Abstract
Hyperlipidemia is a major risk factor for developing atherosclerosis in humans, and epidemiological studies have correlated specific lipoprotein levels with cardiovascular disease risk. Murine models of atherosclerosis rely on the induction of hyperlipidemia for vascular lesions to form, but the pathogenic contributions attributed to different lipoprotein populations are not well defined. To address this issue, we analyzed over 300 LDL receptor (LDLR) deficient mice that have been fed a high-fat diet and for which a full lipoprotein profile and aortic root atherosclerosis values were assessed. Overall, aortic root atherosclerosis is best predicted by plasma VLDL cholesterol levels with less predictive value derived from either LDL or HDL cholesterol. Triglyceride levels are more atherogenic in female mice, especially immune competent females, and depletion of the adaptive immune system leads to a global reduction in plasma lipid levels and aortic root lesion size yet does not appear to alter the atherogenic potential of individual lipoprotein subspecies. In contrast, HDL-cholesterol is a better predictor of aortic root atherosclerosis in apoE-deficient mice. In summary, this large scale analysis of high-fat diet fed LDLR deficient mice highlight the relationship between different plasma lipid components, especially VLDL-cholesterol, and aortic root atherosclerosis.
Keywords: hyperlipidemia, cholesterol, HDL
Atherosclerosis is a vascular site-specific chronic inflammation initiated in response to retained and modified lipids within the arterial wall that can eventually lead to clinically significant endpoints including myocardial infarction, peripheral arterial disease, and stroke (1–3). Although multiple risk factors have been shown to play a significant role in the pathogenesis of atherosclerosis and cardiovascular disease, the determination of plasma lipid levels in humans remains a cornerstone in the clinical assessment of cardiovascular risk. Numerous large-scale epidemiological studies and clinical trials have associated different plasma lipid components with the risk for experiencing future cardiovascular events, with most focusing on total cholesterol, LDL cholesterol, and HDL cholesterol (4–10). These population-based studies and related trials of lipid-lowering agents (especially concerning the statin class of drugs) have firmly established that hypercholesterolemia is a primary and treatable cause of atherosclerosis and cardiovascular disease in humans.
Over the past 20 years, mouse models of atherosclerosis have proven extremely useful in dissecting the cellular and molecular interactions involved in atherogenesis. An almost uniform requirement for these models to develop vascular lesions is the induction of hypercholesterolemia, either through dietary or genetic means (11). It has not been shown whether the same pathogenic lipid relationships that have been assessed in humans also hold true for the development of murine atherosclerosis. To address this issue, we performed a retrospective analysis of over 300 diet-induced hyperlipidemic LDL receptor deficient (LDLR−/−) mice studied in our lab, all of which incorporated individual plasma lipid and aortic root atherosclerosis information. Innominate artery atherosclerosis for 235 of the mice was also examined. A similar analysis was also performed on data from 130 apoE-deficient (apoE−/−) mice. The data for these mice were compiled from a number of studies that have examined the effects of immune deficiency, gender, type of high-fat diet, and immune cell transfers on atherogenesis in the LDLR−/− mouse model (12–16, and C. A. Reardon and G. S. Getz, ,unpublished data).
MATERIALS AND METHODS
Mice and lipid determination
Specific experimental details for each study can be found in references (12–16). Briefly, all mice were housed in specific pathogen-free barrier facilities at the University of Chicago and experimental procedures performed in accordance with NIH and institutional guidelines. Most of the mice examined in this paper were LDLR deficient on the C57BL/6 background. In addition to high-fat diet fed LDLR−/− mice with a fully functional adaptive immune system (12–14), different subgroups included adaptive immune-deficient mice derived from recombination activating gene (RAG)/LDLR double knockouts (12, 13, unpublished data) as well as partially adaptive immune-deficient mice that were derived by either selectively reconstituting the RAG/LDLR double knockout mice with different lymphocyte subsets (15, unpublished data), selectively depleting lymphocyte subsets or function in LDLR−/− mice (14, unpublished data), or by crossing the LDLR−/− mice with other immune-deficient models (unpublished data). In all cases, a standardized protocol was followed: at 8–10 weeks of age, the mice were switched from a standard laboratory low-fat chow diet to a high-fat diet (either the Western type diet, Harlan Teklad TD88137; a milk fat-enriched diet, Harlan Teklad TD97222; or a safflower oil-enriched diet, Harlan Teklad TD97223). After 12 weeks of high-fat diet feeding, the mice were fasted for 4 h and bled via the retro-orbital sinus with the resultant plasma used for determining fasting plasma cholesterol and triglyceride levels using kits from Roche Molecular Diagnostics. Lipoprotein cholesterol profiles were generated from the plasma via fractionation using tandem Superose 6 fast-protein liquid chromatography (FPLC) columns. FPLC fractions were analyzed for cholesterol content and peaks corresponding to VLDL, LDL, and HDL identified. All of the triglyceride in the FPLC fractions was contained in the VLDL fractions and was used to identify the fractions corresponding to VLDL. Preliminary studies of immune competent and RAG deficient apoE deficient (apoE−/−) mice maintained on chow diet for 27 weeks were also analyzed for aortic root and innominate artery atherosclerosis, plasma lipids, and lipoproteins. A total of 130 apoE−/− mice were studied, two-thirds of which were fully backcrossed onto the C57BL/6 background.
Atherosclerosis quantification
At sacrifice the upper arterial vasculatures were perfused with a paraformaldehyde solution under physiologic pressures, dissected from the mouse, mounted in OCT, and serial 10 μm frozen sections collected for Oil Red O staining of neutral lipids (12–16). Aortic root atherosclerosis was assessed as the average of three sections, each separated by 100 μm, beginning at the site of appearance of the coronary artery and valve leaflets. Innominate artery atherosclerosis was determined based on three or four sections, each separated by 100 μm, beginning 350 μm above the aortic arch. Atherosclerosis was quantified from digitally captured images and OpenLab Software, version 1.7.6.
