FACS. Mock-infected mice showed low expression of FIZZ1, but high expression of F4/80 (Fig. 3, top panel). Consistent with the lower right panels of Fig. 2A and 2B, macrophages from mice infected with Ft LVS were alternatively activated as evidenced by the significant (p < 0.001) increase in FIZZ1 expression (Fig. 3, bottom panel).
Figure 3.
In vivo Ft LVS infection results in AA-Mφ. C57BL/6 mice were administered saline or Ft LVS (10,000 CFU) i.p. Three days later, the mice were sacrificed and peritoneal macrophages were harvested and simultaneously stained for FIZZ1 and F4/80. Protein expression was determined by FACS analysis. The numbers in the quadrants indicate the percent of cells within that quadrant and have been rounded to the nearest one-tenth of a percent. Six mice were used for each treatment and data shown are from a single representative mouse.
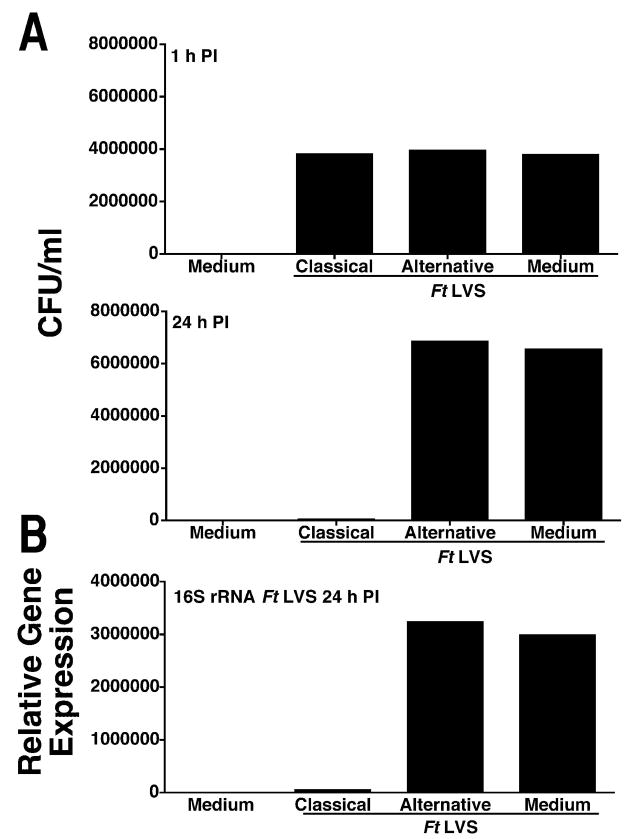
We next tested the hypothesis that Ft LVS survives within macrophages by inducing an AA-Mφ phenotype. Primary murine macrophages were treated with medium only, rIFN-γ and LPS, or rIL-4 for 48 h. Cells were then infected with Ft LVS (MOI = 5) for 1 h followed by 1 h of gentamicin treatment to kill extracellular bacteria. After gentamicin treatment, cells were either immediately lysed or incubated for an addition 24 h in fresh media and then lysed. Intracellular Ft LVS in cell lysates were quantified by colony counts. At 1 h post-infection, all infected cells contained approximately the same number of Ft LVS (Fig. 4A), indicating that there were no differences in bacterial uptake among the three treatment groups. However, by 24 h post-infection (Fig. 4A), CA-Mφ exhibited significantly lower bacterial loads when compared with AA-Mφ induced by either rIL-4 or by infection only. Comparison of the relative gene expression for Ft LVS 16S rRNA, a surrogate marker for colony counts (18), yielded similar results (Fig. 4B). Most importantly, similar numbers of bacterial CFU were recovered from the Ft-infected medium- or IL-4-pretreated macrophages, supporting the hypothesis that Ft LVS induces macrophages to become alternatively activated for its own survival.
Figure 4.
Ft LVS induction of AA-Mφ results in intracellular survival. Peritoneal macrophages from C57BL/6 mice were treated with medium only, rIFN-γ (20 ng/ml) plus LPS (10 ng/ml), or rIL-4 (40 ng/ml) for 48 h. The cells were then infected with Ft LVS (MOI 5) for either 1 or 24 h. Intracellular Ft LVS was determined by (A) colony counts from the plated lysates, or (B) real-time PCR amplification of the Ft LVS 16S rRNA in the same samples as analyzed in (A). All treatments were performed in triplicate, and data are presented as means ± SEM (the SEMs are too small to be seen). Data are derived from a single representative experiment (n = 4).
