Abstract
Induction of antiviral cytotoxic T lymphocytes (CTLs) has been proposed to require cross-presentation of viral antigens derived from infected extralymphatic host cells by antigen-presenting cells (APC). This postulated mechanism of cross-priming is thought to be essential for CTL responses against viruses that do not infect professional APC, e.g., because of absence of the specific virus receptor. Here, we show for the human pathogen poliovirus that naturally nonpermissive murine APC acquire viral RNA in vivo independently of the cellular virus receptor. Uptake of poliovirus or polioviral RNA initiated neosynthesis of viral antigen to an extent sufficient to prime CTLs in vivo, which were detectable 2-3 wk after infection. Our results do not only indicate that experiments studying cross-presentation and cross-priming by using potentially amplifiable or translatable materials need careful examination, but they also question the general biological importance of cross-presentation and cross-priming in antiviral CTL responses.
Two separate pathways have evolved for the presentation of endogenous and exogenous antigens to T cells; they locate to different subcellular compartments and engage distinct MHC molecules (1, 2). In general, endogenously derived peptides are loaded onto MHC class I molecules and presented to CD8+ T cells, whereas exogenous antigens are presented to CD4+ T cells in the context of MHC class II. Cross-presentation of exogenous antigen to CD8+ T cells has been proposed as an alternative mechanism of cytotoxic T lymphocyte (CTL) induction (3, 4). Although loading of antigens onto MHC class I molecules via the external pathway can clearly be demonstrated experimentally (refs. 5-12 and reviewed in refs. 13-16), it remains difficult to evaluate the relative contributions of cross-priming and direct priming to the induction of antiviral CTL responses in vivo, where direct viral infection of antigen-presenting cells (APC) and subsequent endogenous neosynthesis of antigen cannot be formally excluded.
Recently, a study on poliovirus (PV) infection of mice postulated that cross-priming was especially important for induction of CTL responses against viruses that do not infect professional APC (17). These studies presented the most compelling evidence so far for in vivo cross-priming because direct infection of APC seemed to be excluded by absence of the specific virus receptor. The host range of PV is restricted to human (and some primates) because of the expression of a specific cellular membrane protein, the PV receptor (PVR, CD155) (18), which mediates virus cell entry. Species, such as mice, that do not express the PVR are not susceptible to poliomyelitis (18-20). Because the ability of PV to cause clinical disease in vivo or cytopathic effects in cell cultures in vitro strictly correlates with expression of the PVR, it is generally assumed that PV cannot infect cells of PVR-negative hosts. Sigal et al. (17) generated bone marrow (BM) chimeras in which, because of transgenic expression of the PVR on either the donor BM cells and/or recipient host cells, PV could productively replicate either exclusively in nonhematopoietic cells (B6 → PVR BM chimera) or exclusively in hematopoietic cells including APC (PVR → B6 BM chimera), in both (PVR → PVR BM chimera) or neither (B6 → B6 BM chimera). They demonstrated that to induce CTL responses that could be restimulated 3 wk after infection, PVR expression was required on non-APC but, importantly, it was not required on the APC themselves. Based on the assumption that PV cannot infect PVR-negative APC, the authors concluded that PVR-negative APC must cross-present viral antigens.
It is correct that PV infection of murine cells is not possible if it is assessed as the ability of virus to cause cytolysis and viral propagation in cell cultures or clinical disease in vivo. However, we report here that this assumption is not correct if cell entry of viral RNA and translation of virally encoded antigen are evaluated. Our results demonstrate that induction of PV-specific CTL responses depended on endogenous neosynthesis of viral antigens in PVR-negative APC and not on cross-presentation.
Materials and Methods
Viruses and Mice. Trivalent PV vaccine (Sabin) and formalin-inactivated PV vaccine (Salk) were obtained from Berna Biotech (Bern, Switzerland). PV serotype I (Mahoney) was obtained from the Swiss Serum and Vaccine Institute (Bern, Switzerland). PV 160S particles were purified according to published protocols (21). For immunizations with UV-inactivated PV, virus stocks were exposed to UV light radiation with the minimal dose that prevented plaque formation on Vero cell monolayers; this completely inhibited viral translation in Vero cells as judged by the absence of immunohistochemically detectable nonstructural PV proteins 2B and 3D. The mAbs used to detect PV proteins 2B and 3D have been described (22). Ovalbumin-recombinant PV (PV OVA) (23) and PVR-transgenic mice (24) expressing the δ-isoform of the human PVR under control of the β-actin promoter (referred to as cPVR mice in ref. 17) were kindly provided by Raul Andino (University of California, San Francisco). The PVR-transgenic mice were on an outbred Institute for Cancer Research background. C57BL/6 mice were obtained from the Institute of Laboratory Animal Science, University of Zurich. For all experiments PVR-transgenic F1 mice (cPVR × C57BL/6) and nontransgenic C57BL/6 mice were used. Mice were immunized by tail vein injection.
