Abstract
Background
The histone deacetylase (HDAC) inhibitors valproic acid (VPA) and suberoyl bis-hydroxamic acid (SBHA) have recently been demonstrated to be strong Notch1 activators. Upregulation of the Notch1 pathway has been shown to limit growth and suppress hormonal secretion in neuroendocrine (NE) tumors. We hypothesized that HDAC inhibition would be an effective strategy to activate the Notch1 pathway and inhibit growth and hormonal secretion in pheochromocytoma cells.
Methods
Pheochromocytoma PC-12 cells were treated with up to 8 mM VPA or 40 µM SBHA for 2 days. NE tumor markers achaete-scute complex-like 1 (ASCL1) and chromogranin A (CgA) were measured after treatment by Western analysis. Growth was assessed by a cellular proliferation assay, and Western analysis was used to determine the mechanism of growth regulation.
Results
HDAC inhibitor treatment caused a dose-dependent decrease in ASCL1 and CgA while increasing the amount of active Notch1 protein. With 6 day treatment, dose-dependent growth inhibition and cleavage of the apoptotic markers caspase-3 and PARP was observed.
Conclusions
VPA and SBHA effectively upregulate Notch1, suppress NE tumor markers, and decrease growth via apoptosis of pheochromocytoma cells in vitro. Activation of the Notch1 signaling pathway with HDAC inhibitors may represent a new strategy to treat pheochromocytomas.
Introduction
Pheochromocytomas are rare catecholamine-secreting tumors derived from the chromaffin cells of the adrenal medulla.1 The true incidence of pheochromocytoma is unclear: less than 0.5% of patients with hypertensive symptoms actually have a pheochromocytoma2, while as many as 4% of adrenal incidentalomas are pheochromocytomas.3 The incidence of pheochromocytoma may be higher in patients with a history of cancer.4 These hormonally active tumors secrete vasoactive compounds such as norepinephrine, epinephrine, dopamine, and chromogranin A; this can lead to the classic symptomatic triad of episodic headache, sweating, and palpitations.5, 6 Hypertension, which can be life-threatening, is commonly associated with pheochromocytoma.
While medical management can relieve symptoms, treatments for pheochromocytoma are limited. Surgical excision remains the only definitive cure. Chemotherapeutic regimens for malignant disease are unsatisfying at best. Combination therapy with 131I-MIBG and cyclophosphamide, dacarbazine, and vincristine has produced additive effects, but there was no significant long-lasting benefit.7 Moreover, radiofrequency ablation of hepatic and bony metastases has shown symptomatic relief in only some patients.8 As malignant disease has no truly effective treatment, novel approaches must be discovered for these patients.
Notch1 is a multifunctional transmembrane receptor protein that functions as either a tumor suppressor or an oncogene depending on the ceullular context.9, 10 Upon binding a ligand, the Notch1 protein undergoes two proteolytic cleavages, translocates to the nucleus, and binds with the CBF-1 complex to regulate gene expression. Previous work by our group has shown the Notch1 signaling pathway to be inactive in neuroendocrine (NE) tumors, and its overexpression in vitro leads to growth inhibition and a decrease in NE markers.11–13 Recently, we have demonstrated that pharmacologic activation of Notch1 in carcinoid and medullary thyroid cancer cells by the histone deacetylase (HDAC) inhibitors valproic acid (VPA)14, 15 and suberoyl bis-hydroxamic acid (SBHA)16, 17 leads to a decrease in growth and hormonal secretion. However, the role of Notch1 in pheochromocytoma cells is not clear. Active Notch1 is absent in pheochromocytoma cells. Thus, we explored the role of Notch1 in pheochromocytoma cells in vitro. We hypothesized that treatment with VPA and SBHA would be an effective strategy to activate the Notch1 pathway, inhibit growth, and limit hormonal secretion in pheochromocytoma cells.
Methods
Cell culture
Rat pheochromocytoma PC-12 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA). PC-12 cells were maintained in Ham’s F12K media (ATCC), which was supplemented with 15% horse serum (Sigma Aldrich, St. Louis, MO), 2.5% fetal bovine serum (Sigma Aldrich), and 100 IU/mL penicillin and 100 µg/mL streptomycin (Invitrogen, Carlsbad, CA) as previously described.18, 19 The cells were maintained in a humidified atmosphere of 5% CO2 at 37° C.