Failure of Ft LVS to induce AA-Mφ results in prolonged inflammatory response
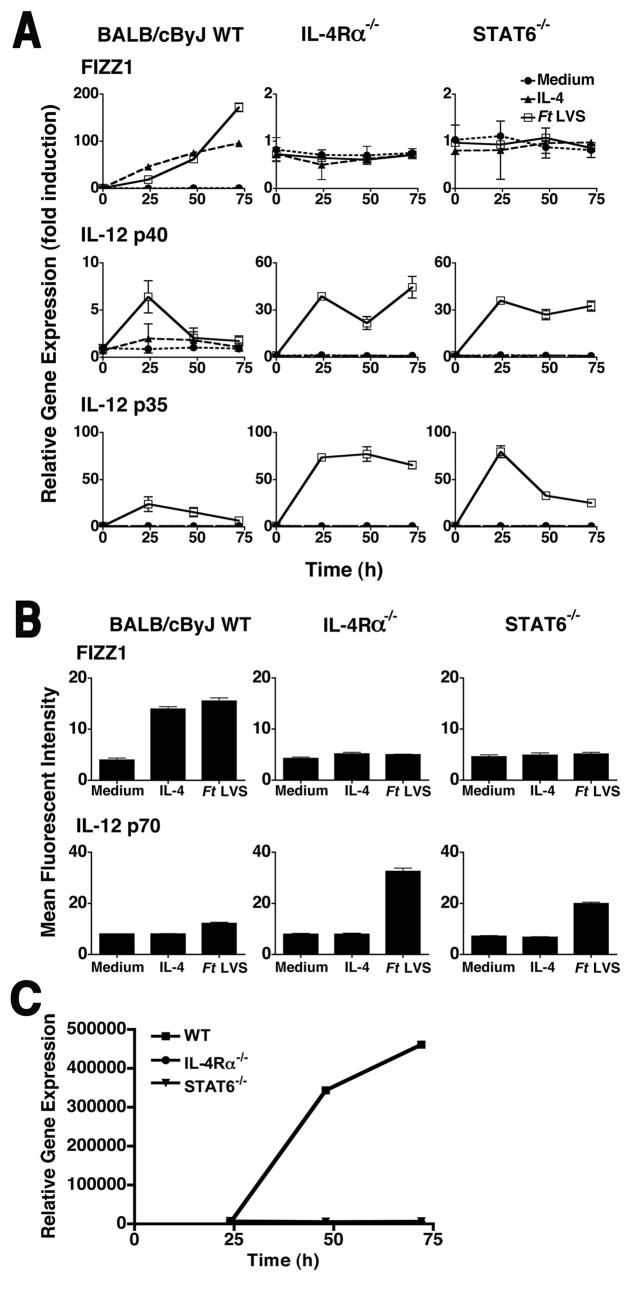
IL-4 and IL-13 use the same signaling pathways to induce AA-Mφ (31–33). Both cytokines signal through the IL-4Rα chain and induce the recruitment of the transcription factor STAT6 that translocates to the nucleus where it activates transcription of STAT6-dependent genes. To assess whether Ft LVS is dependent upon this signaling pathway to induce AA-Mφ, thioglycollate-elicited macrophages derived from WT BALB/cByJ, IL-4Rα−/−, or STAT6−/− mice were stimulated with medium alone, rIL-4 alone, or infected with Ft LVS (MOI = 5) for 24, 48, or 72 h, and AA-Mφ and CA-Mφ gene expression and protein production were measured. As expected, FIZZ1 gene expression increased upon stimulation of WT macrophages with either rIL-4 or Ft LVS (Fig. 5A, top panels). In contrast, macrophages deficient in either IL-4Rα or STAT6 failed to induce FIZZ1 mRNA in response to IL-4 stimulation; this was also observed upon infection of macrophages with Ft LVS. These results support the hypothesis that both IL-4Rα- and STAT6-dependent signaling is required for differentiation of AA-Mφ directly induced by Ft LVS infection. These data were further supported by flow cytometric analyses for detection of FIZZ1 protein in WT, IL-4Rα−/−, and STAT6−/− primary macrophages (Fig. 5B, top graphs). In contrast to the failure of Ft LVS-infected IL-4Rα−/− and STAT6−/− macrophages to develop AA-Mφ markers, Ft LVS infection of IL-4Rα−/− and STAT6−/− macrophages prolonged expression of steady-state levels of IL-12 p35 and IL-12 p40 mRNA that resulted in increased levels of IL-12 p70 protein (Fig. 5B, bottom graphs). Similar enhancements in pro-inflammatory gene expression were observed for TNF-α and IL-1β (data not shown). These data indicate that the failure to induce STAT6 signaling via IL-4Rα leads to the prolongation of the CA-Mφ phenotype. Consistent with these observations, infected WT cells that expressed AA-Mφ markers were highly permissive for Ft LVS replication, while cells deficient in IL-4Rα or STAT6 had significantly lower bacterial loads as measured by relative levels of Ft-specific 16S rRNA (Figure 5C).
Figure 5.
Failure to induce AA-Mφ results in prolonged inflammatory response by the host. (A) Peritoneal macrophages from WT BALB/cByJ, IL-4Rα−/−, or STAT6−/− mice were treated with medium only, rIL-4 (40 ng/ml), or exposed to Ft LVS (MOI = 5) for 0 to 72 h. Total RNA was extracted and analyzed by real-time PCR for genes associated with AA-Mφ and CA-Mφ. Gene expression is reported as relative gene expression normalized to untreated control samples. All treatments were performed in triplicate, and data are presented as means ± SEM. (B) Peritoneal macrophages from WT BALB/cByJ, IL-4Rα−/−, or STAT6−/− mice were treated with medium only, rIL-4 (40 ng/ml) or exposed to Ft LVS (MOI = 5) for 48 h. Cells were harvested and stained for expression of either FIZZ1 or IL-12 p70 protein and subjected to FACS analysis. All treatments were performed in triplicate, and data are presented as means ± SEM. Data are derived from a single representative experiment (n = 3). (C) Real-time PCR for Ft LVS 16S rRNA was carried out using the same samples as (A). Gene expression is reported as relative gene expression.