Data analysis
The LDLR deficient data set included a total of 321 mice (164 males and 157 females), with each mouse representing a separate lipid and atherosclerosis measurement with the exception of 88 paired mice that were treated as 44 discrete samples. Each pair of mice had their plasma pooled for FPLC lipoprotein determination, which was then coupled with the average of the atherosclerosis assessments for the two mice. Each paired sample was treated as a single data point with appropriate weighting when determining the linear regression for each lipid association. Similar data was obtained from 27-week-old chow-fed apoE−/− mice (n = 130; 66 males and 64 females), with 70 paired mice that were treated as 35 discrete samples. All data are expressed as mean ± SD. Unpaired two-tailed t-tests were used to determine differences between groups (probability values of P < 0.01 were considered statistically significant for these studies). Linear regression was used to determine the relationship between variables, and the squared correlation coefficient (R2) was used to measure strength of relationship. Differences in slopes of regression lines were tested using a Wald test for interaction. All data processing and statistical analyses were performed using STATA Statistical software, StatView, and Microsoft Excel.
RESULTS
Lipid and lipoprotein profile determinations of LDLR−/− mice
The mean terminal vital parameters including plasma lipid and root atherosclerosis measurements for the LDLR−/− mice included in this data set as well as subgroup breakdowns are shown in Table 1. The fasting plasma cholesterol levels attained in these LDLR−/− mice fed a high-fat diet were on average approximately seven times that seen in normocholesterolemic humans.
TABLE 1.
Vital parameters for LDLR−/− mice
| Total Cholesterol | Triglycerides | VLDLc | LDLc | HDLc | Non-HDLc:HDLc | Mass | Aortic Root Atherosclerosis | |
|---|---|---|---|---|---|---|---|---|
| All mice (n = 321) | 1500 ± 587 | 639 ± 444 | 668 ± 451 | 718 ± 216 | 110 ± 36 | 14.3 ± 8.8 | 29.2 ± 6.7 | 192,412 ± 107,491 |
| Gender subgroups | ||||||||
| Males (n = 164) | 1543 ± 566 | 769 ± 487 | 629 ± 412 | 783 ± 224 | 124 ± 36 | 12.7 ± 7.3 | 33.6 ± 5.6 | 173,987 ± 96,662 |
| Females (n = 157) | 1449 ± 610 | 486 ± 328a | 714 ± 491 | 641 ± 178a | 93 ± 27a | 16.1 ± 9.9a | 24.1 ± 3.4a | 214,174 ± 115,666a |
| Adaptive immunity subgroups | ||||||||
| Competent (n = 148) | 1695 ± 701 | 747 ± 506 | 797 ± 567 | 780 ± 242 | 109 ± 38 | 16.9 ± 10.9 | 30.4 ± 6.3 | 242,956 ± 112,934 |
| Partial deficient (n = 55) | 1439 ± 426 | 776 ± 402c | 616 ± 242 | 712 ± 212 | 111 ± 36 | 13.0 ± 5.8 | 31.3 ± 7.0c | 156,257 ± 80,309b,c |
| Deficient (n = 118) | 1269 ± 362d | 424 ± 262d | 522 ± 280d | 637 ± 143d | 111 ± 32 | 11.4 ± 4.9d | 26.6 ± 6.2d | 143,310 ± 78,815d |
All values are displayed as mean ± SD. Lipid parameters are expressed as mg/dl, mass in grams, and atherosclerosis as μm2.
P < 0.01 for males vs. females.
P < 0.01 for adaptive immune competent vs. adaptive immune partial deficient.
P < 0.01 for adaptive immune partial deficient vs. adaptive immune deficient.
P < 0.01 for adaptive immune competent vs. adaptive immune deficient.
Total data set analysis of LDLR−/− mice
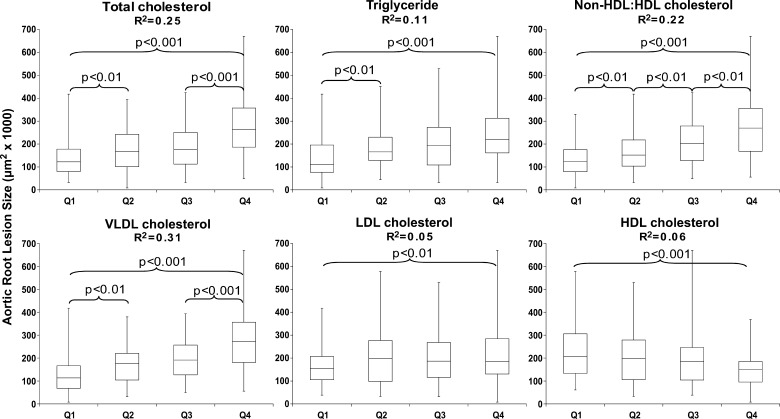
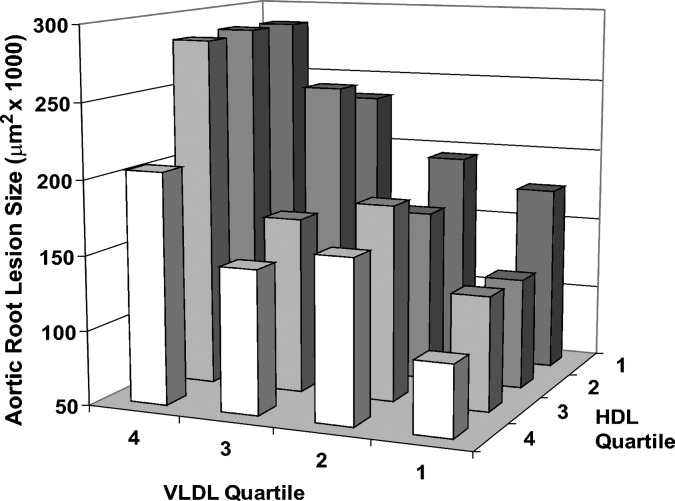
When analyzing the complete LDLR−/− data set, box and whisker plots were constructed relating each progressive quartile for each individual lipid variable to aortic root atherosclerosis as shown in Fig. 1. As expected, aortic root atherosclerosis was overall positively associated with terminal total cholesterol, triglycerides, VLDL cholesterol, and LDL cholesterol, while negatively associated with HDL cholesterol levels. Interestingly, no association was observed between the body mass of the mouse and aortic root lesion size (data not shown). The calculated ratio of non-HDL cholesterol to HDL cholesterol was also positively related to aortic root lesion size, with significant differences observed between each stepwise quartile. Based on the squared correlation coefficient (R2 value) calculated from the linear regression derived from the scatter plot for each relation, VLDL cholesterol ended up being the best predictor of aortic root atherosclerosis with much lower predictive value seen with LDL or HDL cholesterol. To see if the negative association seen with HDL cholesterol was independent of the stronger positive association seen with VLDL cholesterol, a cross-classification based on these two lipid measures was performed. As shown in Fig. 2, within each quartile of VLDL cholesterol, the resultant aortic root lesion size increased with decreasing quartiles of HDL cholesterol. This relationship is even more apparent when aortic root atherosclerosis associated with each quartile of VLDL is plotted against upper and lower half of HDL cholesterol (see supplementary Figure I). Innominate artery atherosclerosis in 235 LDLR−/− mice was not strongly correlated with any of the lipid parameters studied (see supplementary Table I).