Western blot analysis
PC-12 pheochromocytoma cells were treated with either VPA (Sigma Aldrich) or SBHA (Biomol, Plymouth Meeting, PA), and whole cell lysates were prepared as previously described.20 An equal volume of dimethyl sulfoxide (DMSO, Sigma Aldrich, MO) was used as a control. Total protein concentrations were quantified with a bicinchoninic acid assay kit (Pierce Biotechnology, Rockford, IL). Denatured cellular extracts were resolved by SDS-PAGE, transferred onto 0.4 µm nitrocellulose membranes (Schleicher and Schuell, Keene, NH), blocked in milk, and incubated with appropriate antibodies.
The antibody dilutions were prepared as follows: 1:500 for chromogranin A (CgA; Zymed Laboratories, San Francisco, CA); 1:1,000 for Notch1 (Santa Cruz Biotechnology, Santa Cruz, CA), mammalian achaete-scute complex-like 1 (ASCL1; BD Biosciences, San Diego, CA), poly-ADP ribose phosphate (PARP), cleaved caspase-3 (Cell Signaling Technology, Beverly, MA); and 1:10,000 for glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Trevigen, Gaithersburg, MD).
Horseradish peroxidase conjugated goat anti-rabbit IgG (1:2000, Cell Signaling Technology) secondary antibody was used for cleaved caspase-3, CgA, GAPDH, Notch1, and PARP, while goat anti-mouse IgG (1:200, Pierce Biotechnology) secondary antibody was used for ASCL1. For visualization of the protein signal, Immunstar (Bio-Rad Laboratories, Hercules, CA) was used for CgA, PARP, and GAPDH. SuperSignal West Femto (Pierce Biotechnology) was used for ASCL1, Notch1, and cleaved caspase-3 as per the manufacturer's instructions.
Luciferase reporter assay
To determine the functional activity of Notch1, PC-12 pheochromocytoma cells were transiently transfected with CBF-1 luciferase constructs as previously described.21 Cells were plated onto 6-well plates in triplicate and allowed to adhere overnight. CBF-1 luciferase reporter plasmid was cotransfected with cytomegalovirus β-galactosidase (CMV-β-gal) utilizing Lipofectamine 2000 Transfection Reagent (Invitrogen). After incubating overnight, cells were treated with either 8 mM VPA or 40 µM SBHA for 2 days. Cells were then harvested and lysed, and luciferase and β-galactosidase assays (Promega, Madison, WI) were performed in accordance with the manufacturer's instructions. Luciferase levels were measured using a Monolight 2010 Luminometer (Analytical Luminescence Laboratory, San Diego, CA). β-galactosidase activity was measured using a spectrophotometer at 420 nm (µQuant; Bio-Tek Instruments, Winooski, VT).
Cell proliferation assay
Cell proliferation was measured by the methylthiazolyldiphenyl-tetrazolium bromide (MTT; Sigma-Aldrich) rapid colorimetric assay. Cells were seeded in equal amounts in quadruplicate on 24-well plates, and incubated overnight. The cells were then treated with VPA or SBHA in concentrations up to 8 mM or 40 µM, respectively, and were incubated for up to 6 days. Every 2 days, treatment media was changed, and the MTT assay was performed by replacing the standard medium with 250 µl of serum-free medium containing MTT (0.5 mg/mL) and incubating at 37°C. After 4 hours of incubation, 750 µL DMSO was added to each well and mixed thoroughly. The plates were then measured at 540 nm using a spectrophotometer (µQuant; Bio-Tek Instruments).
Statistical analysis
As appropriate to presented data, one-way analysis of variance (ANOVA) and the independent samples T test were performed using SPSS (Version 11; SPSS, Inc., Chicago, IL). A P value of ≤ 0.05 was considered to be significant.
