Abstract
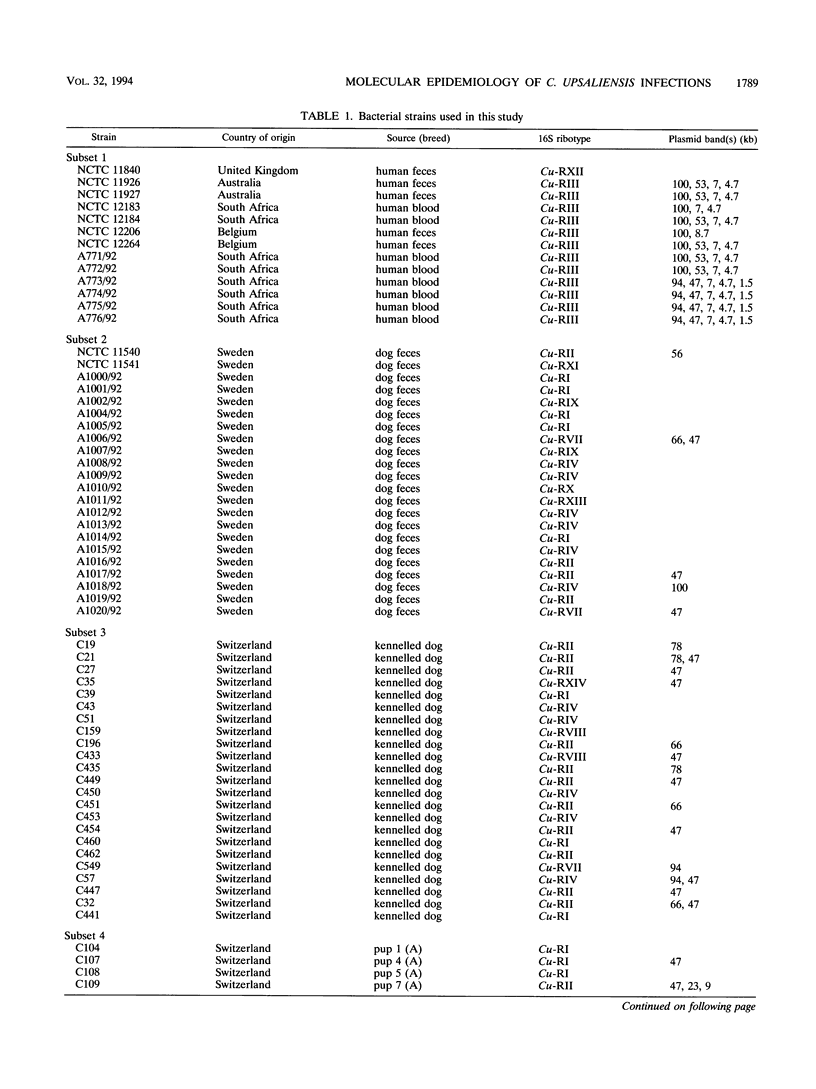
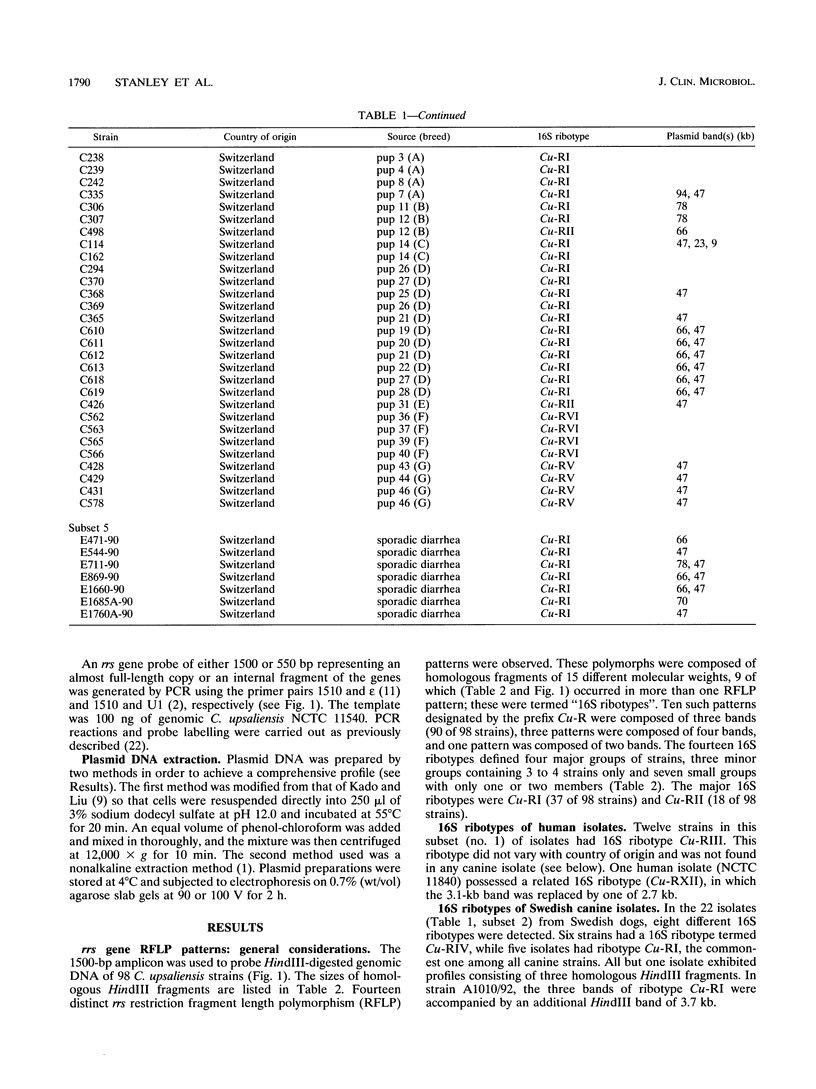
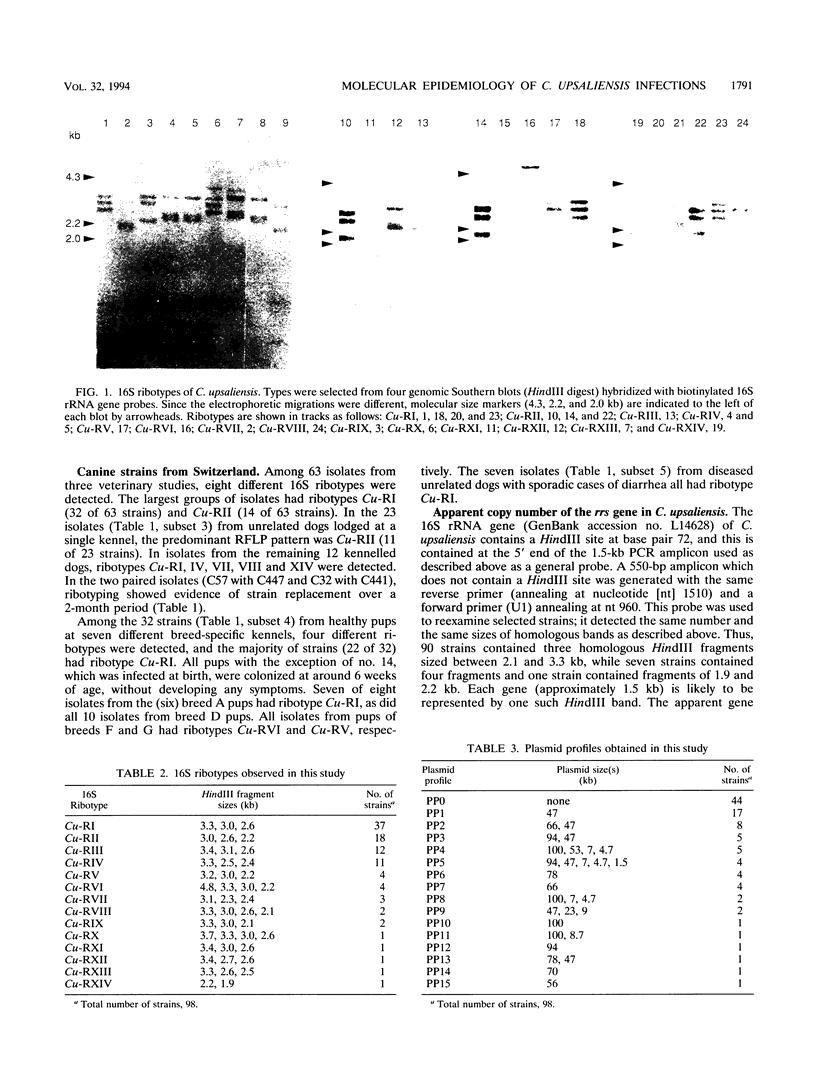
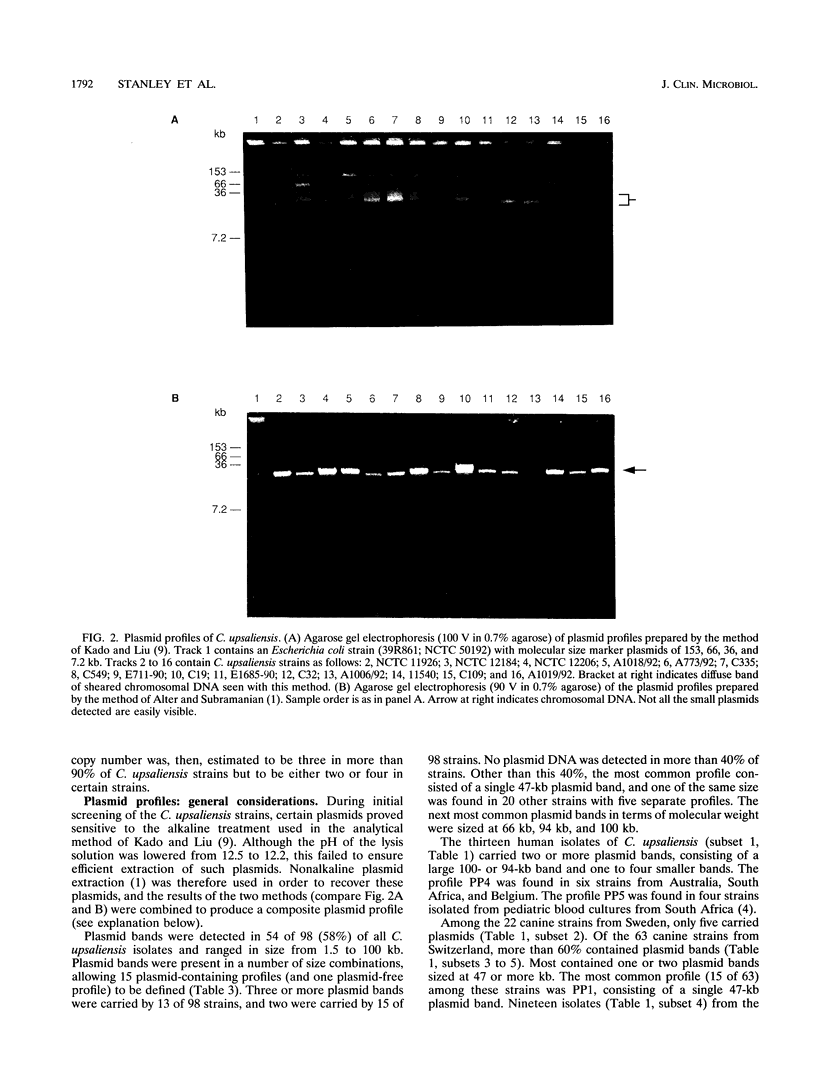
The utility of combined 16S rRNA (rrs) gene restriction fragment length polymorphism and plasmid profiles to differentiate between and within Campylobacter upsaliensis of human and canine origin was examined. Fourteen distinct rrs gene restriction fragment length polymorphs consisting of bands sized between 1.9 and 4.8 kb were observed. The copy number of the 16S rRNA gene was three in most strains of C. upsaliensis. Plasmids were found in almost 60% of the strains; ranging in size from 1.5 to 100 kb, they gave 15 distinct plasmid profiles. All isolates from humans contained one or more plasmids, as did strains isolated from dogs with sporadic diarrhea. The two commonest 16S ribotypes were divided into eight and nine subgroups by plasmid profiling. The genotyping of canine isolates from three veterinary surveys detected both multiple infections and reinfection of dogs. Except for one, each of the isolates from humans constituted a single and unique 16S ribotype, and these more frequently carried plasmids than did canine strains. Ribotypes of human strains were not found among canine isolates. These results suggest that host-specific genotypic differences may exist among strains of C. upsaliensis, for example, intraspecific clones or clone complexes pathogenic for humans.