Fig. 1.
Box and whisker plots relating terminal plasma lipid parameters to aortic root atherosclerosis as assessed in LDLR−/− mice after 12 weeks of high-fat diet feeding. Each plot encompasses the entire data set (n = 321 mice) divided into progressive quartiles (Q1–Q4) based on increasing plasma lipid levels for each variable (n∼80 mice per quartile). Displayed is the squared correlation coefficient (R2 value) for the corresponding linear regression from the scatter plot (not shown) relating each lipid variable to resultant aortic root atherosclerosis. Statistically significant differences between adjacent quartiles as well as the Q1 to Q4 comparison are shown. ANOVA comparing means of Q1 through Q4 exhibited statistical significance (P < 0.001) for each parameter except LDL Cholesterol (P = 0.052). Error bars represent SD.
Fig. 2.
The extent of aortic root atherosclerosis is independently associated with both high levels of VLDL cholesterol and low levels of HDL cholesterol in the circulation. This chart is based on the entire data set (n = 321 mice) with each individual bar representing approximately 20 mice. Progressive quartiles for VLDL cholesterol and HDL cholesterol are based on increasing levels of cholesterol for each lipid variable.
Similar results were obtained when only mice fed the Western type diet (n = 179) were analyzed. The parameters with the highest squared correlation coefficient for aortic root atherosclerosis are non-HDLc/HDL ratio (R2 = 0.30), VLDL cholesterol (R2 = 0.25), and total cholesterol (R2 = 0.15). The R2 values for triglycerides, LDL cholesterol, and HDL cholesterol are >0.11. Within each quartile of VLDL cholesterol, aortic root lesion size increased with decreasing HDL cholesterol (see supplementary Figure I).
LDLR-deficient subgroup analysis: gender
The entire data set was next analyzed on the basis of gender. As compared with male LDLR−/− mice, females have significantly lower levels of plasma triglycerides, LDL cholesterol, and HDL cholesterol but a larger aortic root atherosclerosis burden (Table 1).
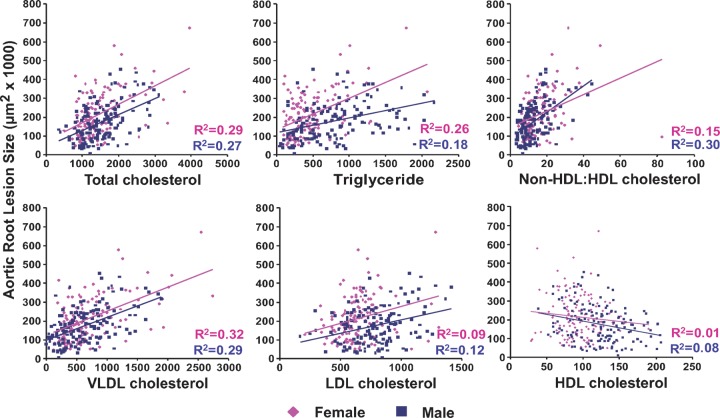
Scatter plots relating each lipid variable to resultant aortic root atherosclerosis were constructed with linear regression and squared correlation coefficient values displayed (Fig. 3). Overall, a similar pattern between each lipid parameter and aortic root atherosclerosis was observed for each gender as was noted for the data set as a whole. Squared correlation coefficient values between genders were similar, but male mice did demonstrate slightly stronger associations for LDL cholesterol and HDL cholesterol than female mice, which were recapitulated in the non-HDL cholesterol to HDL cholesterol relationship. On the other hand, the triglyceride levels in female mice were a better predictor of aortic root atherosclerosis than observed in males. Across the different plasma lipid components, female mice tended to have larger aortic root atherosclerotic lesions than male mice at any given lipid level, represented by the positive vertical displacement of the female linear regression line relative to that of males. On the other hand, the slopes of the linear regression lines for each association were overall similar between males and females. Only the plasma triglyceride linear regression demonstrated a significant difference in slope, implying that rising triglyceride levels may exert progressively more of an atherogenic pressure in females than males.
Fig. 3.
Subgroup analysis based on gender. Each graph plots the lipid variable (mg/dl) against the resultant aortic root atherosclerosis for both males (blue squares) and females (pink diamonds). Each color coded linear regression (blue for males and pink for females) has the corresponding squared correlation coefficient (R2 value) displayed. The slope of the female triglyceride regression line is significantly different than the slope of the male triglyceride regression line (P < 0.001).