Results
Treatment of pheochromocytoma cells with HDAC inhibitors upregulates Notch1
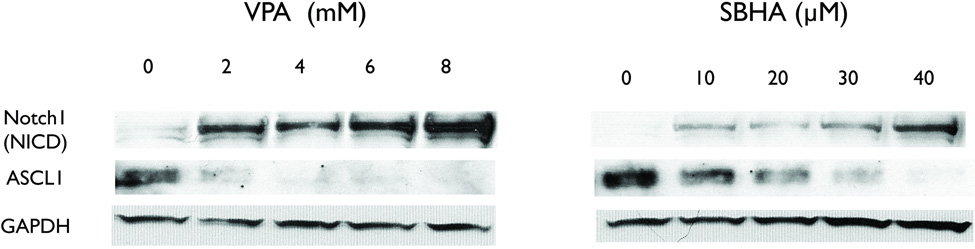
Western analysis was used to determine the effect on the Notch1 pathway by HDAC inhibitors in PC-12 cells. At baseline, PC-12 cells expressed no appreciable amount of the cleaved, active portion of Notch1 (NICD, Figure 1). Thus, pheochromocytoma cells are like other NE tumors that do not have active Notch1: gastrointestinal carcinoid, pulmonary carcinoid, medullary thyroid cancer, and small cell lung cancer cell lines.9, 11, 12, 21, 22 With 2 day treatment of increasing concentrations of either VPA or SBHA, the amount of NICD increased compared to control. Moreover, a decrease in achaete scute complex-like 1 (ASCL1), a downstream target of Notch1, was observed. ASCL1 is a basic helix-loop-helix transcription factor that promotes neuronal differentiation and is negatively regulated by Notch1. This dose-dependent response is consistent with what we have observed in other NE cells15, 16, 22, but a higher concentration was required to achieve this in pheochromocytoma cells.
Figure 1.
HDAC inhibitor treatment upregulates Notch1 in pheochromocytoma cells. Treatment for 2 days with increasing concentrations of VPA and SBHA increases the amount of active, cleaved Notch1 protein (NICD). The downstream target achaete scute complex-like 1 (ASCL1) also decreased with treatment. GAPDH is shown as a loading control. VPA, valproic acid, SBHA, suberoyl bis-hydroxamic acid, GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
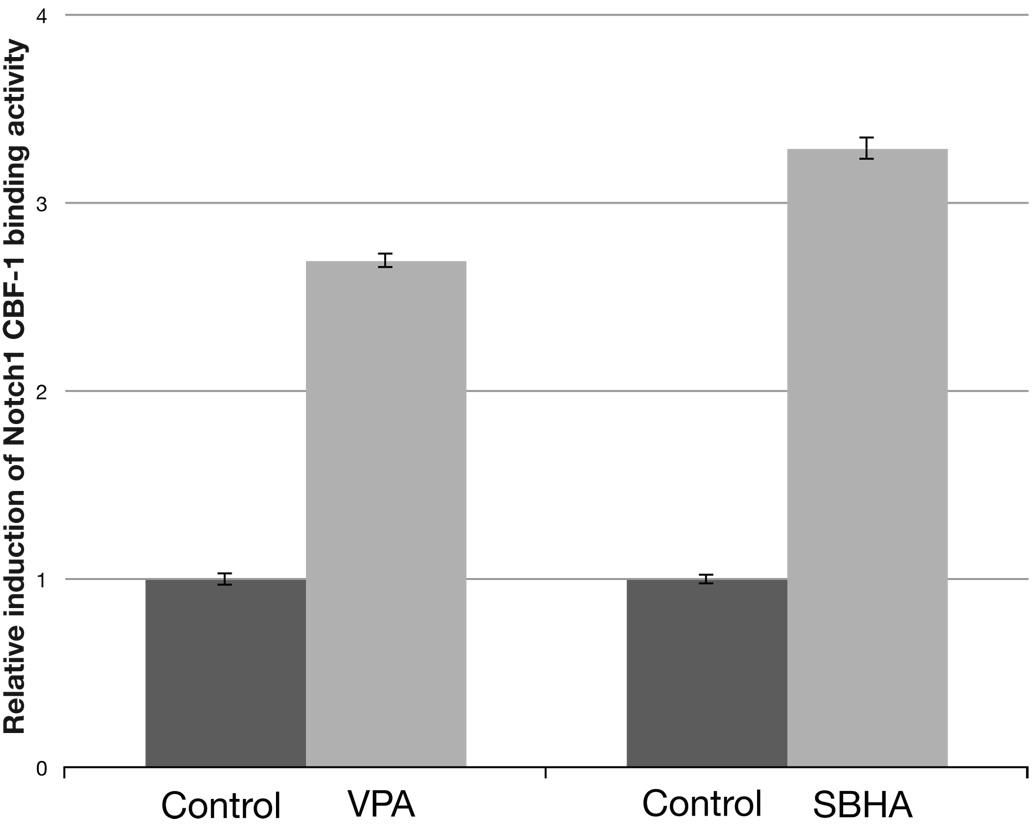