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter D. C., Subramanian K. N. A one step, quick step, mini prep. Biotechniques. 1989 May;7(5):456–458. [PubMed] [Google Scholar]
- Barry T., Powell R., Gannon F. A general method to generate DNA probes for microorganisms. Biotechnology (N Y) 1990 Mar;8(3):233–236. doi: 10.1038/nbt0390-233. [DOI] [PubMed] [Google Scholar]
- Bukanov N. O., Berg D. E. Ordered cosmid library and high-resolution physical-genetic map of Helicobacter pylori strain NCTC11638. Mol Microbiol. 1994 Feb;11(3):509–523. doi: 10.1111/j.1365-2958.1994.tb00332.x. [DOI] [PubMed] [Google Scholar]
- Da Silva Tatley F. M., Lastovica A. J., Steyn L. M. Plasmid profiles of "Campylobacter upsaliensis" isolated from blood cultures and stools of paediatric patients. J Med Microbiol. 1992 Jul;37(1):8–14. doi: 10.1099/00222615-37-1-8. [DOI] [PubMed] [Google Scholar]
- Ezquerra E., Burnens A. P., Frith K., Costas M., Stanley J. Molecular genotype analysis of Salmonella bovismorbificans. Mol Cell Probes. 1993 Feb;7(1):45–54. doi: 10.1006/mcpr.1993.1006. [DOI] [PubMed] [Google Scholar]
- Goossens H., De Boeck M., Coignau H., Vlaes L., Van den Borre C., Butzler J. P. Modified selective medium for isolation of Campylobacter spp. from feces: comparison with Preston medium, a blood-free medium, and a filtration system. J Clin Microbiol. 