Neutralization of IL-4 and IL-13 blocks Ft LVS-induced AA-Mφ activation and intracellular Ft replication
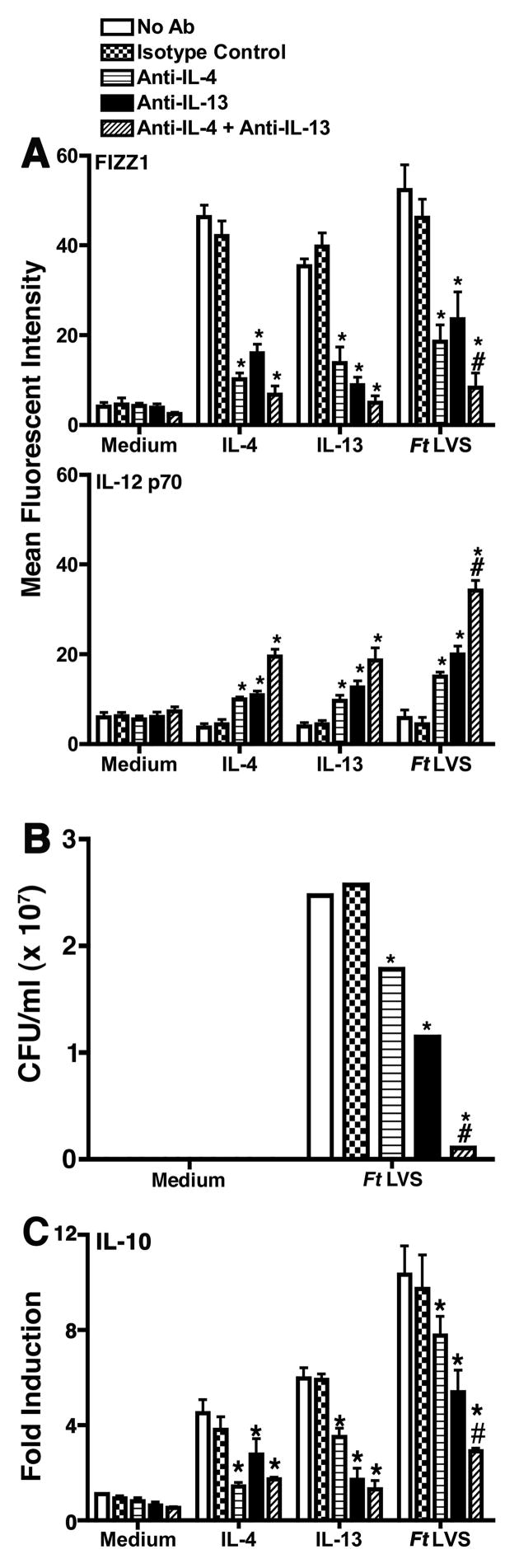
Since macrophages deficient in IL-4Rα or STAT6 failed to become alternatively activated, we hypothesized that neutralization of the cytokines required for AA-Mφ activation would be expected to yield similar results. To test this hypothesis, WT macrophages were treated without or with medium only, an isotype control IgG, anti-IL-4 and/or anti-IL-13 antibodies. Treatment of cells with either anti-IL-4 or anti-IL-13 antibodies, but not medium or the isotype control antibody, significantly (p < 0.001) inhibited expression of FIZZ1 in cells treated with rIL-4 or rIL-13, or infected with Ft LVS, and the presence of both neutralizing antibodies completed inhibited FIZZ1 mRNA (data not shown) and protein expression in infected cells (Figure 6A). Conversely, macrophages stimulated with rIL-4 or rIL-13, or infected with Ft LVS, subsequent to antibody treatment, exhibited a compensatory increase in the expression of IL-12 p40 mRNA (data not shown) and IL-12 p70 protein (Figure 6A). This observation further supports the data obtained in macrophages derived from IL-4Rα−/− and STAT6−/− mice and indicates that macrophage-derived IL-4 and IL-13 drive AA-Mφ differentiation in Ft LVS-infected cells, and conversely, neutralization of IL-4 and IL-13 produced by the macrophages results in a CA-Mφ phenotype. Antibody neutralization of either IL-4 or IL-13 resulted in significantly lower bacterial counts in comparison to cells treated with medium only or with the isotype control antibody; however, neutralization of both cytokines resulted in nearly complete inhibition of bacterial replication (Figure 6B). Finally, antibody-mediated neutralization of IL-4 and IL-13 led to a diminished capacity of Ft LVS-infected macrophages to produce IL-10 (Figure 6C), thus mitigating the potential effects of this anti-inflammatory cytokine on induction proinflammatory cytokines such as IL-12.
Figure 6.
Neutralization of IL-4 and IL-13 during Ft LVS infection results in prolonged macrophage classical activation. (A) Peritoneal macrophages were cultured in medium, treated with rIL-4 (40 ng/ml) or rIL-13 (40 ng/ml), or infected with Ft LVS (MOI = 5). Cultures were also treated with medium only or an isotype-matched IgG control antibody or neutralizing anti-IL-4 (100 μg), anti-IL-13 (100 μg), or both antibodies for 48 h. Cells were harvested and stained for expression of FIZZ1 and IL-12 p70 protein and subjected to FACS analysis. All treatments were performed in triplicate, and data are presented as means ± SEM. Data are represented as a single representative experiment (n = 3). (B) Peritoneal macrophages were cultured in medium alone, or infected with Ft LVS. Cultures were either treated with medium only or an isotype control IgG or neutralizing anti-IL-4, anti-IL-13, or both for 48 h. Intracellular Ft LVS was determined by colony counts from plated lysates. (C) Peritoneal macrophages were treated the same as in (A). Total RNA was extracted and analyzed by real-time PCR for IL-10. Gene expression is reported as fold induction normalized to untreated control samples. All treatments were performed in triplicate, and data are presented as means ± SEM. Statistical significance for comparison between one neutralizing antibody and control samples is represented as *, while statistical significance for treatment with both neutralizing antibodies is represented as #.