Cells. Cell lines used in this study were obtained from the American Type Culture Collection. A murine fibrosarcoma cell line expressing the PVR was isolated from PVR-transgenic mice injected s.c. with methylcholanthrene; the cells were subcloned in vitro. BM-derived dendritic cells (DCs) were prepared as described (25). To isolate splenic DCs, spleens of infected mice were cut into small pieces and digested for 60 min with Collagenase D from Clostridium histolyticum (1 mg/ml in Iscove's modified Dulbecco's medium/5% FCS; Roche Diagnostics). After removing remaining aggregates by centrifugation, single cell suspensions were stained with anti-CD11c microbeads (Miltenyi Biotec, Auburn, CA), and CD11c+ cells were positively selected by using an autoMACS (Miltenyi Biotec). DC preparations were >90% pure.
For in vitro infections, PV was added to cell suspensions or monolayers in six-well tissue culture plates at a multiplicity of infection of 10-50. After 2 h, cells were washed twice with balanced salt solution to remove unbound virus and then incubated with medium at 37°C and 5% CO2. Aliquots of the supernatant were taken at indicated time points and stored at -80°C until virus titers were determined as plaques on Vero cell monolayers.
RNA Transfection. BM-derived DCs and L929 fibroblasts were transfected with 1 μg of virion-extracted PV RNA by using the DEAE-dextran transfection method (26). Cells and supernatant were collected 10 h after transfection and freeze-thawed once before virus titers were analyzed by plaque assay.
T Cell-Mediated Cytotoxicity. Single cell suspensions were prepared from spleens 7 days after immunization except where otherwise stated. For in vitro restimulation, 4 × 106 responder spleen cells were incubated with peptide-labeled and irradiated stimulator cells in the presence of 25 units/ml recombinant IL-2. Then 2 × 105 thioglycollate-elicited macrophages or 2 × 106 spleen cells were used as stimulators. After 5 days, serial dilutions of effector spleen cells were tested for cytolytic activity in a standard 5-h chromium release assay against EL-4 (thymoma cell line, H-2b) or MC57 (fibroblast cell line, H-2b) target cells that had been labeled with chromium and the appropriate peptides. PV-specific and ovalbumin-specific CTL responses were measured against peptides corresponding to amino acids 22-30 of the PV polyprotein and amino acids 257-264 of chicken ovalbumin, respectively. No cytotoxicity was detected after restimulation of spleen cells from naive, nonimmunized mice, which served as negative control in all restimulations. Percentage of peptide-specific lysis was calculated as (specific release - spontaneous release) × 100/(maximum release - spontaneous release).
Neutralizing Abs. Sera of immunized mice were prediluted 40-fold with MEM containing 2% FCS. Serial 2-fold dilutions of sera were preincubated with an equal volume of medium containing 500 plaque-forming units (pfu)/ml PV at 37°C and 5% CO2 for 90 min. One hundred microliters of this mixture was transferred onto Vero cell monolayers grown in 96-well plates. After incubation for 90 min at 37°C and 5% CO2, wells were overlaid with medium containing 0.1% methyl cellulose and incubated for an additional 24-36 h. The overlay was then removed, and plates were fixed and stained with 0.5% crystal violet. The neutralizing Ab titer was defined as the highest serum dilution that reduced the number of plaques by 50%.
Plaque Assay. Organs of infected mice were homogenized in 2 ml of MEM (2% FCS), and cellular debris was removed by centrifugation at 3,000 rpm and 4°C for 20 min. Blood samples were freeze-thawed once. Ten-fold serial dilutions of samples containing virus were plated onto Vero cell monolayers and incubated for 90 min at 37°C and 5% CO2 before an overlay containing 0.1% methyl cellulose was added. After 24-36 h, the plates were stained with 0.5% crystal violet and plaques were counted.