1986 Nov;24(5):840–843. doi: 10.1128/jcm.24.5.840-843.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens H., Pot B., Vlaes L., Van den Borre C., Van den Abbeele R., Van Naelten C., Levy J., Cogniau H., Marbehant P., Verhoef J. Characterization and description of "Campylobacter upsaliensis" isolated from human feces. J Clin Microbiol. 1990 May;28(5):1039–1046. doi: 10.1128/jcm.28.5.1039-1046.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens H., Vlaes L., Butzler J. P., Adnet A., Hanicq P., N'Jufom S., Massart D., de Schrijver G., Blomme W. Campylobacter upsaliensis enteritis associated with canine infections. Lancet. 1991 Jun 15;337(8755):1486–1487. doi: 10.1016/0140-6736(91)93182-9. [DOI] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N. W., Lombardi R., Bingham H., Hani E., Louie H., Ng D., Chan V. L. Fine mapping of the three rRNA operons on the updated genomic map of Campylobacter jejuni TGH9011 (ATCC 43431). J Bacteriol. 1993 Nov;175(22):7468–7470. doi: 10.1128/jb.175.22.7468-7470.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastovica A. J., Ambrosio R. E. Plasmid profiles of clinical isolates of Campylobacter with no or weak catalase activity. S Afr Med J. 1986 Sep 13;70(6):337–338. [PubMed] [Google Scholar]