To confirm that the increase seen by Western analysis in NICD also translated to a measurable increase in Notch1 activity, we performed a standard luciferase reporter assay incorporating the CBF-1 binding site. As shown in figure 2, the Notch1 activity was approximately three times greater in PC-12 cells after a 2 day treatment with both VPA (8 mM) and SBHA (40 µM). This change was statistically significant (P < 0.001, independent samples T test). Data from both Western analysis and luciferase assays confirm that treatment of PC-12 cells by HDAC inhibitors increases cleaved Notch1 protein, and more importantly, that Notch1 is functionally active.
Figure 2.
Treatment with HDAC inhibitors increases the amount of active Notch1 as measured by relative luciferase activity. After 2 days of treatment with 8 mM VPA and 40 µM SBHA, an approximately 3-fold induction of Notch1 activity was observed. The increase was statistically significant (P < 0.001, independent samples T test). The experiment was performed in triplicate. VPA, valproic acid. SBHA, suberoyl bis-hydroxamic acid.
HDAC inhibitors decrease neuroendocrine markers
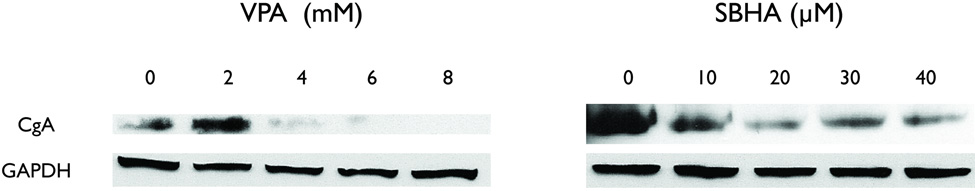
Reductions in CgA levels are correlated with decreases in hormonal secretion.18, 19 CgA is an acidic glycoprotein cosecreted with hormones by NE tumors. Additionally, we have demonstrated a decrease in CgA mediated by Notch1 activation by VPA and SBHA in carcinoid cells.15, 16 We therefore wanted to see if VPA and SBHA had the same effect on CgA in PC-12 pheochromoctyma cells. As shown in figure 3, treatment of PC-12 cells with increasing amounts of VPA and SBHA decreased CgA, suggesting an overall decrease in hormonal secretion.
Figure 3.
