Abstract
Human cells are known to be more refractory than rodent cells against oncogenic transformation in vitro. To date, the molecular mechanisms underlying such resistance remain largely unknown. The combination of simian virus 40 early region and H-Ras V12 has been effective for transformation of rat embryo fibroblasts, but not for human cells. However, the additional ectopic expression of the telomerase catalytic subunit (hTERT) was reported to be capable of causing transformation of normal human cells. In this study, however, we demonstrate that the combined expression of the above-mentioned three genetic elements is not always sufficient to transform normal human diploid fibroblasts (HDF). Although the expression and function of these introduced genetic elements were essentially the same, among four HDF, TIG-1 and TIG-3 were resistant to transformation. The other two (BJ and IMR-90) showed transformed phenotypes, but they were much restricted compared with rat embryo fibroblasts in expressing simian virus 40 early region and H-Ras V12. In correlation with these phenotypes, TIG-1 and TIG-3 remained diploid after the introduction of these genetic elements, whereas BJ and IMR-90 became highly aneuploid. These results strongly suggest that the lack of telomerase is not the sole reason for the refractory nature of HDF against transformation and that normal human cells have still undefined intrinsic mechanisms rendering them resistant to oncogenic transformation.
Studying the process of transformation of normal cells into malignant cells induced by oncogenes or carcinogens in vitro is undoubtedly a very powerful approach to elucidate the basic mechanism of cancer. So far, however, this type of experiment has been made extensively with chicken or rodent cells but only very limitedly with human cells, because it is empirically known that human cells are more resistant to oncogenic transformation than cells of any other species (1-3). For example, whereas normal rodent cells can be easily transformed by several combinations of two oncogenes [e.g., activated Ras and Myc, activated Ras and adenovirus E1A, and activated Ras and simian virus 40 (SV40) T antigen (4, 5, 30)], normal human cells have never been reproducibly transformed by such oncogene combinations (2, 3). Such findings suggest that the cells of humans, a species having a relatively long lifespan, have evolutionarily acquired relatively stringent mechanisms counteracting malignant transformation. It has been speculated that the difference in susceptibility to transformation between human and rodent cells may be due to differences in DNA repair capacity, response to oxidative stress, maintenance of genome stability, or epigenetic control of gene expression such as by DNA methylation (1-3, 6). Recent rapid progress in the field of cellular-aging research suggests that a difference between human and rodent cells in the regulation of the expression of the telomerase catalytic subunit (TERT) is a possible explanation for this species difference in transformation. TERT expression is tightly suppressed in most somatic cells of humans (7), whereas it is constitutively expressed in rodent somatic cells (8). This constitutive TERT expression and extremely long telomere are assumed to be the biological basis for the relatively frequent spontaneous immortalization observed in rodent cells, which property is supposed to be an essential prerequisite for malignant transformation (9). In fact, ectopic expression of TERT has been proved to be capable of immortalizing many types of normal human cells (10, 11). Hahn et al. (9, 12) were the first to report that normal human cells could be transformed by the expression of SV40 early region (SV40 ER) and activated H-Ras only after ectopic expression of hTERT. However, Seger et al. (13) recently reported the transformation of normal human fibroblasts by the combined expression of adenovirus E1A, activated Ras, and MDM2 without the additional expression of hTERT. In addition, chicken embryo fibroblasts, which lack telomerase activity in culture, can be completely transformed by many single viral ongogenes such as v-src (14-16). Thus, no simple correlation seems to exist between the telomerase expression and the transformability, and the role of telomerases in oncogenic transformation of nonestablished normal fibroblasts still remains to be clarified.
Recently, it was reported that a considerable heterogeneity exists among human diploid fibroblasts (HDF) in the control of p16INK4 expression on oxidative stress, depending on the origin of tissues (17). In particular, skin-derived BJ fibroblasts, which have been frequently used for transformation assays, have extremely high antioxidant capacity and slow telomere shortening (18).
Additionally, whereas human embryonic kidney epithelial cells were transformed by the combined expression of hTERT, SV40 ER, and activated H-Ras without signs of extensive genomic instability, all the BJ fibroblasts transformed by use of the same protocol were reported to be highly aneuploid (19, 20), raising the question as to whether the combination of these genetic elements is actually sufficient for the complete transformation of HDF. Therefore, to clarify the general minimal number of genetic events required for the conversion of normal HDF into fully malignant cells, it is very important to see whether the reported means to transform some given HDF is really effective for many other HDF.