- Lastovica A. J., Le Roux E., Penner J. L. "Campylobacter upsaliensis" isolated from blood cultures of pediatric patients. J Clin Microbiol. 1989 Apr;27(4):657–659. doi: 10.1128/jcm.27.4.657-659.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton D., Moreno M., Owen R. J., Stanley J. 16S rrn gene copy number in Helicobacter pylori and its application to molecular typing. J Appl Bacteriol. 1992 Dec;73(6):501–506. doi: 10.1111/j.1365-2672.1992.tb05012.x. [DOI] [PubMed] [Google Scholar]
- Owen R. J., Desai M., Garcia S. Molecular typing of thermotolerant species of Campylobacter with ribosomal RNA gene patterns. Res Microbiol. 1993 Nov-Dec;144(9):709–720. doi: 10.1016/0923-2508(93)90035-z. [DOI] [PubMed] [Google Scholar]
- Owen R. J., Hernandez J. Genotypic variation in 'Campylobacter upsaliensis' from blood and faeces of patients in different countries. FEMS Microbiol Lett. 1990 Oct;60(1-2):5–10. doi: 10.1016/0378-1097(90)90335-n. [DOI] [PubMed] [Google Scholar]
- Owen R. J., Hernandez J. Occurrence of plasmids in "Campylobacter upsaliensis" (catalase negative or weak group) from geographically diverse patients with gastroenteritis or bacteraemia. Eur J Epidemiol. 1990 Jun;6(2):111–117. doi: 10.1007/BF00145782. [DOI] [PubMed] [Google Scholar]
- Patton C. M., Shaffer N., Edmonds P., Barrett T. J., Lambert M. A., Baker C., Perlman D. M., Brenner D. J. Human disease associated with "Campylobacter upsaliensis" (catalase-negative or weakly positive Campylobacter species) in the United States. J Clin Microbiol. 1989 Jan;27(1):66–73. doi: 10.1128/jcm.27.1.66-73.