Ft LVS infection of macrophages alters the cytokine milieu to favor development of the AA-Mø phenotype
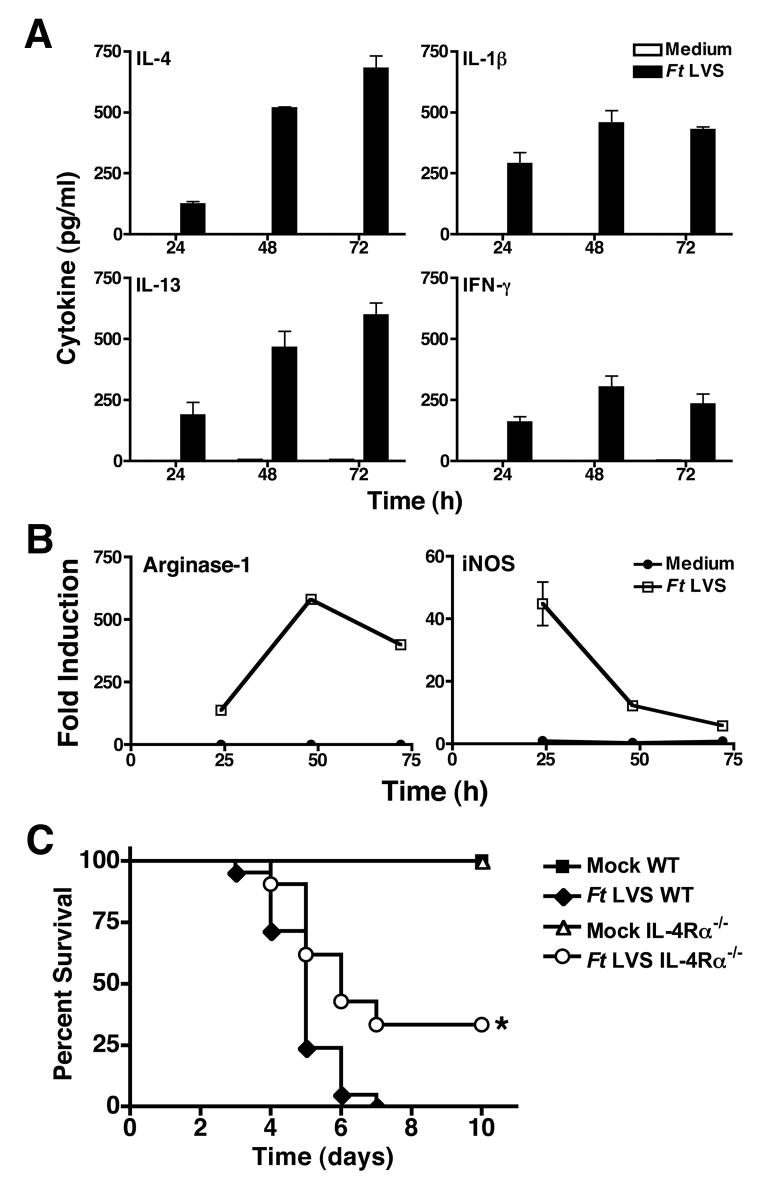
Ft LVS infection (MOI = 5) strongly induced both IL-4 and IL-13, with maximum production detected 72 h post-infection (Figure 7A). Ft LVS also induced the production of both IL-1β and IFN-γ, however, production of these cytokines peaked at 48 h, with a slight decrease by 72 h (Figure 7A). These observations are consistent with the hypothesis that Ft LVS-infection results in a cytokine milieu that favors the development of an AA-Mø phenotype.
Figure 7.
Production of IL-4 and IL-13 promote the AA-Mφ phenotype while attenuating the CA-Mφ phenotype during Ft LVS infection. Peritoneal macrophages were treated with medium alone or infected with Ft LVS (MOI = 5). (A) Cell supernatants were harvested at the indicated times and analyzed by ELISA for production of IL-4, IL-13, IL-1β, and IFN-γ. (B) Total RNA was extracted and analyzed by real-time PCR for arginase-1 and iNOS mRNA. Gene expression is reported as fold induction normalized to untreated control samples. All treatments were performed in triplicate, and data are presented as means ± SEM of 4 individual experiments. (C) BALB/cByJ WT and IL-4Rα−/− mice were i.p. inoculated with either saline or Ft LVS (10,000 CFU). Survival was monitored for up to 10 days. Data is pooled from 4 separate experiments with 4–5 mice/treatment/experiment. Statistical significance for comparison between Ft LVS-infected WT and Ft LVS-infected IL-4R−/− mice is represented as * (p < 0.001).
CA-Mφ produce iNOS that enables the cell to kill many intracellular parasites through the production of NO.. AA-Mφ counteract iNOS activity by producing arginase-1 that competes with iNOS for the same substrate, arginine, thereby allowing survival of intracellular microbes (34,35). Arginase-1 and iNOS mRNA were also measured concurrently in Ft-infected macrophages. These two gene products were reciprocally regulated: arginase-1 mRNA expression was detectable by 24 h and increased to its peak at 48–72 h, while iNOS mRNA decreased from its peak at 24 h to background levels by 72 h (Figure 7B). This correlates well with the kinetics of IL-4 and IL-13 production in Ft-infected macrophages (Figure 7A) that facilitate differentiation of AA-Mφ, thus enabling the survival and replication of Ft LVS (Figure 4).
Failure to develop AA-Mφ improves survival in vivo
Although AA-Mφ induction is beneficial to the Ft, we hypothesized that the failure to induce AA-Mφ in vivo may protect against lethal infection with Ft by allowing CA-Mφ to control Ft replication. To test this hypothesis, BALB/cByJ WT and IL-4Rα−/− mice were injected i.p. with saline (mock) or Ft LVS (10,000 CFU) and survival monitored for 10 days. All of the mock-infected WT and IL-4Rα−/− mice survived; however, 5% of Ft-infected WT mice died on day 3, with 100% mortality by day 7. In contrast, 10% of IL-4Rα−/− mice challenged with Ft LVS died at day 4 post-infection, and 33% of the mice survived through day 10 (p < 0.001). This data suggests that the prevention of AA-Mφ induction during Ft LVS infection increases survival.