Treatment with the HDAC inhibitors VPA and SBHA reduces CgA. Western blot analysis showed a decrease in levels of chromogranin A (CgA), a marker of hormonal secretion. VPA, valproic acid, SBHA, suberoyl bis-hydroxamic acid, GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Pheochromocytoma cell growth is significantly limited by HDAC inhibitors
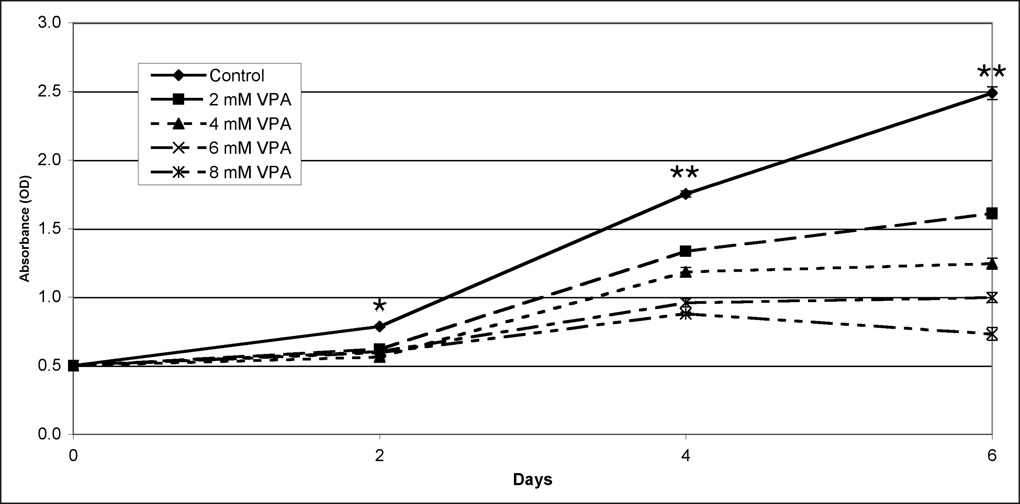
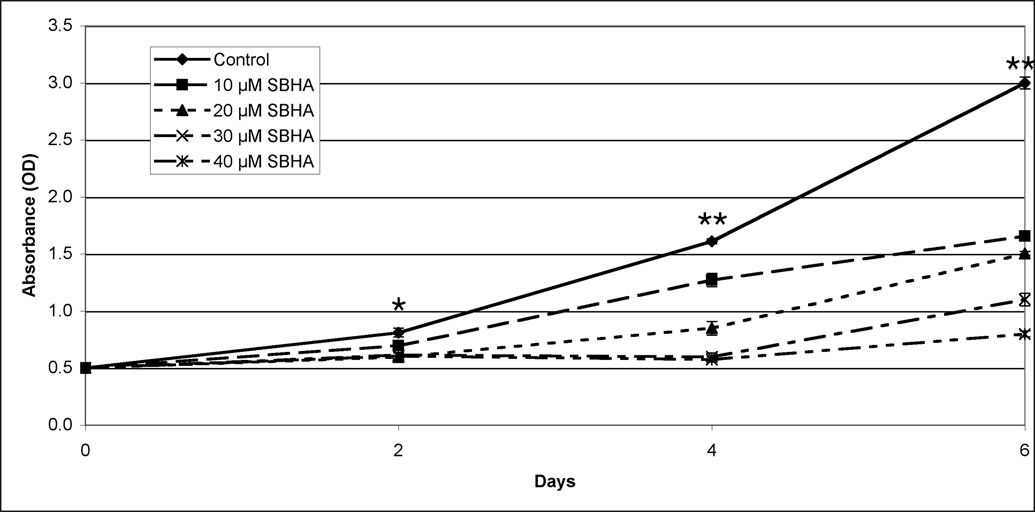
Our group has demonstrated growth inhibition associated with an increase in Notch1 protein in other NE cells lines.11, 15, 16, 21 As a result, we wanted to see if this increase was also associated with growth inhibition in PC-12 cells. The MTT growth assay was used to determine the impact of VPA and SBHA on pheochromocytoma cell growth. Growth inhibition varied directly with higher concentrations of both HDAC inhibitors (Figure 4). After 6 days of treatment, 8 mM VPA inhibited growth by nearly 70%, while 40 µM SBHA inhibited growth by more than 70%. Importantly, growth was suppressed significantly (P < 0.001, one-way ANOVA) after only 2 days by the lowest doses used. Thus, HDAC inhibitors are a potent treatment for growth inhibition of pheochromocytoma cells.
Figure 4.
Treatment with VPA (4a) and SBHA (4b) limits growth of PC-12 pheochromocytoma cells. PC-12 cells were treated with the indicated concentrations of HDAC inhibitors for up to 6 days, and cell viability was determined by the MTT colorimetric growth assay. Points represent mean +/− SE. All treatments are significantly different from control as soon as day 2 (* = P < 0.01, ** = P < 0.001, one-way ANOVA). VPA, valproic acid. SBHA, suberoyl bis-hydroxamic acid.