LDLR deficient subgroup analysis: adaptive immune status
The entire data set was then analyzed with respect to the status of the adaptive immune system of the mouse, which was manipulated either genetically or experimentally in a number of the included studies. Three classifications used to group the mice were (1) adaptive immune competent where no manipulation of the adaptive immune system was involved (2), adaptive immune deficient where the mice were entirely depleted of any functional T- or B-lymphocytes by crossing the LDLR−/− mouse onto the RAG-deficient background, and (3) partially adaptive immune deficient where only a subset of the adaptive immune system was defective through either genetics (e.g., crossing onto a CD1d−/− background) or through selective cell population reconstitution (e.g., adoptive transfer of B-1 B-lymphocytes to RAG−/−LDLR−/− mice). As compared with adaptive immune competent mice, the adaptive immune-deficient mice demonstrate significantly lower mean plasma total cholesterol, triglyceride, VLDL cholesterol, and LDL cholesterol levels with a concurrent significant decrease in aortic root lesion size (Table 1). Interestingly, other than having a significantly higher plasma triglyceride level than the adaptive immune-deficient mice, the somewhat heterogeneous partially adaptive immune-deficient group tended to have both plasma lipid levels and aortic root atherosclerosis lesion burden that fell between those measured for the adaptive immune competent and adaptive immune-deficient mice (Table 1).
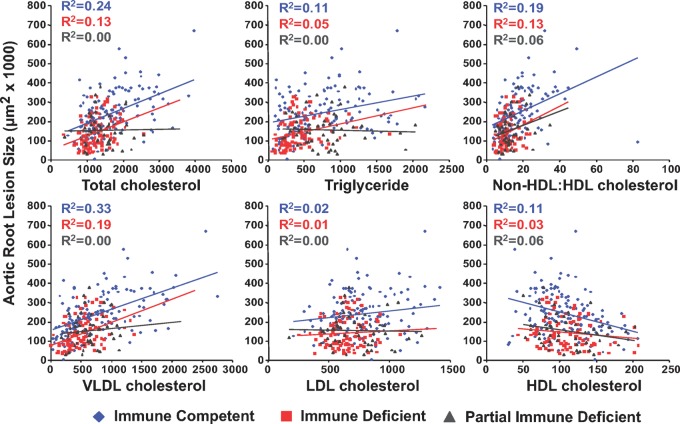
As with the gender subgroup analysis, scatter plots relating each lipid variable to resultant aortic root atherosclerosis were constructed with linear regression and squared correlation coefficient values for each group displayed (Fig. 4). Across all lipid measures, the adaptive immune-deficient mice demonstrated the same direction of association but lower squared correlation coefficients than the adaptive immune competent mice. Adaptive immune-deficient mice did tend to have lower plasma lipid levels across the board, but for any given plasma lipid level the adaptive immune-deficient mice had less aortic root atherosclerosis than their adaptive immune-competent counterparts, represented by the negative vertical displacement of the adaptive immune-deficient mice linear regression line relative to that of the adaptive immune-competent mice. No difference in slope was observed between these two groups, indicating that the atherogenic pressure exerted by increasing levels of these pathogenic lipoproteins is similar between these two groups. When examining the somewhat heterogeneous subgroup of adaptive immune-partial-deficient mice, it was observed that none of the proatherogenic lipid measures held any predictive value for resultant aortic root atherosclerosis (a squared correlation coefficient of zero), though HDL cholesterol still did hold a negative relation with aortic root lesion size with a recapitulation of this relationship seen in the nonHDL to HDL cholesterol ratio.
Fig. 4.
Subgroup analysis based on adaptive immune status. Each graph plots the lipid variable (mg/dl) against the resultant aortic root atherosclerosis for adaptive immune competent (blue diamonds), adaptive immune-deficient (red squares), and adaptive immune partially deficient (gray triangles) mice. Each color coded linear regression (blue for adaptive immune competent, red for adaptive immune deficient, and gray for adaptive immune partially deficient mice) has the corresponding squared correlation coefficient (R2 value) displayed.