Detection of PV RNA. In situ hybridization with a fluorochrome-labeled RNA probe of negative polarity (nucleotides 4460-7440) was performed on paraformaldehyde-fixed and tritonpermeabilized cytospins of splenic DCs as published (27).
Results
Primed PV-Specific CTL Responses in Nonpermissive, PVR-Negative Mice. We compared CTL responses of PVR-transgenic (ref. 24, the cPVR mouse strain used in ref. 17) and nontransgenic C57BL/6 mice after i.v. immunization with 3 × 107 pfu of WT PV I (Mahoney). Spleen cells were isolated 7 days after immunization, restimulated in vitro on PV peptide-labeled stimulator cells, and analyzed for cytolytic activity in a standard chromium release assay. Surprisingly, the immunized control nontransgenic C57BL/6 mice exhibited primed PV-specific CTL responses (Fig. 1, PV wt), although these mice did not show any clinical signs of polioviral infection. The induced CTL responses were comparable to that of PVR-transgenic mice, in which PV productively replicates. CTL priming in nontransgenic C57BL/6 mice was not limited to a single PV strain but could also be detected in mice immunized with other virus strains, such as PV III Saukett (data not shown), the Sabin vaccine strains (Fig. 1, PV Sabin), and a recombinant PV (Fig. 1, PV OVA).
Fig. 1.
PV-specific CTL responses in PVR-transgenic (squares) and nontransgenic (circles) C57BL/6 mice. Mice were immunized i.v. with 3 × 107 pfu of WT PV I Mahoney (PV wt), the trivalent Sabin vaccine (PV Sabin), or PV OVA. Spleen cells were isolated on day 7 after infection and restimulated in vitro for 5 days. PV-specific cytotoxic activity was measured by using PV peptide-labeled (filled symbols) and nonlabeled (open symbols) EL-4 target cells. Data of individual mice are shown.
CTL Priming in PVR-Negative Mice Is Not Caused by Adaptation of PV to Murine Tissue. PV is an RNA virus that can readily mutate, and mouse-adapted mutants of certain PV strains have been described (28). To exclude rapid or unintended adaptation to murine cells, the different PV strains used were tested in vitro for cytopathic effects and viral replication in cultured cell lines that did or did not express the PVR (Fig. 2). PV replicated in human and monkey cell lines, which naturally express the PVR, and in a PVR-transgenic murine cell line (Fig. 2a). All three cell lines were lysed by the infection (data not shown). In contrast, productive PV replication was not detected in several cell lines of murine origin as assessed by the virus titer in the supernatant (Fig. 2b) or by cytopathic effects (data not shown). In addition, productive PV replication in spleen cells (Fig. 2c) or BM-derived DCs (Fig. 2d) from PVR-transgenic (24) or nontransgenic C57BL/6 mice incubated with PV in vitro strictly correlated with the transgenic expression of the PVR. Susceptibility of mice to poliomyelitic disease after intracerebral inoculation of PV also strictly depended on PVR-expression (data not shown). Presentation of PV-derived CTL epitopes by PVR-negative cells could therefore not be attributed to PV variants adapted to murine cells but resulted from PVR-independent uptake of WT virus into nonpermissive cells.
Fig. 2.
In vitro growth curves of PV in cells either expressing (filled symbols) or not expressing (open symbols) the PVR. Viral propagation was measured in cell cultures of PVR-expressing human, monkey, or PVR-transgenic mouse cell lines (a); murine PVR-negative cell lines (b); and spleen cells (c) or BM-derived DCs (d) from PVR-transgenic and nontransgenic mice. Cells were infected at a multiplicity of infection of 10-50 for 2 h, washed twice, and incubated with fresh medium. Aliquots of supernatant were taken at the indicated time points, and virus titer was determined in a plaque assay.