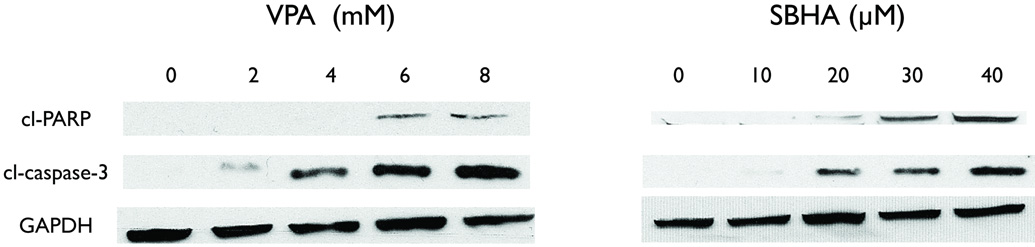
HDAC inhibitors induce apoptosis in pheochromocytoma cells in vitro
After observing that HDAC inhibitors significantly limited growth in PC-12 cells, we studied the mechanism of this inhibition. We utilized a Western blot for PARP and caspase-3. Both of these proteins are well-known markers of the apoptotic pathway, and their cleavage is indicative of apoptosis. After 2 days of treatment by HDAC inhibitors, increased cleavage was noted in both PARP and caspase-3 (Figure 5). These increases were dose-dependent and suggest that the cell death observed by MTT was due to apoptosis. This suggests that HDAC inhibitors cause growth inhibition via apoptosis.
Figure 5.
The mechanism of growth inhibition by VPA and SBHA is apoptosis. PC-12 cells were treated with the indicated concentrations of HDAC inhibitors for 2 days, and total cell lysates were prepared. An increase in the cleavage of these two proteins suggests that apoptosis induced cell death. GAPDH was used as a loading control. VPA, valproic acid, SBHA, suberoyl bis-hydroxamic acid, GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Discussion
Pheochromocytomas are rare catecholamine-secreting tumors of the adrenal medulla for which surgical resection is the only cure. Unfortunately, not all pheochromocytomas are resectable, and up to 10% of patients have malignant disease. For those patients with surgically unresectable tumors, medical treatment remains elusive. While chemotherapeutic regimens have shown some promise, there is an urgent need for pharmacologic treatments of greater efficacy. The HDAC inhibitors VPA and SBHA have been shown to be effective in other NE tumors, including gastrointestinal carcinoid, pulmonary carcinoid, small cell lung cancer, and medullary thyroid cancer.14–17, 22 Here we present the results of treatment of pheochromocytoma cells with VPA and SBHA.
In this study, we have shown that pharmacologic activation of the Notch1 signaling pathway in pheochromocytoma cells leads to growth inhibition and hormonal suppression. Pheochromocytoma PC-12 cells express very little active Notch1 at baseline. Importantly, we observed an increase in the amount of cleaved, active Notch1 protein (NICD) with increasing doses of both HDAC inhibitors. This was also shown to be functionally active NICD by the measurement of CBF-1 binding activity. Moreover, treatment with HDAC inhibitors led to a suppression of NE hormones and markers. This could be therapeutically useful if it leads to decreased hormonal secretion, as these patients often suffer from symptoms such as sweating, headache, tachycardia, and hypertension.
Treatment with HDAC inhibitors significantly suppressed growth in pheochromocytoma cells. Even at the lowest doses tested, there was a statistically significant growth inhibition after 2 days of treatment. After 6 days, growth was inhibited approximately 70% by both drugs at the highest doses tested. An increase in the cleavage of caspase-3 and PARP indicated that apoptosis was responsible for growth inhibition by both drugs (Figure 5). Depending on the NE cancer, HDAC inhibitors appear to inhibit growth differently: in gastrointestinal and pulmonary carcinoid cells, these drugs induce cell cycle arrest15, 16, while they induce apoptosis in medullary thyroid cancer.14, 17 Not only is treatment with HDAC inhibitors associated with hormonal suppression, it is associated with significant growth inhibition via apoptosis.
Notch1 activation is clearly an important component of the treatment of NE tumors. We have already demonstrated that activation leads to growth inhibition and hormonal suppression.9, 11, 12, 21 Our results in pheochromocytoma cells confirm results we have previously shown with HDAC inhibition activating the Notch1 signaling pathway.15, 16 Previous studies have shown that Notch1 is indeed responsible for growth inhibition in NE tumors.9, 11, 12 Thus, it is reasonable to propose that it is the cause of growth inhibition in pheochromocytoma cells as well.