Discussion
Macrophage activation is central for controlling the replication of intracellular pathogens like Francisella. However, despite the fact that Ft LVS initially induces a robust inflammatory response, the mice nonetheless die, usually 4–5 days after i.p. infection. Our observations suggest that Ft may avoid macrophage-mediated killing by directly altering the macrophage differentiation state from one that is “classically activated” and highly microbicidal to one that is “alternatively activated,” and therefore, permissive to microbial survival and replication at the expense of the host. Ft LVS infection of murine macrophages induced TLR2-dependent expression of “AA-Mφ” markers (e.g., FIZZ1, Arg-1, Ym1, SR) that followed expression of “CA-Mφ” markers (e.g., IL-12, iNOS).
The innate immune response to Ft is complex and involves multiple pattern recognition receptors. Despite the fact that every single host gene that we have examined to date requires TLR2 signaling for its expression (e.g., TNF-α, IL-1β, IFN-γ, IFN-β, iNOS, IL-6, KC, MCP-1, RANTES and others (18,20)), in addition to those shown in Figure 1), we have more recently observed that expression of a subset of these TLR2-dependent genes additionally requires the organism to escape from the phagosome, implying that cytosolic sensing and signaling is also necessary. Using an Ft LVS mutant that is unable to escape from the phagosome, in conjunction with IFN-β-deficient macrophages, we further demonstrated that those genes whose induction required both TLR2 and cytosolic sensing were also IFN-β-dependent. In addition to this uncharacterized cytosolic sensor, activation of the inflammasome, as has been reported for the response to F. novicida (36), is also necessary for secretion of IL-1β in response to Ft LVS, despite the fact that induction of IL-1β mRNA is solely TLR2-dependent (37). In preliminary experiments, we have observed that TLR2 signaling by synthetic triacylated lipopeptide is also not sufficient for induction of all AA-Mø markers (e.g., FIZZ-1, arginase-1, and mannose; data not shown). Experiments are ongoing to identify subsets of genes that encode AA-Mø markers that can be induced by Ft infection solely through TLR2 versus those that require additional signaling pathways for their expression.
Induction of AA-Mφ by treatment of macrophages with rIL-4, rIL-13, or by infection with Ft LVS promoted replication of intracellular Ft, in contrast to CA-Mφ derived by IFN-γ + LPS treatment. In vivo, infection of mice with Ft LVS also led to induction of AA-Mφ. Multiple groups have shown the importance of IFNs in the control of Francisella infection (36–39). IFN-γ−/− mice have been shown to be highly susceptible to otherwise sublethal doses of Ft (40). Cole et al. showed that even though endogenous IFN-β contributed to control intracellular Ft replication within the first 24 h of infection, Ft was able to overcome this inflammatory signal and replicate over time. Anthony et al. first showed pre-treatment of cells with IL-4 or GM-CSF permitted Ft replication (41). Our data expand and clarify these previous findings by showing that the cytokine treatments impact the antimicrobial state of the macrophages through the induction of either classical or alternative activation. We have further shown that infection of the macrophages by Ft only is sufficient to elicit an AA-Mφ phenotype in the absence of exogenous IL-4. The concept of macrophage plasticity has long since been recognized and Gratchev et al. showed that the cytokine milieu facilitates the macrophages to change phenotypes (42). Our data clearly demonstrate that Ft LVS-infected macrophages change phenotypes as evidenced by a switch from a CA-Mφ producing and secreting IL-12 p70 to AA-Mφs that produce multiple markers associated with alternative activation, e.g., FIZZ1, Arg-1, etc. (Figure 2).
IL-4 and IL-13 drive many immune and anti-inflammatory responses that are associated with a Th2 response (31,42). The IL-4Rα chain is essential for signaling by both cytokines. Ft LVS failed to induce alternative activation in IL-4Rα−/− or STAT6−/− macrophages and prolonged expression of CA-Mφ activation markers, indicating that IL-4- and/or IL-13-mediated signaling underlie AA-Mφ differentiation in the Ft-infected cells. Neutralization of either IL-4 or IL-13 blunted AA-Mφ activation during Ft LVS infection, but prolonged CA-Mφ activation. Since these same events were seen in both the RAW 267.4 cell line and in macrophages derived from Rag2−/− mice (data not shown), our neutralization data strongly support the conclusion that the macrophages are, in fact, the source of the IL-4 and IL-13 and that both cytokines contribute to AA-Mφ differentiation in infected macrophages. Leiby et al. previously reported that neutralization of IL-4 with a MAb enhanced survival of mice i.p. infected with Ft LVS compared with control mice (39). Although they reported that, “the differences were not statistically significant,” the potential contribution of the compensatory activity of IL-13 induced during infection, which also drives AA-Mφ activation (Figure 6), was not examined in their studies. Our data expands upon their findings by showing that neutralization of both IL-4 and IL-13 is required to limit intracellular replication of Ft maximally, and that mice deficient in IL-4Rα, that fail to differentiate AA-Mφ, have significantly higher survival rates (p < 0.001) compared to WT mice (Figure 7C).
Apart from the fact that Ft LVS infection also greatly increases intracellular replication (Figure 4), upregulation of mannose receptor expression (Figure 2) may also contribute to the dissemination of this pathogen. Schulert and Allen reported that the mannose receptor mediates uptake of Ft, and more efficiently so in the presence of complement (43). Thus, increased uptake of Ft via mannose receptors may facilitate spread from cell to cell for survival as well as replication. IL-4 production and secretion by infected cells might be envisioned to act on adjacent cells to increase mannose receptor expression, thus allowing more efficient uptake of Ft once it escapes a neighboring cell. This mechanism might also apply to the observed increase in scavenger receptors in infected macrophages that have also been shown to enhance uptake of this pathogen (44).