To gain further insight into these controversial points, by the experiments reported herein, we systematically analyzed different kinds of normal HDF, including BJ, for their susceptibility to oncogene-mediated transformation.
Materials and Methods
Cells. TIG-1 (21), TIG-3 (22), and IMR-90 (23) cells are human diploid embryonic lung fibroblasts. BJ cells are human diploid foreskin fibroblasts (10). TIG-1 and TIG-3 cells were obtained from the Japanese Collection of Research Bioresources, and IMR-90 cells were obtained from American Type Culture Collection. BJ cells were kindly provided by J. R. Smith (Baylor College of Medicine, Houston). These diploid human fibroblasts were not clonal and were maintained as populations. All these cells have a finite lifespan, and were used at population doublings between 30 and 40. Rat embryo fibroblasts (REF) were prepared from 16-day-old Fisher rat embryos, and were used at population doublings <7. The ecotropic retrovirus packaging cell line Plat-E (24) was kindly donated by T. Kitamura (University of Tokyo). The cervical carcinoma cell line HeLa was obtained from Japanese Collection of Research Bioresources. All the cells described above were cultured in DMEM supplemented with 10% FBS.
Retroviral Vectors. pCX4 series of retroviral vectors were improved from pCX (25), a murine leukemia virus-based vector, to eliminate the production of fusion proteins resulting from initiation at upstream ATG codons within the gag region of the vectors. Six kinds of pCX4 vectors with different drug-selection markers were constructed. pCX4bsr, pCX4neo, pCX4pur, pCX4hyg, pCX4bleo, and pCX4.1hisD confer resistance to blasticidin S, G418, puromycin, hygromycin, zeocin, and histidinol, respectively. The complete sequences of these vectors are available from the GenBank database [accession nos. AB086384 (pCX4bsr), AB086385 (pCX4neo), AB086386 (pCX4pur), AB086387 (pCX4hyg), AB086388 (pCX4bleo), and AB086389 (pCX4.1hisD)]. An activated mutant of human H-ras, H-ras V12, was subcloned into pCX4pur. The hTERT cDNA (12) was kindly provided by R. Weinberg (Whitehead Institute, Cambridge, MA) and subcloned into pCX4neo. The SV40 ER encoding both large and small T antigens derived from pZipneoSV40T (26), a gift from P. Sharp (Massachusetts Institute of Technology, Cambridge, MA), was subcloned into pCX4bsr. The adenovirus-5 E1A-coding region was obtained from Japanese Collection of Research Bioresources and subcloned into pCX4bsr. The human MDM2 cDNA (27) was kindly provided by S. Berberich (Wright State University, Dayton, OH) and subcloned into pCX4.1hisD. The murine ecotropic retrovirus receptor (EcoVR) cDNA was subcloned into pCX4hyg. Details of all of the retroviral constructs mentioned above are available on request.
Retroviral-Mediated Gene Transfer. Retroviral vector plasmids were used for transfection of Plat-E, an ecotropic murine leukemia virus-packaging cell line, by means of Fugene6 (Boehringer Mannheim) according to the manufacturer's directions. Two days after the transfection, culture supernatants were collected, filtered, supplemented with 8 μg/ml polybrene, and used for infection. Two days after the infection, drug selection of infected cells was started, and the selected populations were used in all the experiments. For human fibroblasts, the murine ecotropic retrovirus receptor was first introduced by using an amphotropic vector produced from Plat-E cotransfected with pCX4hyg/EcoVR and the amphotropic retrovirus-packaging constructs pGP + pE-Ampho (Takara Bio, Shiga, Japan). This procedure made human fibroblasts susceptible to the subsequent infection with ecotropic viral vectors. The infection efficiencies were >80% as judged by using GFP-expressing retroviral vectors. Infected cell populations were selected in blasticidin S (10 μg/ml), G418 (500 μg/ml), puromycin (2 μg/ml), or hygromycin (200 μg/ml) for ≈1 week.
Telomere Length Assays. Telomere lengths were analyzed by the terminal restriction fragment length assay with the TeloTAGGG Telomere Length Assay Kit (Roche Diagnostics) according to the manufacturer's directions.