The mechanism of action of HDAC inhibitors remains unresolved. While they have been shown to modify transcription by increasing histone acetylation and altering chromatin structure23, the exact mechanism for the restoration of tumor suppressor genes such as Notch1 is unclear. It may act directly through transcriptional regulation, affect transcriptional repressors or degradation enzymes, or influence cells through yet another unknown mechanism.
Of possible further interest are other signaling pathways already investigated in pheochromocytoma. Pharmacologic activation of the raf-1 pathway18 and inhibition of the GSK3β pathway19 have similar effects on phenotype to that of Notch1 activation: both pathways decrease the amount of ASCL1 and CgA while inhibiting growth. Given these commonalities, there may be some interaction between all three of these seemingly unrelated pathways. If the effects are synergistic, this could denote a possible combination therapy for pheochromocytoma.
In conclusion, the limited treatment options available for patients with malignant or unresectable pheochromocytomas necessitate new pharamacologic modalities. Notch1 upregulation has been shown an effective treatment of NE tumors, and the activation of this the pathway could be a possible therapeutic target for patients with phechromocytoma. This study demonstrates that pharmacologic activation of the Notch1 signaling pathway by the HDAC inhibitors VPA and SBHA is possible in pheochromocytoma cells. Moreover, such in vitro treatment led to a decrease in hormonal markers ASCL1 and CgA and significant growth inhibition via apoptosis. These drugs may represent a novel strategy in the treatment of patients with unresectable or malignant disease.
Acknowledgments
Grant support: Joel T. Adler is a Howard Hughes Medical Institute Research Training Fellow and is supported by the University of Wisconsin General Clinical Research Center. Additional support from a Research Scholars Grant from the American Cancer Society, National Institutes of Health Grants DK063015, DK064735, DK066169, CA117117, and CA109053, the George H. A. Clowes, Jr., Memorial Research Career Development Award of the American College of Surgeons, a Carcinoid Cancer Foundation research award (to H. C.), the Clincial Investigator’s Award from the Society of Surgical Oncology, a Doctors Cancer Foundation grant, and by a University of Wisconsin Medical School grant (to M. K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adler JT, Meyer-Rochow GY, Chen H, et al. Pheochromocytoma: current approaches and future directions. Oncologist. 2008 doi: 10.1634/theoncologist.2008-0043. In press. [DOI] [PubMed] [Google Scholar]
- 2.Stein PP, Black HR. A simplified diagnostic approach to pheochromocytoma. A review of the literature and report of one institution's experience. Medicine (Baltimore) 1991;70:46–66. doi: 10.1097/00005792-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Mantero F, Terzolo M, Arnaldi G, et al. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab. 2000;85:637–644. doi: 10.1210/jcem.85.2.6372. [DOI] [PubMed] [Google Scholar]
- 4.Adler JT, Mack E, Chen H. Isolated adrenal mass in patients with a history of cancer: remember pheochromocytoma. Ann Surg Oncol. 2007;14:2358–2362. doi: 10.1245/s10434-007-9426-4. [DOI] [PubMed] [Google Scholar]
- 5.Manger WM. The vagaries of pheochromocytomas. Am J Hypertens. 2005;18:1266–1270. doi: 10.1016/j.amjhyper.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 6.Manger WM, Gifford JRW. Clinical and experimental pheochromocytoma. Cambridge, MA: Blackwell Science; 1996. [Google Scholar]