In addition to wound repair and regulation of the immune response (8), AA-Mφ provide host resistance to certain parasites (45,46). AA-Mφ have developed multiple functions for resistance such as acidic mammalian chitinase that induces eotaxin and MCP-1 during infection with Heligosomoides polygyrus, and in Schistosoma mansonii egg granulomas (47–49). FIZZ2, which is expressed exclusively in the gastrointestinal tract and induced during infection with Trichuris muris, interferes with parasite chemotaxis towards host tissues (50,51). In addition to its role in angiogenesis, Ym1 acts as a chemotactic factor for eosinophils (52), which play an essential role in anti-helminth responses (53). The outcome of parasitic infections is also dependent on both the parasite as well as the susceptibility of the host. C57BL/6 mice are resistant to infection with Trypanosoma congolense because they have a predominantly CA-Mφ type environment during the early stages of infection that allows nitric oxide, generated by iNOS, to control growth of the parasite; however, AA-Mφs develop later in infection and contribute to wound repair. BALB/c mice infected with T. congolense have a simultaneous mix of CA-Mφ and AA-Mφ during the early stages of infection, resulting in uncontrolled parasite growth (54). Our data show Ft LVS is able to strongly induce AA-Mφ in both C57BL/6 as well as BALB/c backgrounds. Both strains are susceptible to Ft and typically succumb to infection within 4–7 days (Figure 7C, 55). However, the inability of IL-4Rα−/− mice to develop AA-Mφ correlates with increased survival, presumably due to prolonged activation of the CA-Mφ phenotype and control of intracellular replication.
Collectively, our data supports a novel paradigm for the host response to the intracellular bacterium, Ft LVS: initially, the host interaction with Ft LVS results in a brisk up-regulation of proinflammatory genes that is generally associated with development of CA-Mφ. With time, however, the organism ultimately “redirects” macrophage differentiation to that of an AA-Mφ phenotype by inducing the production of IL-4 and IL-13 that ultimately facilitates intracellular survival of the organism with a concomitant mitigation of the proinflammatory response. Thus, the balance of “environmental” signals induced during Ft infection of macrophages results in a constantly evolving host immune response that can be either beneficial or detrimental to the host and pathogen alike. This organism clearly takes advantage of the plasticity of macrophages to differentiate for its own survival and suggest new approaches for therapeutic intervention.
Acknowledgments
We wish to thank Dr. Karen Elkins for her thoughtful comments during the preparation of this manuscript.
Footnotes
This work was supported in part by NIH grants AI18797 and AI157168 (SNV) and AI38985 and AI59775 (ADK)
Abbreviations used in this paper: LVS, live vaccine strain; FIZZ1, found in inflammatory zone 1; AA-Mφ, alternatively activated macrophages; CA-Mφ, classically activated macrophages; HPRT, hypoxanthine phosphoribosyltransferase; MOI, multiplicity of infection; WT, wild-type
Disclosures
The authors have no financial conflict of interest.
References
- 1.Tarnvik A. Nature of protective immunity to Francisella tularensis. Rev Infect Dis. 1989;11:440–451. [PubMed] [Google Scholar]
- 2.Ellis J, Oyston PC, Green M, Titball RW. Tularemia. Clin Microbiol Rev. 2002;15:631–646. doi: 10.1128/CMR.15.4.631-646.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Friedlander AM, Hauer J, Layton M, et al. Tularemia as a biological weapon: medical and public health management. J Am Med Assoc. 2001;285:2763–2773. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 4.Elkins KL, Cowley SC, Bosio CM. Innate and adaptive immunity to Francisella tularensis live vaccine strain. Microbes Infect. 2003;5:135–142. doi: 10.1016/s1286-4579(02)00084-9. [DOI] [PubMed] [Google Scholar]
- 5.Eigelsbach HT, Downs CM. Prophylactic effectiveness of live and killed tularemia vaccines. I. Production of vaccine and evaluation in the white mouse and guinea pig. J Immunol. 1961;87:415–425. [PubMed] [Google Scholar]
- 6.Sjöstedt A. Intracellular survival mechanisms of Francisella tularensis, a stealth pathogen. Microbes Infect. 2006;8:561–567. doi: 10.1016/j.micinf.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Loegering DJ, Drake JR, Banas JA, McNealy TL, Mc Arthur DG, Webster LM, Lennartz MR. Francisella tularensis LVS grown in macrophages has reduced ability to stimulate the secretion of inflammatory cytokines by macrophages in vitro. Microb Pathog. 2006;41:218– 225. doi: 10.1016/j.micpath.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 9.Colton CA, Mott RT, Sharpe H, Xu Q, van Nostrand WE, Vitek MP. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J Neuroinflammation. 2006;3:27–38. doi: 10.1186/1742-2094-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ponomarev ED, Maresz K, Tan Y, Dittel BN. CNS-derived interleukin-4 is essential for the regulation of autoimmune inflammation and induces a state of alternative activation in microglial cells. J Neurosci. 2007;27:10714–10721. doi: 10.1523/JNEUROSCI.1922-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahnert AP, Seiler M, Stein S, Bandermann K, Hahnke H, Mollenkopf, Kaufmann SHE. Alernative activation deprives macrophages of a coordinated defense program to Mycobacterium tuberculosis. Eur J Immunol. 2006;36:631–647. doi: 10.1002/eji.200535496. [DOI] [PubMed] [Google Scholar]