Priming of CTL Responses Against a Nonstructural PV-Encoded Protein. We next evaluated whether neosynthesis of virally coded antigen in PVR-negative cells or cross-presentation of antigen derived from the injected virus particle was responsible for the priming of PV-specific CTL responses in conventional mice. For this purpose, we were given and used the same PV OVA strain (23) that had been studied (17). In PV OVA, a 600-nt sequence corresponding to the carboxy-terminal half of chicken ovalbumin flanked by artificial protease recognition sites was inserted at the P1 and P2 junction of the PV genome (23). Upon infection of cells, the ovalbumin sequence is translated as part of the polioviral polyprotein and is then released into the cytosol after further cleavage of the polyprotein by viral proteases. Because the ovalbumin protein is not present in the injected virus particle, ovalbumin-specific CTL responses can be induced only after the recombinant ovalbumin has been translated as part of the viral genome in infected cells. Infection of mice with PV OVA therefore allows discrimination between conventional MHC class I antigen presentation of endogenously derived peptides and cross-presentation of exogenous antigen. Nontransgenic PVR-negative mice mounted CTL responses against ovalbumin after immunization with PV OVA (Fig. 3a). The detection of ovalbumin-specific CTL responses was not caused by in vitro priming because of inclusion of IL-2 in our in vitro cultures. Restimulation of PV OVA immunized C57BL/6 and PVR-transgenic mice gave similar ovalbumin-specific cytolytic activity irrespective of the presence of IL-2 (Fig. 3b, 1°PV OVA), whereas ovalbumin-specific CTL responses could not be restimulated from spleens of naive, nonimmunized, or PV WT immunized C57BL/6 mice that had not encountered ovalbumin in vivo (Fig. 3b, naive and 1°PV wt). Thus, priming of PV-specific CTLs in the nontransgenic C57BL/6 mice was a result of cellular neosynthesis of PV encoded proteins within PVR-negative cells.
Fig. 3.
(a) Ovalbumin-specific CTL responses in PVR-transgenic (squares) and nontransgenic (circles) C57BL/6 mice after immunization with PV OVA. Mice were infected i.v. with either 5 × 107 or 5 × 106 pfu of PV OVA as indicated. Spleen cells were isolated at 7 and 21 days after infection, restimulated in vitro for 5 days with ovalbumin peptide-loaded stimulator cells, and tested for cytolytic activity on ovalbumin peptide-labeled (filled symbols) and nonlabeled (open symbols) target cells. Data of individual mice are shown. (b) Ovalbumin-specific CTL responses were not primed during in vitro restimulation. Spleen cells were isolated 7 days after immunization from PV OVA-immunized PVR-transgenic mice (▪), PV OVA-immunized nontransgenic C57BL/6 mice (•), PV wt-immunized C57BL/6 mice (⋄), or naive C57BL/6 mice (▿). Ovalbumin-specific cytotoxicity was assessed after 5 days of in vitro stimulation on ovalbumin peptide-loaded stimulator cells. Symbols represent mean ± SEM for groups of three mice.
Compared to PVR-transgenic mice, PVR-negative mice required higher amounts of PV for CTL priming as 5 × 107 pfu PV OVA primed equally well in both mouse strains, whereas a 10-fold lower virus dose (5 × 106 pfu) induced CTL responses in PVR-transgenic mice but only very weak CTL-priming in the nontransgenic C57BL/6 mice (Fig. 3a). In addition, 3 wk after infection with 5 × 107 pfu of virus, CTL activity could not be restimulated from spleens of infected PVR-negative (nontransgenic) mice, whereas this was still possible in PVR-transgenic mice (Fig. 3a).
Presence of Polioviral RNA Within Professional APC of PVR-Negative Mice. The priming of ovalbumin-specific CTL responses in conventional mice already indicated that translation of PV-encoded antigens was not restricted to PVR-expressing cells. To more directly demonstrate virus entry into PVR-negative cells, CD11c+ splenic DCs were isolated from PV-infected C57BL/6 mice by magnetic bead cell sorting, and the presence of viral RNA was investigated by using a fluorochrome-labeled riboprobe (27). Viral RNA was not detected in splenic DCs of naive, nonimmunized C57BL/6 mice or in that of mice injected with PV 20 min before isolation (Fig. 4 a and b), although this DC preparation contained high titers of presumably extracellular virus (data not shown). However, viral RNA was detected in nontransgenic C57BL/6 mice in a small proportion of DCs isolated at 12 h after infection (Fig. 4c) and in 30-40% of DCs isolated at 21 h after infection (Fig. 4d). Both the strength and pattern of the signal were comparable to that observed in DCs of control PVR-transgenic mice isolated at 12 h after infection (80% positive; Fig. 4f). Whether the increase of the signal over time observed in immunized nontransgenic mice resulted from continuous uptake of virus in vivo or amplification of viral RNA within the cell was not determined. However, the presence of polioviral RNA within DCs of nontransgenic C57BL/6 mice illustrates that APC of nonpermissive mice are capable of acquiring polioviral RNA independently of the cellular PVR.