- 7.Sisson JC, Shapiro B, Shulkin BL, Urba S, Zempel S, Spaulding S. Treatment of malignant pheochromocytomas with 131-I metaiodobenzylguanidine and chemotherapy. Am J Clin Oncol. 1999;22:364–370. doi: 10.1097/00000421-199908000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Pacak K, Fojo T, Goldstein DS, et al. Radiofrequency ablation: a novel approach for treatment of metastatic pheochromocytoma. J Natl Cancer Inst. 2001;93:648–649. doi: 10.1093/jnci/93.8.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunnimalaiyaan M, Chen H. Tumor suppressor role of Notch-1 signaling in neuroendocrine tumors. Oncologist. 2007;12:535–542. doi: 10.1634/theoncologist.12-5-535. [DOI] [PubMed] [Google Scholar]
- 10.Sjolund J, Manetopoulos C, Stockhausen MT, Axelson H. The Notch pathway in cancer: differentiation gone awry. Eur J Cancer. 2005;41:2620–2629. doi: 10.1016/j.ejca.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, Chen H. Overexpression of the NOTCH1 intracellular domain inhibits cell proliferation and alters the neuroendocrine phenotype of medullary thyroid cancer cells. J Biol Chem. 2006;281:39819–39830. doi: 10.1074/jbc.M603578200. [DOI] [PubMed] [Google Scholar]
- 12.Kunnimalaiyaan M, Yan S, Wong F, Zhang YW, Chen H. Hairy Enhancer of Split-1 (HES-1), a Notch1 effector, inhibits the growth of carcinoid tumor cells. Surgery. 2005;138:1137–1142. doi: 10.1016/j.surg.2005.05.027. discussion 42. [DOI] [PubMed] [Google Scholar]
- 13.Nakakura EK, Sriuranpong VR, Kunnimalaiyaan M, et al. Regulation of neuroendocrine differentiation in gastrointestinal carcinoid tumor cells by notch signaling. J Clin Endocrinol Metab. 2005;90:4350–4356. doi: 10.1210/jc.2005-0540. [DOI] [PubMed] [Google Scholar]
- 14.Greenblatt DY, Cayo M, Adler JT, et al. Valproic acid activates notch1 signaling and induces apoptosis in medullary thyroid cancer cells. Annals of Surgery. 2008 doi: 10.1097/SLA.0b013e3181758d0e. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenblatt DY, Vaccaro AM, Jaskula-Sztul R, et al. Valproic acid activates notch-1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. Oncologist. 2007;12:942–951. doi: 10.1634/theoncologist.12-8-942. [DOI] [PubMed] [Google Scholar]
- 16.Greenblatt DY, Cayo M, Ning L, et al. Suberoyl bishydroxamic acid inhibits cellular proliferation by inducing cell cycle arrest in carcinoid cancer cells. J Gastrointest Surg. 2007;11:1515–1520. doi: 10.1007/s11605-007-0249-1. discussion 20. [DOI] [PubMed] [Google Scholar]
- 17.Ning L, Greenblatt DY, Jaskula-Sztul R, Kunnimalaiyaan M, Chen H. SBHA activates notch1 signaling and induces apoptosis in medullary thyroid cancer cells. Oncologist. 2008 doi: 10.1097/SLA.0b013e3181758d0e. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kappes A, Vaccaro A, Kunnimalaiyaan M, Chen H. ZM336372, a Raf-1 activator, inhibits growth of pheochromocytoma cells. J Surg Res. 2006;133:42–45. doi: 10.1016/j.jss.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Kappes A, Vaccaro A, Kunnimalaiyaan M, Chen H. Lithium ions: a novel treatment for pheochromocytomas and paragangliomas. Surgery. 2007;141:161–165. doi: 10.1016/j.surg.2006.12.005. discussion 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sippel RS, Carpenter JE, Kunnimalaiyaan M, Lagerholm S, Chen H. Raf-1 activation suppresses neuroendocrine marker and hormone levels in human gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G245–G254. doi: 10.1152/ajpgi.00420.2002. [DOI] [PubMed] [Google Scholar]
- 21.Kunnimalaiyaan M, Traeger K, Chen H. Conservation of the Notch1 signaling pathway in gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G636–G642. doi: 10.1152/ajpgi.00146.2005. [DOI] [PubMed] [Google Scholar]
- 22.Platta CS, Greenblatt DY, Kunnimalaiyaan M, Chen H. Valproic acid activates the notch1 signaling pathway and inhibits growth in small cell lung cancer cells. J Surg Res. 2008 doi: 10.1016/j.jss.2008.03.008. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cress WD, Seto E. Histone deacetylases, transcriptional control, and cancer. J Cell Physiol. 2000;184:1–16. doi: 10.1002/(SICI)1097-4652(200007)184:1<1::AID-JCP1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]