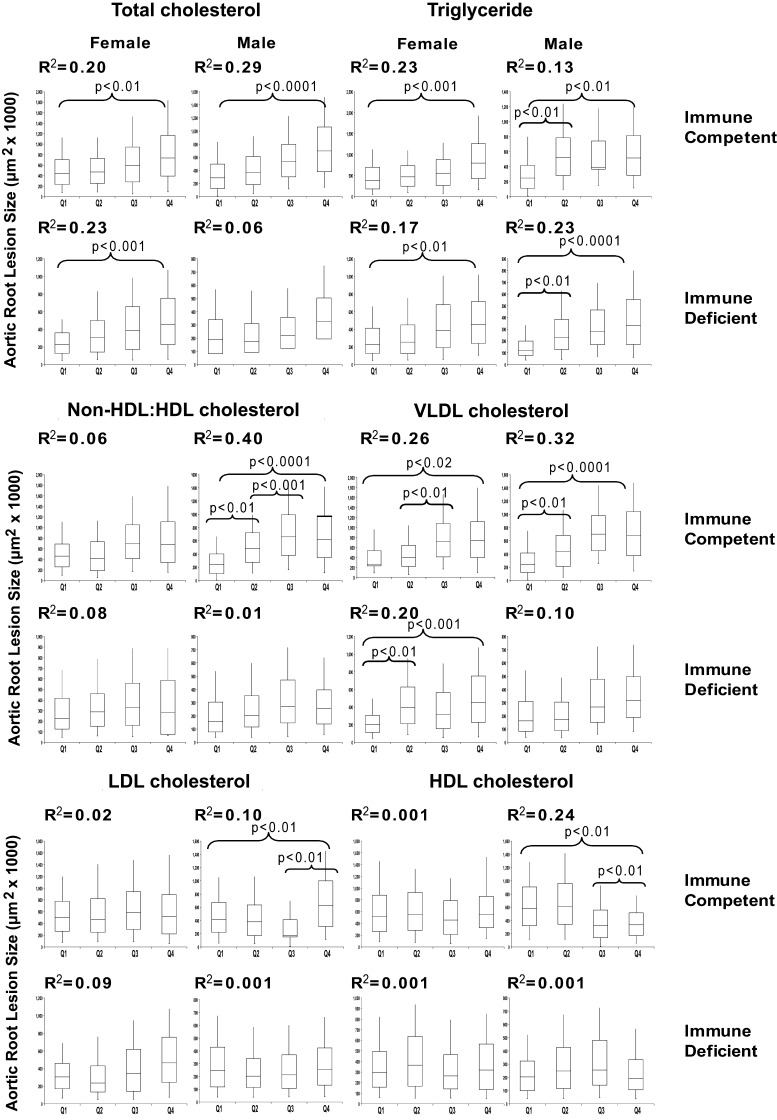
To study the interaction between gender and immune status, we show the box and whisker plot for the immune competent and immune-deficient mice stratified by gender (the partially immune-deficient mice were excluded from this analysis) (Fig. 5). For these analyses, each box contains 14–20 animals. Here it is seen that triglyceride and VLDL cholesterol are associated with aortic root atherosclerosis in males and females of the immune-competent and -deficient subsets. Total cholesterol was associated with aortic root atherosclerosis in both genders of immune- competent mice but only female immune-deficient mice, while LDL cholesterol was poorly associated with this atherosclerosis in all subsets. The negative association with HDL cholesterol was only seen with the immune-competent males. The immune-deficient mice of either gender showed little negative association with aortic root atherosclerosis. The highest correlation coefficients were seen for the non-HDLc/ HDL cholesterol ratio (R2 = 0.40) and VLDL cholesterol (R2 = 0.32) in immune competent male mice.
Fig. 5.
Box and whisker plots relating terminal plasma lipid parameters to aortic root atherosclerosis in immune competent and fully immune incompetent LDLR−/− mice separated by gender. Each plot encompasses the data set divided into progressive quartiles (Q1–Q4) based on increasing plasma lipid levels for each variable (n∼12–18 mice per quartile). Displayed is the squared correlation coefficient (R2 value) for the corresponding linear regression from the scatter plot (not shown) relating each lipid variable to resultant aortic root atherosclerosis. Statistically significant differences between adjacent quartiles as well as the Q1 to Q4 comparison are shown. Error bars represent SD.
Total data set analysis of apoE−/− mice
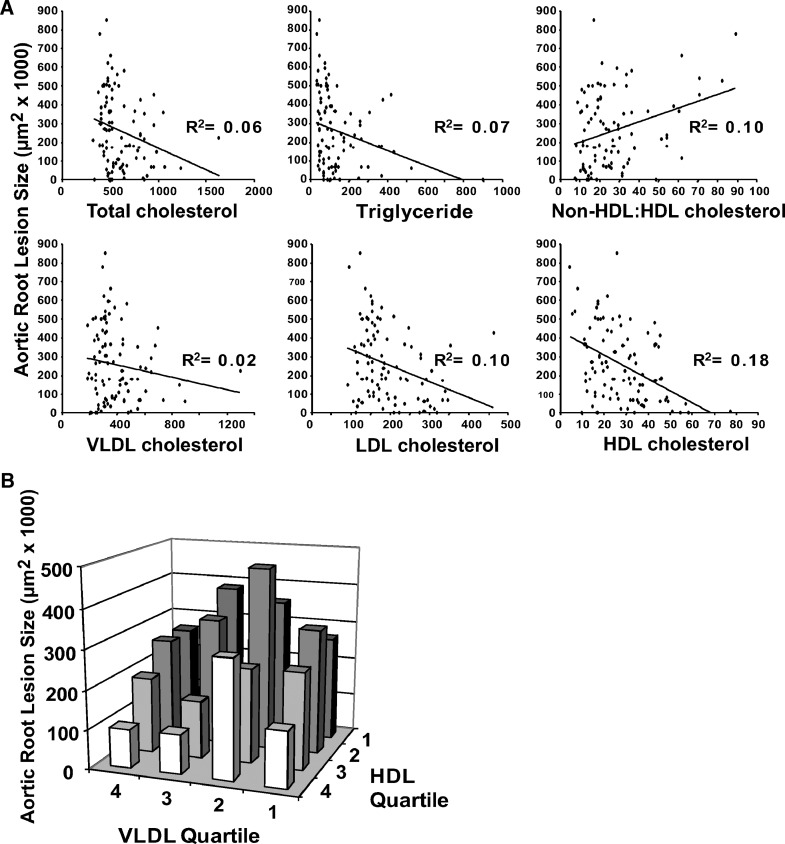
To determine whether these risk relationships also apply to another model of murine atherosclerosis, we undertook a similar analysis of apoE−/− mice maintained on chow diet. Both aortic root and innominate artery atherosclerosis were examined in male and female immune-competent and -deficient mice at 27 weeks of age. The summary of these data is shown in Table 2. This preliminary analysis is confounded in that only 64% of the animals were fully backcrossed into the C57BL/6 background; the others were predominantly in that background (16). The scatter plot for total cholesterol, HDL cholesterol and the ratio of non-HDL cholesterol/HDL cholesterol in relation to aortic root atherosclerosis are shown in Fig. 6A. In contrast to the LDLR−/− mice, aortic root atherosclerosis was surprisingly negatively correlated with total cholesterol in these animals that were preselected for an initial total cholesterol of 350–600 mg/dl. On the other hand, apoE−/− animals revealed a more robust negative correlation with HDL cholesterol level (R2 = 0.18) than was observed for the LDLR−/− mice (R2 = 0.06). Also the non-HDL cholesterol/HDL cholesterol ratio for the apoE−/− mice showed a lower correlation (R2 = 0.10). The R2 values for these parameters are not significantly associated with innominate artery atherosclerosis (see supplementary Table I). As we did for the LDLR−/− mice, we also examined the cross correlation of VLDL cholesterol and HDL cholesterol in influencing aortic root atherosclerosis in apoE−/− mice (Fig. 6B). For this murine model also, at each quartile of VLDL-cholesterol there is an inverse relationship between HDL-cholesterol and aortic root atherosclerosis. Similar trends were observed when only the fully backcrossed apoE−/− mice were analyzed (data not shown).