- 12.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80:1298–1307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polumuri SK, V, Toshchakov Y, Vogel SN. Role of phosphatidylinositol-3 kinase in transcriptional regulation of TLR-induced IL-12 and IL-10 by Fc gamma receptor ligation in murine macrophages. J Immunol. 2007;179:236–246. doi: 10.4049/jimmunol.179.1.236. [DOI] [PubMed] [Google Scholar]
- 14.Sutterwala FS, Noel GJ, Clynes R, Mosser DM. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J Exp Med. 1997;185:1977–85. doi: 10.1084/jem.185.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McIntire FC, Sievert HW, Barlow GH, Finley RA, Lee AY. Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry. 1967;6:2363–2373. doi: 10.1021/bi00860a011. [DOI] [PubMed] [Google Scholar]
- 16.Elkins KL, Winegar RK, Nacy CA, Fortier AH. Introduction of Francisella tularensis at skin sites induces resistance to infection and generation of protective immunity. Microb Pathog. 1992;13:417–421. doi: 10.1016/0882-4010(92)90085-3. [DOI] [PubMed] [Google Scholar]
- 17.Salkowski CA, Kopydlowski K, Blanco J, Cody MJ, McNally R, Vogel SN. IL-12 is dysregulated in macrophages from IRF-1 and IRF-2 knockout mice. J Immunol. 1999;163:1529–1536. [PubMed] [Google Scholar]
- 18.Cole LE, Elkins KL, Michalek SM, Qureshi N, Eaton LJ, Rallabhandi P, Cuesta N, Vogel SN. Immunological consequences of Francisella tularensis live vaccine strain infection: role of the innate immune response in infection and immunity. J Immunol. 2006;176:6888–6899. doi: 10.4049/jimmunol.176.11.6888. [DOI] [PubMed] [Google Scholar]
- 19.Cuesta N, Salkowski CA, Thomas KE, Vogel SN. Regulation of lipopolysaccharide sensitivity by IFN regulatory factor-2. J Immunol. 2003;170:5739–5747. doi: 10.4049/jimmunol.170.11.5739. [DOI] [PubMed] [Google Scholar]
- 20.Cole LE, Shirey KA, Barry E, Santiago A, Rallabhandi P, Elkins KL, Puche AC, Michalek SM, Vogel SN. Toll-like receptor 2-mediated signaling requirements for Francisella tularensis live vaccine strain infection of murine macrophages. Infect Immun. 2007;75:4127–4137. doi: 10.1128/IAI.01868-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stenmark S, Sunnemark D, Bucht A, Sjöstedt A. Rapid local expression of interleukin-12, tumor necrosis factor α, and γ interferon after cutaneous Francisella tularensis in tularemia-immune mice. Infect Immun. 1999;67:1789–1797. doi: 10.1128/iai.67.4.1789-1797.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forestal CA, Benach JL, Carbonara C, Italo JK, Lisinski TJ, Furie MB. Francisella tularensis selectively induces proinflammatory changes in endothelial cells. J Immunol. 2003;171:2563–2570. doi: 10.4049/jimmunol.171.5.2563. [DOI] [PubMed] [Google Scholar]
- 23.Golovliov I, Sandstrom G, Ericsson M, Sjöstedt A, Tarnvik A. Cytokine expression in the liver during the early phase of murine tularemia. Infect Immun. 1995;63:534–538. doi: 10.1128/iai.63.2.534-538.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golovliov I, Kuoppa K, Sjöstedt A, Tarnvik A, Sandstrom G. Cytokine expression in the liver of mice infected with a highly virulent strain of Francisella tularensis. FEMS Immunol Med Microbiol. 1996;13:239–244. doi: 10.1111/j.1574-695X.1996.tb00244.x. [DOI] [PubMed] [Google Scholar]
- 25.Welch JS, Escoubet-Lozach L, Sykes DB, Liddiard K, Greaves DR, Glass CK. TH2 cytokines and allergic challenge induce Ym1 expression in macrophages by a STAT6-dependent mechanism. J Biol Chem. 2002;277:42821–42829. doi: 10.1074/jbc.M205873200. [DOI] [PubMed] [Google Scholar]
- 26.Loke P, MacDonald AS, Robb A, Maizels RM, Allen JE. Alternatively activated macrophages induced by nematode infection inhibit proliferation via cell-to-cell contact. Eur J Immunol. 2000;30:2669–2678. doi: 10.1002/1521-4141(200009)30:9<2669::AID-IMMU2669>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 27.Raes G, Noël W, Beschin A, Brys L, De Baetselier P, Gholamreza Hassansadeh G. FIZZ1 and Ym as tools to discriminate between differentially activated macrophages. Develop Immunol. 2002;9:151–159. doi: 10.1080/1044667031000137629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleetwood AJ, Lawrence T, Hamilton JA, Cook AD. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J Immunol. 2007;15:5245–5252. doi: 10.4049/jimmunol.178.8.5245. [DOI] [PubMed] [Google Scholar]
- 29.Mantovani A, Sica A, Locati M. New vistas on macrophage differentation and activation. Eur J Immunol. 2007;37:14–16. doi: 10.1002/eji.200636910. [DOI] [PubMed] [Google Scholar]
- 30.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization:new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 31.Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connection maps. Science. 2003;300:1527–1528. doi: 10.1126/science.1085458. [DOI] [PubMed] [Google Scholar]
- 32.Mohrs M, Ledermann B, Köhler G, Dorfmüller A, Gessner A, Brombacher B. Differences between IL-4 and IL-4 receptor-a-deficient mice in chronic Leishmaniasis reveal a protective role for IL-13 receptor signaling. J Immunol. 1999;162:7302–7308. [PubMed] [Google Scholar]
- 33.Stein MS, Keshav N, Harris, Gordon S. IL-4 potently enhances murine macrophage mannose receptor activity; a marker for alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munder M, Eichmann K, Morán JM, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol. 1999;163:3771–3777. [PubMed] [Google Scholar]