Fig. 4.
Polioviral RNA is present in APC of PVR-negative mice. Splenic CD11c+ DCs were purified by magnetic bead cell sorting (purity >90%) from nontransgenic PVR-negative mice before (a) and 20 min (b), 12 h (c), 21 h (d), and 36 h (e) after injection of PV WT and then hybridized with a PV-specific fluorochrome-labeled riboprobe (27). (f) PVR-transgenic splenic DCs isolated at 12 h after infection with PV WT served as positive controls.
We therefore assessed the ability of PVR-negative DCs to support PV replication once the positive-stranded RNA genome was delivered into the cytosol. BM-derived DCs of nontransgenic mice were transfected with 1 μg of virion-extracted RNA by using the DEAE-dextran method (26). Ten hours after transfection, the cultures of PV RNA-transfected PVR-negative DCs contained infectious PV as indicated by plaque formation on monolayers of susceptible cells (Fig. 5a). PVR-negative L929 mouse fibroblasts were transfected as positive controls (Fig. 5b). We considered it very unlikely that viral RNA could have survived the 10-h incubation period in serum-containing media at 37°C; however, to rule out the possibility that we were transferring remaining, potentially infectious PV RNA, we added 2 μg of viral RNA directly onto HeLa cell monolayers and assessed plaque formation. PV RNA alone did not give rise to any plaques (Fig. 5c). Thus, the infectivity detected in cultures of transfected C57BL/6 DCs indicates the generation of infectious virus progeny in PVR-negative APC.
Fig. 5.
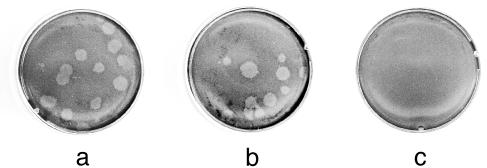
PVR-negative DCs support PV replication from cytosolic viral RNA. PVR-negative BM-derived DCs (a) and PVR-negative L929 mouse fibroblast cells (b) were transfected with 1 μg of virion-extracted RNA (26). Cultures were harvested 10 h after transfection, freeze-thawed once, and plated onto monolayers of PVR-expressing, PV-susceptible cells. (c) Direct plating of viral RNA onto PV-susceptible monolayers did not lead to plaque formation.
Inhibition of Viral Translation Abolishes PV-Specific CTL Priming. To evaluate the importance of cellular antigen neosynthesis for priming of PV-specific CTL responses in PVR-negative mice, WT PV and PV OVA were UV-inactivated to prevent translation of the PV genome. Efficiency of UV inactivation was confirmed by the absence of immunohistochemically detectable nonstructural PV proteins and lack of plaque formation in PVR-expressing cell cultures exposed to the inactivated viruses (data not shown). Impairment of viral translation completely abolished CTL priming in both PVR-transgenic and nontransgenic PVR-negative mice. Neither PV-specific nor ovalbumin-specific CTL responses could be restimulated 7 days after immunization with either UV-inactivated PV or UV-inactivated PV OVA (Fig. 6 a and b). In addition, repeated immunizations with formalin-inactivated PV failed to prime CTL responses (Fig. 6d), although this treatment provided sufficient amounts of exogenous antigen for loading of MHC class II presentation as indicated by induction of strong neutralizing IgG Ab responses to PV that are strictly CD4+ T cell-dependent (Fig. 6e). Similar results were obtained with purified PV (Fig. 6c). Purified 160S PV particles, which are depleted of free viral RNA and of cellular debris potentially containing viral antigens synthesized in cells used to grow virus stocks, primed CTL responses in nontransgenic C57BL/6 mice. However, if translation of the viral RNA was inhibited by UV irradiation of purified virions before injection, no CTL responses were primed. Thus, neither contamination by viral antigens nor by nonencapsidated RNA but the presence of infectious PV particles was essential for the induction of CTL responses in nontransgenic C57BL/6 mice.
Fig. 6.