TABLE 2.
Vital parameters for apoE−/− mice
| Total Cholesterol | Triglycerides | VLDLc | LDLc | HDLc | nonHDLc:HDLc | Mass | Aortic Root Atherosclerosis | Innominate Artery Atherosclerosis |
|---|---|---|---|---|---|---|---|---|
| 610 ± 215 | 137 ± 130 | 387 ± 173 | 193 ± 73 | 27 ± 13 | 26.7 ± 17 | 28.9 ± 5.8 | 262,530 ± 195,268 | 97,683 ± 73,980 |
All values are displayed as mean ± SD. Lipid parameters are expressed as mg/dl, mass in grams, and atherosclerosis as μm2. N = 130 mice.
Fig. 6.
ApoE−/− mice. A: Analysis of aortic root atherosclerosis. Each graph plots the plasma lipid level (mg/dl) against the resultant aortic root atherosclerosis and has the corresponding squared correlation coefficient (R2 value) displayed. B: Progressive quartiles for VLDL cholesterol and HDL cholesterol and the corresponding aortic root atherosclerotic lesion size are plotted.
DISCUSSION
As presented in this reanalysis of primary data from over 300 high-fat diet fed LDLR−/− mice and 130 apoE−/− mice studied in our laboratory, it is clear that plasma lipid parameters are able to predict resulting aortic root atherosclerosis, the arterial site most often assessed in murine atherosclerosis studies. For LDLR−/− mice, the correlative relationships identified between components of plasma lipoprotein profile demonstrated that VLDL cholesterol proves to be the best indicator of root atherosclerosis in this model. Total cholesterol, triglyceride, LDL cholesterol, and low HDL cholesterol levels also are positively correlated with aortic root lesion size, but to a lesser extent. Similar but more subtle differences in atherogenic drive for different plasma lipid components were also observed when animals were stratified by both gender and adaptive immune status. Thus, although the overall atherogenic qualities of various plasma lipid components follows that observed in human studies, the differences reported here for LDLR−/− mice, especially VLDL cholesterol levels, may be related to subtle mechanistic differences in the atherogenic process that takes place in this murine model.
This first analysis specifically looks at aortic root and innominate artery atherosclerosis in LDLR−/− mice fed a high-fat diet and relate the size of these lesions to the terminal lipid levels and lipoprotein profile. The aortic root is typically the initial locus of atherosclerotic lesion development in mice and is the most commonly assessed site for murine studies of atherosclerosis (17, 18). Although we report here data relating plasma lipid levels to atherosclerosis at this site, our lab and others have also studied the innominate artery or brachiocephalic trunk as a second arterial site prone to develop human-like atherosclerotic lesions (19, 20). In this data set, 235 of the mice also had lesion data for the innominate artery. The associations between plasma lipid levels and atherosclerosis at this site seemed to resemble those seen for the aortic root but the squared correlation coefficients were much lower (see supplementary Table I). This finding supports the notion that the atherogenic response to various risk factors may differ by vascular site (19). This variable atherogenic response to plasma lipid levels may also relate to the type of hypercholesterolemic mouse model studied, because the lipid associations for these LDLR−/− mice did not hold when analyzing apoE-deficient mice. HDL seems to be a more important negative risk factor for aortic root atherosclerosis in the apoE−/− mice than in the LDLR−/− mice. In addition there appears to be a relationship between VLDL cholesterol and aortic root atherosclerosis at the highest half of the HDL distribution. It does appear to be important to consider the arterial site and the mechanism by which the lesions are induced when interpreting studies involving murine atherogenesis.
This large-scale analysis of the LDLR−/− mice is unique in that it had the power to identify trends and associations between plasma lipid levels and resultant aortic root atherosclerosis across a number of experimental conditions. Over 300 mice were included in this data set, a number that greatly exceeds the vast majority of published murine atherosclerosis studies. Although any environmental and genetic variability was minimized, the individual experimental groups of mice studied still represent a somewhat heterogeneous population with respect to dietary and immunological manipulations, as evidenced by the varying correlations with aortic root atherosclerosis in some of the parameters by gender and immune status. This type of variability is inherent in any population-based study. Our data set represents a retrospective murine epidemiological study that highlights a correlative (but not necessarily a mechanistic) relationship between plasma lipid levels and aortic root atherosclerosis.
Here we unexpectedly find that the development of aortic root atherosclerosis in LDLR−/−mice is more closely related to VLDL cholesterol levels than LDL or HDL levels, both of which are key players in the development of atherosclerosis in humans. This finding could be due to the higher relative levels of VLDL cholesterol in these high-fat diet fed LDLR−/− mice as compared with humans. The complex vascular lesions that develop in these mice form over the span of weeks rather than years as in humans. The absence of cholesterol ester transfer protein in mice effectively attenuates the functional link between the apolipoprotein B containing lipoproteins (mainly LDL and VLDL) and HDL. This deficiency may describe the lower correlation values seen for LDL and HDL in this model relative to that of VLDL.