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton C. M., Wachsmuth I. K., Evins G. M., Kiehlbauch J. A., Plikaytis B. D., Troup N., Tompkins L., Lior H. Evaluation of 10 methods to distinguish epidemic-associated Campylobacter strains. J Clin Microbiol. 1991 Apr;29(4):680–688. doi: 10.1128/jcm.29.4.680-688.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Baquar N., Threlfall E. J. Genotypes and phylogenetic relationships of Salmonella typhimurium are defined by molecular fingerprinting of IS200 and 16S rrn loci. J Gen Microbiol. 1993 Jun;139(Pt 6):1133–1140. doi: 10.1099/00221287-139-6-1133. [DOI] [PubMed] [Google Scholar]
- Stanley J., Burnens A. P., Linton D., On S. L., Costas M., Owen R. J. Campylobacter helveticus sp. nov., a new thermophilic species from domestic animals: characterization, and cloning of a species-specific DNA probe. J Gen Microbiol. 1992 Nov;138(11):2293–2303. doi: 10.1099/00221287-138-11-2293. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., Eaton M., Yan W., Chang N. Genome maps of Campylobacter jejuni and Campylobacter coli. J Bacteriol. 1992 Apr;174(7):2332–2337. doi: 10.1128/jb.174.7.2332-2337.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee W., Anderson B. N., Ross B. C., Dwyer B. Atypical campylobacters associated with gastroenteritis. J Clin Microbiol. 1987 Jul;25(7):1248–1252. doi: 10.1128/jcm.25.7.1248-1252.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]