Nonreplicating forms of PV (filled symbols) fail to induce PV-specific CTL responses in both PVR-transgenic (squares) and nontransgenic (circles) C57BL/6 mice. Mice were immunized with either 3 × 107 pfu of live or UV-inactivated PV OVA (a), 3 × 107 pfu of live or UV-inactivated WT PV I (b), 8 × 106 pfu of live or UV-inactivated purified 160S PV particles (c), or six repetitive injections of formalin-inactivated PV (PV form) (d and e). Spleen cells were isolated 7 days after the primary immunization and restimulated in vitro for 5 days before peptide-specific lysis was determined against the indicated peptides. Open symbols represent CTL responses induced by the replicating forms of the respective viruses within the same experiment. (e) Repetitive immunization with formalin-inactivated PV induced PV-neutralizing IgM (filled symbols) and IgG (open symbols) responses in PVR-transgenic (diamonds) and nontransgenic (triangles) mice. Symbols represent mean ± SEM for groups of three mice.
Discussion
We report here in naturally nonpermissive mice that APC acquire polioviral RNA and prime PV-specific CTL responses independently of the PVR. Uptake of PV or polioviral RNA into PVR-negative host cells did not lead to disease but was sufficient to initiate de novo synthesis of virally encoded antigen and to induce PV-specific and ovalbumin-specific CTL responses in nontransgenic PVR-negative mice. Moreover, the strong correlation between the induction of CTL responses and the necessity for viral RNA to be translated (as shown for ovalbumin) suggests that low levels of endogenous synthesis of viral antigen are necessary for CTL priming.
We have demonstrated synthesis of viral antigen by a functional CTL read-out; however, until now we were unsuccessful in isolating nonstructural PV antigens or ovalbumin from C57BL/6 mice treated with PV or PV OVA. Whether this is purely a quantitative methodological problem remains unanswered. Nevertheless, our experiments using inactivated viruses confirmed the dependence of CTL priming on translation of viral genes. Inactivated virus, which only differs from live virus in its inability to initiate translation of viral genes but contains defined amounts of viral protein, did not prime CTL responses in either nontransgenic or PVR-transgenic mice. By purifying PV, we rendered contaminating free RNA or viral antigens, which might be contained in the crude virus preparations very unlikely to be responsible for CTL priming in nontransgenic mice. Moreover, cross-priming was not observed even after repetitive injections, although sufficient exogenous antigen was provided to be loaded onto the MHC class II pathway.
Our results differ from a previous report (17) that did not detect CTL responses in BM chimera lacking PVR expression (B6→B6) after inoculation of similar virus doses. However, this was probably not because of the absence of CTL priming in PVR-negative mice but might be explained by the shorter duration of CTL responses induced by PV-exposed PVR-negative APC. In our experiments primed CTLs could be restimulated from nontransgenic C57BL/6 spleens for up to 2 wk after infection but not after 3-4 wk (Fig. 3) as evaluated (17). At this later time point, CTL priming is readily missed (see below). In addition, PV-specific CTL responses in nontransgenic mice not only differed from those in PVR-transgenic mice with respect to their duration, but also required higher doses of virus (2-5 × 107 pfu). A 10-fold lower virus inoculum yielded only very weak priming in nontransgenic mice, whereas PVR-transgenic mice still mounted CTL responses (Fig. 3). This could explain why CTL responses were restimulated from B6→PVR but not from B6→B6 chimeras 3 wk after infection (17) because these chimeras were exposed to different resulting virus doses. In B6→PVR BM chimera PV would productively replicate in PVR-expressing non-BM-derived cells and thereby amplify the initial virus inoculum, leading to a significantly higher virus dose and prolonged presence of virus. This, in turn, would increase the number of antigen-expressing PVR-negative APC and prolong the time period during which APC present viral antigen. In contrast, productive PV replication does not occur in completely PVR-negative B6→B6 BM chimeric mice. Thus, the two different chimera models evaluate the ability of nontransgenic APC to take up PV and express virus-encoded antigen (i.e., translate the positive-stranded RNA genome) for CTL induction in two drastically quantitatively different situations. In view of our data, the failure to restimulate CTL responses from completely PVR-negative chimeras 3 wk after infection (17) may not unequivocally prove absence of endogenous synthesis of PV antigen in PVR-negative APC but more likely reflects that the CTL responses primed in such chimeras were missed by testing at too late a time point (29). This finding of short-lived CTL memory under conditions in which antigen seems limiting supports the notion that antigen is a key factor in maintaining immunological CTL memory particularly if protection is examined (30).