The first major subgroup analysis performed on the LDLR−/− data set was on the basis of gender. As previously reported, female mice tend to have larger lesions at the aortic root than males (21–23). At any given plasma lipid level the linear regression line for females is vertically displaced upwards for each lipid parameter assessed (Fig. 3). This is most strikingly shown for plasma triglyceride levels. Female LDLR−/− mice have significantly lower plasma triglyceride levels than their male counterparts (Table 1), yet have a much higher squared correlation coefficient relating triglyceride levels to aortic root atherosclerosis (Fig. 3). Furthermore, the slope of the female linear regression line was significantly different than that of the male mice; suggesting that plasma triglycerides may be more of a risk factor for females. This risk difference has been observed in humans as well: data from the Framingham study demonstrated that plasma triglyceride levels as an individual risk factor were associated with coronary heart disease only in women (24). Differences in the lipid composition of VLDL could account for the differences in the atherogenic potential between males and females. The VLDL cholesterol to plasma triglyceride ratio is significantly higher in female mice compared with male mice (1.65 ± 0.84 vs. 0.90 ± 0.50, P < 0.001).
The second major subgroup analysis on the LDLR−/− data set was on the basis of adaptive immune status, a long-term interest of our laboratory. The absence of functional T- and B-lymphocytes leads to a reduction and proportional redistribution of plasma lipid levels in the LDLR−/− and apoE−/− mouse models (12, 13, 16). The adaptive immune system can play a role in regulating systemic lipid levels (14). T and B-lymphocytes have been implicated in the pathogenesis of atherogenesis (1, 2). In the absence of both T- and B-lymphocytes, there appears to be a net reduction of atherogenic plasma lipid levels as well as a reduction of the inflammatory cytokine profile driving lesion development. The heterogeneous partially adaptive immune-deficient group lacked the strong correlation between the proatherogenic plasma lipids and aortic root lesion size, perhaps because these immunological manipulations may have modulated the lesion inflammatory reaction independent of any lipid changes. The proportion of total cholesterol represented by VLDL cholesterol in immune-deficient mice (41%) is lower than that seen with immune competent mice (47%), and this could contribute to lower correlation coefficient for VLDL cholesterol by aortic root atherosclerosis in the immune-deficient group.
Excluding the partial immune-deficient group, we stratified aortic root atherosclerosis by both gender and immune status. Similar correlations were seen for total cholesterol, triglyceride, and VLDL cholesterol for both males and females, regardless of immune status except for total cholesterol in immune-deficient males (Fig. 5). On the other hand relatively high correlations were seen for HDL cholesterol and especially for nonHDLc/HDL cholesterol ratio only in male immune competent mice. This latter risk is consistent with the recent report by Kastelein and colleagues (25) who showed the value of the apoB/apoA-I ratio as a positive risk factor.
This report is a retrospective correlative study that does not nor is able to delineate mechanistic underpinnings to any of the observed associations described. This said, the reported relationships may help to direct future experiments that are able to draw a mechanistic explanation for these findings. Another limitation of this study is that the measured plasma lipid levels and full lipoprotein profile were assessed only at the time of sacrifice. This single time point measurement may not necessarily capture steady-state lipid levels or reflect subtle changes in either lipid levels or the lipoprotein profile that occur during lesion development.
Analysis of the apoE−/− mice data set revealed that it is HDL cholesterol levels that have the strongest predictive value for resultant aortic root atherosclerosis, with high levels of HDL cholesterol correlating with smaller aortic root lesions. This suggests that as opposed to the LDLR−/− model where atherogenic lipid influx may be the more critical component of the atherogenic process, the major drive for lesion growth in apoE−/− mice may be the inability of lesion macrophage foam cells to effectively efflux cholesterol from the growing atherosclerotic plaque. Therefore, it is important to consider both the arterial site and the mechanism by which the lesions are induced in interpreting studies involving murine atherogenesis. Because of the limitation in pool size, we were not able to undertake a subset analysis of the apoE−/− mice based on gender or immune status.
In conclusion, this analysis summarizes a large body of work relating individual plasma lipid components to the development of atherosclerosis in mice: a first of its kind. Although hypercholesterolemia is indeed associated with the extent of atherosclerotic lesion development in the vasculatures of LDLR−/− mice, it is clear that plasma lipid parameters are insufficient on their own to adequately predict aortic root lesion size in mice. This speaks to the complex cellular and molecular interactions at play in atherogenesis. Though of a much more preliminary nature, the differences between the relative risk relationship of lipid and lipoprotein levels and aortic root atherosclerosis in LDLR−/− and apoE−/− mice and the much different response of the innominate artery atherosclerosis adds to the notion of the complexity of atherogenesis at least in these two murine models. Our analysis does reveal the somewhat unexpected findings in the LDLR−/− mice, including the primacy of VLDL cholesterol in promoting lesion development, the stronger influence of triglycerides in female than male mice, and the global effects of adaptive immune deficiency on plasma lipid levels. Additional prospective experimental murine studies will be needed to provide mechanistic explanations for these findings.
Supplementary Material
Abbreviations
FPLC, fast-protein liquid chromatography
LDLR, LDL receptor
RAG, recombination activating gene
Published, JLR Papers in Press, October 28, 2008.
Footnotes
This work was supported by the Cardiovascular Pathophysiology and Biochemistry Training grant HL007237 and the Medical Scientist Training Program grant GM007281 for P.A.V; NIH Clinical and Translational Science Award UL1 RR024999 for R.A.T; and NIH R01 grants HL056827, HL068661, and HL085516 for G.S.G.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of a figure and a table.