- 35.Meghari S, Berruyer C, Lepidi H, Galland F, Naquet P, Mege JL. Vanin-1 controls granuloma formation and macrophage polarization in Coxiella burnetii infection. Eur J Immunol. 2007;37:24–32. doi: 10.1002/eji.200636054. [DOI] [PubMed] [Google Scholar]
- 36.Henry TA, Brotcke DS, Weiss LJ, Thompson, Monack DM. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J Exp Med. 2007;204:987–994. doi: 10.1084/jem.20062665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cole LE, Santiago A, Barry E, Kang T, Shirey K, Roberts ZJ, Elkins KL, Cross A, Vogel SN. The Macrophage Proinflammatory Response to Francisella tularenesis LVS Requires Coordination of Multiple Signaling Pathways. J Immunol. 180:6885–91. doi: 10.4049/jimmunol.180.10.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anthony L, Ghadirian E, Nestel F, Kongshavn PA. The requirment for gamma interferon in resistance of mice to experimental tularemia. Microb Pathog. 1989;7:421–428. doi: 10.1016/0882-4010(89)90022-3. [DOI] [PubMed] [Google Scholar]
- 39.Leiby D, Fortier A, Crawford R, Schreiber RD, Nacy CA. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect Immun. 1992;60:84–89. doi: 10.1128/iai.60.1.84-89.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elkins KL, Rhinehart-Jones TR, Culkin SJ, Yee D, Winegar RK. Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect Immun. 1996;64:3288–3293. doi: 10.1128/iai.64.8.3288-3293.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anthony LSD, Morrissey PJ, Nano FE. Growth inhibition of Francisella tularensis live vaccine strain by IFN-γ-activated macrophages is mediated by reactive nitrogen intermediates derived from L-arginine metabolism. J Immunol. 1992;148:1829–1834. [PubMed] [Google Scholar]
- 42.Gratchev A, Kzhyshkowska J, Köthe K, Muller-Molinet I, Kannookadan S, Utikal J, Goerdt S. Mφ1 and Mφ2 can be re-polarized by Th2 or Th1 cytokines, respectively, and respond to exogenous danger signals. Immunobiol. 2006;211:473–486. doi: 10.1016/j.imbio.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 43.Schulert G, Allen L-AH. Differential interaction of mononuclear phagocytes by Francisella tularesnsis: role of the macrophage mannose receptor. J Leukoc Biol. 2006;80:563–571. doi: 10.1189/jlb.0306219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pierini NR. Uptake of serum-opsonized Francisella tularensis by macrophages can be mediated by class A scavenger receptors. Cell Microbiol. 2006;8:1361–1370. doi: 10.1111/j.1462-5822.2006.00719.x. [DOI] [PubMed] [Google Scholar]
- 45.Finkleman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, Schopf L, Urban JF., Jr Interleukin-4 and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev. 2004;201:139–155. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 46.Anthony RM, Rutizky LI, Urban JF, Jr, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anthony RM, Urban JF, Jr, Alem F, Hamed HA, Rozo CT, Boucher JL, van Rooijen N, Gause WC. Memory TH2 cells induce alternative activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pesce J, Kaviratne M, Ramalingam TR, Thompson RW, Urban JF, Jr, Cheever AW, Young DA, Collins M, Grusby MJ, Wynn TA. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2003;116:2044–2055. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, Hamid Q, Elias JA. Acidic mammalian chitinase in asmathic Th2 inflammation and IL-13 pathway activation. Science. 2004;304:1678–82. doi: 10.1126/science.1095336. [DOI] [PubMed] [Google Scholar]
- 50.Artis D, Wang ML, Keilbaugh SA, He W, Brenes M, Swain GP, Knight PA, Donaldson DD, Lazar MA, Miller HR, Schad GA, Scott P, Wu GD. RELMβ/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci USA. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hogan SP, Seidu L, Blanchard C, Groschwitz K, Mishra A, Karow ML, Ahrens R, Artis D, Murphy AJ, Valenzuela DM, Yancopoulos GD, Rothenberg ME. Resistin-like molecule β regulates innate colonic function: barrier integrity and inflammation susceptibility. J Allery Clin Immunol. 2006;118:257–268. doi: 10.1016/j.jaci.2006.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang NC, Hung SI, Hwa KY, Kato I, Chen JE, Liu CH, Chang AC. A macrophage protein, Ym1, transiently expressed during inflammation is a novel mammalian lectin. J Biol Chem. 2001;276:17497–17506. doi: 10.1074/jbc.M010417200. [DOI] [PubMed] [Google Scholar]
- 53.Gause WC, Urban JF, Jr, Stadecker MJ. The immune response to parasitic helminthes: insights from murine models. Trends Immunol. 2003;24:269–277. doi: 10.1016/s1471-4906(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 54.Noël W, Gh GH, Raes G, Namangala B, Daems I, Brys L, Brombacher F, de Baestelier P, Beschin A. Infection stage-dependent modulation of macrophage activation in Trypanosoma congelese-resistant and -susceptible mice. Infect Immun. 2002;70:6180–87. doi: 10.1128/IAI.70.11.6180-6187.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dreisbach VC, Cowley S, Elkins KL. Purified lipopolysaccharide from Francisella tularensis live vaccine strain (LVS) induces protective immunity against LVS infection that requires B cells and gamma interferon. Infect Immun. 2000;68:1988–96. doi: 10.1128/iai.68.4.1988-1996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]