Productive PV infection leading to generation of viral progeny, cytopathic effects, and disease is clearly restricted to naturally susceptible hosts expressing the PVR (18-20). How can then uptake of PV or polioviral RNA in vivo and neosynthesis of virally encoded antigens in conventional mice be explained? We can only speculate how this PVR-independent cell entry might happen. On the one hand, other molecules (possibly specifically expressed on DCs) may substitute for PVR function in PVR-negative mice. Experiments with chimeric molecules comprised of the PVR and membrane molecules such as CD4 (31) or intercellular adhesion molecule-1 (32) have demonstrated that heterologous expression of a single PVR domain is sufficient for mediating PV infection. In addition, infection intermediates of PV are capable of infecting PVR-negative Chinese hamster ovary cells in vitro (33, 34). The conformational change of the PV capsid necessary for this process has been suggested to occur under physiological conditions (35), and thus could take place, at low rate, in vivo. On the other hand, our findings may represent a more general property of DCs. It is conceivable that DCs, which are privileged APC that function as sentinels of the immune system (36), could “nonspecifically” acquire pathogens or translatable material for antigen presentation to T cells. Whether or not this involves a yet unidentified receptor remains to be clarified.
In conclusion, our studies signal possible complications of seemingly straight forward experimental approaches particularly when potentially replicating or translatable materials are used to study cross-priming (11, 12, 37, 38). Although the present experiments do not exclude that any cross-priming can take place [e.g., in the BM chimeras (17) or for high dosed antigens (5, 6, 8-11)], our results nevertheless render cross-priming of antiviral CTL responses too inefficient or insufficient to be generalized as an important physiological process.
Acknowledgments
We thank R. Andino and S. Mandl for kindly providing cPVR-transgenic mice (24) and PV OVA (23), as well as for generous discussions and several rounds of manuscript review, and K. McCoy, M. van den Broek, and N. Harris for helpful discussions and critical reading of the manuscript. This work was funded by grants from the Swiss National Science Foundation, the Deutsche Forschungsgemeinschaft (to S.F.), and the Kanton Zurich.
Abbreviations: APC, antigen-presenting cells; BM, bone marrow; CTL, cytotoxic T lymphocyte; DC, dendritic cell; PV, poliovirus; PV OVA, ovalbumin-recombinant PV; PVR, PV receptor; pfu, plaque-forming unit.
References
- 1.Watts, C. (1997) Annu. Rev. Immunol. 15, 821-850. [DOI] [PubMed] [Google Scholar]
- 2.Yewdell, J., Anton, L. C., Bacik, I., Schubert, U., Snyder, H. L. & Bennink, J. R. (1999) Immunol. Rev. 172, 97-108. [DOI] [PubMed] [Google Scholar]
- 3.Bevan, M. J. (1976) J. Exp. Med. 143, 1283-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bevan, M. J. (1987) Nature 325, 192-194. [DOI] [PubMed] [Google Scholar]
- 5.Kovacsovics-Bankowski, M. & Rock, K. L. (1995) Science 267, 243-246. [DOI] [PubMed] [Google Scholar]
- 6.Reis e Sousa, C. & Germain, R. N. (1995) J. Exp. Med. 182, 841-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez, A., Regnault, A., Kleijmeer, M., Ricciardi-Castagnoli, P. & Amigorena, S. (1999) Nat. Cell Biol. 1, 362-368. [DOI] [PubMed] [Google Scholar]
- 8.Schirmbeck, R., Melber, K. & Reimann, J. (1995) Eur. J. Immunol. 25, 1063-1070. [DOI] [PubMed] [Google Scholar]
- 9.Carbone, F. R. & Bevan, M. J. (1990) J. Exp. Med. 171, 377-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Storni, T., Lechner, F., Erdmann, I., Bachi, T., Jegerlehner, A., Dumrese, T., Kundig, T. M., Ruedl, C. & Bachmann, M. F. (2002) J. Immunol. 168, 2880-2886. [DOI] [PubMed] [Google Scholar]