References
- 1.Hansson G. K., A. K. Robertson, and C. Söderberg-Nauclér. 2006. Inflammation and atherosclerosis. Annu Rev Pathol. 1 297–329. [DOI] [PubMed] [Google Scholar]
- 2.Getz G. S., P. A. VanderLaan, and C. A. Reardon. 2007. The immune system and murine atherosclerosis. Curr. Drug Targets. 8 1297–1306. [DOI] [PubMed] [Google Scholar]
- 3.Hansson G. K. 2005. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352 1685–1695. [DOI] [PubMed] [Google Scholar]
- 4.Sharrett A. R., C. M. Ballantyne, S. A. Coady, G. Heiss, P. D. Sorlie, D. Catellier, and W. Patsch, and the Atherosclerosis Risk in Communities Study Group. 2001. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 104 1108–1113. [DOI] [PubMed] [Google Scholar]
- 5.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). 2002. Circulation. 106: 3143–3421. [PubMed]
- 6.Grundy S. M., J. I. Cleeman, C. N. Merz, H. B. Brewer Jr, L. T. Clark, D. B. Hunninghake, R. C. Pasternak, S. C. Smith Jr, and J. N. Stone. 2004. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 110 227–239. [DOI] [PubMed] [Google Scholar]
- 7.Ridker P. M., N. Rifai, N. R. Cook, G. Bradwin, and J. E. Buring. 2005. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 294 326–333. [DOI] [PubMed] [Google Scholar]
- 8.Nam B. H., W. B. Kannel, and R. B. D'Agostino. 2006. Search for an optimal atherogenic lipid risk profile: from the Framingham Study. Am. J. Cardiol. 97 372–375. [DOI] [PubMed] [Google Scholar]
- 9.Kannel W. B., and P. W. Wilson. 1992. Efficacy of lipid profiles in prediction of coronary disease. Am. Heart J. 124 768–774. [DOI] [PubMed] [Google Scholar]
- 10.Stampfer M. J., F. M. Sacks, S. Salvini, W. C. Willett, and C. H. Hennekens. 1991. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N. Engl. J. Med. 325 373–381. [DOI] [PubMed] [Google Scholar]
- 11.Getz G. S., and C. A. Reardon. 2006. Diet and murine atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 26 242–249. [DOI] [PubMed] [Google Scholar]
- 12.Reardon C. A., L. Blachowicz, J. Lukens, M. Nissenbaum, and G. S. Getz. 2003. Genetic background selectively influences innominate artery atherosclerosis: immune system deficiency as a probe. Arterioscler. Thromb. Vasc. Biol. 23 1449–1454. [DOI] [PubMed] [Google Scholar]
- 13.Reardon C. A., L. Blachowicz, G. Gupta, J. Lukens, M. Nissenbaum, and G. S. Getz. 2006. Site-specific influence of polyunsaturated fatty acids on atherosclerosis in immune incompetent LDL receptor deficient mice. Atherosclerosis. 187 325–331. [DOI] [PubMed] [Google Scholar]
- 14.Lo J. C., Y. Wang, A. V. Tumanov, M. Bamji, Z. Yao, C. A. Reardon, G. S. Getz, and Y. X. Fu. 2007. Lymphotoxin beta receptor-dependent control of lipid homeostasis. Science. 316 285–288. [DOI] [PubMed] [Google Scholar]
- 15.VanderLaan P. A., C. A. Reardon, Y. Sagiv, L. Blachowicz, J. Lukens, M. Nissenbaum, C. R. Wang, and G. S. Getz. 2007. Characterization of the natural killer T-cell response in an adoptive transfer model of atherosclerosis. Am. J. Pathol. 170 1100–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reardon C. A., L. Blachowicz, T. White, V. Cabana, Y. Wang, J. Lukens, J. Bluestone, and G. S. Getz. 2001. Effect of immune deficiency on lipoproteins and atherosclerosis in male apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 21 1011–1016. [DOI] [PubMed] [Google Scholar]
- 17.Ishibashi S., J. L. Goldstein, M. S. Brown, J. Herz, and D. K. Burns. 1994. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J. Clin. Invest. 93 1885–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reardon C. A., and G. S. Getz. 2001. Mouse models of atherosclerosis. Curr. Opin. Lipidol. 12 167–173. [DOI] [PubMed] [Google Scholar]
- 19.VanderLaan P. A., C. A. Reardon, and G. S. Getz. 2004. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler. Thromb. Vasc. Biol. 24 12–22. [DOI] [PubMed] [Google Scholar]
- 20.Rosenfeld M. E., M. M. Averill, B. J. Bennett, and S. M. Schwartz. 2008. Progression and disruption of advanced atherosclerotic plaques in murine models. Curr. Drug Targets. 9 210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caligiuri G., A. Nicoletti, X. Zhou, I. Törnberg, and G. K. Hansson. 1999. Effects of sex and age on atherosclerosis and autoimmunity in apoE-deficient mice. Atherosclerosis. 145 301–308. [DOI] [PubMed] [Google Scholar]
- 22.Zabalawi M., S. Bhat, T. Loughlin, M. J. Thomas, E. Alexander, M. Cline, B. Bullock, M. Willingham, and M. G. Sorci-Thomas. 2003. Induction of fatal inflammation in LDL receptor and ApoA-I double-knockout mice fed dietary fat and cholesterol. Am. J. Pathol. 163 1201–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taleb S., O. Herbin, H. Ait-Oufella, W. Verreth, P. Gourdy, V. Barateau, R. Merval, B. Esposito, K. Clément, P. Holvoet, et al. 2007. Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 27 2691–2698. [DOI] [PubMed] [Google Scholar]
- 24.Gordon T., W. P. Castelli, M. C. Hjortland, W. B. Kannel, and T. R. Dawber. 1977. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am. J. Med. 62 707–714. [DOI] [PubMed] [Google Scholar]
- 25.Kastelein J. J. P., W. A. van der Steeg, I. Holme, M. Gafney, N. B. Cater, P. Barter, P. Deedwania, A. G. Olsson, S. M. Boekholdt, D. A. Demicco, et al; TNT and IDEAL Study Groups. 2008. Lipids, apolipoproteins, and their ratios in relation to cardiovascular events with statin treatment. Circulation. 117 3002–3009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.