- 11.Bachmann, M. F., Kundig, T. M., Freer, G., Li, Y., Kang, C. Y., Bishop, D. H., Hengartner, H. & Zinkernagel, R. M. (1994) Eur. J. Immunol. 24, 2228-2236. [DOI] [PubMed] [Google Scholar]
- 12.Albert, M. L., Sauter, B. & Bhardwaj, N. (1998) Nature 392, 86-89. [DOI] [PubMed] [Google Scholar]
- 13.Rock, K. L. (1996) Immunol. Today 17, 131-137. [DOI] [PubMed] [Google Scholar]
- 14.Yewdell, J. W., Norbury, C. C. & Bennink, J. R. (1999) Adv. Immunol. 73, 1-77. [DOI] [PubMed] [Google Scholar]
- 15.Heath, W. R. & Carbone, F. R. (2001) Annu. Rev. Immunol. 19, 47-64. [DOI] [PubMed] [Google Scholar]
- 16.Zinkernagel, R. M. (2002) Eur. J. Immunol. 32, 2385-2392. [DOI] [PubMed] [Google Scholar]
- 17.Sigal, L. J., Crotty, S., Andino, R. & Rock, K. L. (1999) Nature 398, 77-80. [DOI] [PubMed] [Google Scholar]
- 18.Mendelsohn, C., Johnson, B., Lionetti, K. A., Nobis, P., Wimmer, E. & Racaniello, V. R. (1986) Proc. Natl. Acad. Sci. USA 83, 7845-7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLaren, L. C., Holland, J. J. & Syverton, J. T. (1959) J. Exp. Med. 109, 475-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren, R. B., Costantini, F., Gorgacz, E. J., Lee, J. J. & Racaniello, V. R. (1990) Cell 63, 353-362. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi-Koll, U., Wiegers, K. J. & Drzeniek, R. (1975) J. Gen. Virol. 26, 307-319. [DOI] [PubMed] [Google Scholar]
- 22.Egger, D., Pasamontes, L., Bolten, R., Boyko, V. & Bienz, K. (1996) J. Virol. 70, 8675-8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandl, S., Sigal, L. J., Rock, K. L. & Andino, R. (1998) Proc. Natl. Acad. Sci. USA 95, 8216-8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crotty, S., Hix, L., Sigal, L. J. & Andino, R. (2002) J. Gen. Virol. 83, 1707-1720. [DOI] [PubMed] [Google Scholar]
- 25.Inaba, K., Inaba, M., Romani, N., Aya, H., Deguchi, M., Ikehara, S., Muramatsu, S. & Steinman, R. M. (1992) J. Exp. Med. 176, 1693-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teterina, N. L., Zhou, W. D., Cho, M. W. & Ehrenfeld, E. (1995) J. Virol. 69, 4245-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolten, R., Egger, D., Gosert, R., Schaub, G., Landmann, L. & Bienz, K. (1998) J. Virol. 72, 8578-8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia, Q., Ohka, S., Iwasaki, K., Tohyama, K. & Nomoto, A. (1999) J. Virol. 73, 6041-6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfizenmaier, K., Jung, H., Starzinski-Powitz, A., Rollinghoff, M. & Wagner, H. (1977) J. Immunol. 119, 939-944. [PubMed] [Google Scholar]
- 30.Kundig, T. M., Bachmann, M. F., Oehen, S., Hoffmann, U. W., Simard, J. J., Kalberer, C. P., Pircher, H., Ohashi, P. S., Hengartner, H. & Zinkernagel, R. M. (1996) Proc. Natl. Acad. Sci. USA 93, 9716-9723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selinka, H. C., Zibert, A. & Wimmer, E. (1992) J. Virol. 66, 2523-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selinka, H. C., Zibert, A. & Wimmer, E. (1991) Proc. Natl. Acad. Sci. USA 88, 3598-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curry, S., Chow, M. & Hogle, J. M. (1996) J. Virol. 70, 7125-7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang, Y., Hogle, J. M. & Chow, M. (2000) J. Virol. 74, 8757-8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, Q., Yafal, A. G., Lee, Y. M., Hogle, J. & Chow, M. (1994) J. Virol. 68, 3965-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banchereau, J. & Steinman, R. M. (1998) Nature 392, 245-252. [DOI] [PubMed] [Google Scholar]
- 37.Buseyne, F., Le Gall, S., Boccaccio, C., Abastado, J. P., Lifson, J. D., Arthur, L. O., Riviere, Y., Heard, J. M. & Schwartz, O. (2001) Nat. Med. 7, 344-349. [DOI] [PubMed] [Google Scholar]
- 38.Motta, I., Andre, F., Lim, A., Tartaglia, J., Cox, W. I., Zitvogel, L., Angevin, E. & Kourilsky, P. (2001) J. Immunol. 167, 1795-1802. [DOI] [PubMed] [Google Scholar]