Protein Analysis. Immunoblotting and immunoprecipitation were performed as described (28). Anti-SV40 large T antigen mouse monoclonal antibody (PAb101) and anti-Rb mouse monoclonal antibody (G3-245) were purchased from Pharmingen. Anti-SV40 small T antigen mouse monoclonal antibody (Ab-3) was purchased from Oncogene Research Products (San Diego); and anti-Ras mouse monoclonal antibody was from Transduction Laboratories (Lexington, KY). Anti-hTERT goat polyclonal antibody (L-20) was from Santa Cruz Biotechnology, and anti-phospho-mitogen-activated protein kinase (MAPK) (against diphosphorylated ERK1/2) mouse monoclonal antibody was from Sigma. Anti-MAPK (ERK1/2) rabbit polyclonal antibody was purchased from Promega, and anti-p53 rabbit polyclonal antibody was obtained from Cell Signaling Technology (Beverly, MA).
Soft-Agar Colony Formation Assays. Single-cell suspensions of 2 × 104 cells were plated per 60-mm culture dish in 3 ml of DMEM containing 10% FCS and 0.36% agar on a layer of 5 ml of the same medium containing 0.7% agar. Plates were fed weekly with 0.5 ml of DMEM/10% FCS. Three weeks after plating, colonies were stained with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, and photographs of the stained colonies were taken as described (25).
Tumorigenicity Assays. After 1 × 106 cells had been injected s.c. into 6- to 8-week-old female nude athymic mice (BALB/c nu/nu), 2D tumor sizes were measured once a week. The tumor volume (mm3) was calculated by using the following formula: (length × width2)/2.
Flow Cytometry. DNA contents were analyzed with a FACScan cytofluorometer by using a CycleTEST kit (Becton Dickinson) according to the manufacturer's directions. The MODFIT LT cell cycle analysis software (Verity Software House, Topsham, ME) was used for cell cycle and ploidy analysis.
Results
Expression and Function of hTERT, SV40 ER, and H-Ras V12 Retrovirally Transduced into HDF. In an attempt to analyze systematically the transformation of human cells in comparison with the transformation of rodent cells, we prepared four different well characterized normal HDF cell populations (TIG-1, TIG-3, BJ, and IMR-90) expressing receptors for mouse ecotropic retroviruses. We introduced many foreign genes into these HDF and REF at equally high efficiencies (>80%) by using the same ecotropic retroviral vector preparations. Thus, this system allowed us to directly compare the transformation efficiency between human and rodent normal fibroblasts.
Initially, the four HDF were infected with retroviral vector expressing hTERT. Then, we introduced the two elements, SV40 ER, encoding both large and small T antigens, and an activated H-Ras V12, in the hTERT-expressing HDF and in REF. Infected cell populations were selected with each appropriate drug between infections. Because of the high-infection efficiency, regularly ≈10 million cells transduced with all of these genetic elements were obtained within five population doublings; and, thus, we could avoid prolonged selection periods that might increase the probability of secondary undefined genetic alterations.
Expression of large and small T antigens and activated H-Ras were at almost equal levels in these HDFs and REF (Fig. 1). We also readily detected hTERT protein in the transduced HDF by immunoblotting (Fig. 1). The length of telomeres in these infected HDF were significantly longer than the length of those in uninfected HDF with similar population doublings and were equivalent to the very long telomeres in REF, clearly showing the functional expression of hTERT in these cells (Fig. 2).
Fig. 1.
Expression of hTERT, SV40 T antigens, and Ras. Total cell lysates were prepared from four HDF (TIG-1, TIG-3, BJ, and IMR-90) infected with retroviral vectors expressing hTERT (T), SV40 ER (S), and H-RasV12 (R), from REF infected with retroviral vectors expressing S and R, and from uninfected TIG-3. Ten micrograms of each total cell lysate was subjected to immunoblot analysis with the antibodies indicated on the right.
Fig. 2.
Elongation of telomeres in human fibroblasts expressing hTERT. Genomic DNA was prepared from four HDF (TIG-1, TIG-3, BJ, and IMR-90) infected with retroviral vectors expressing hTERT (T), SV40 ER (S), and HRasV12 (R), from REF infected with retroviral vectors expressing S and R, and from uninfected TIG-3. Two micrograms of each genomic DNA was digested with HinfI and RsaI and hybridized with a telomere-specific oligonucleotide probe.
SV40 large T antigen can inactivate two major tumor suppressor genes, p53 and Rb, through direct binding (29). As shown in Fig. 3, p53 and Rb were coimmunoprecipitated with SV40 large T antigen in both the transduced HDF and REF, indicating that such bindings actually took place in our experimental setting. Moreover, the immunoblotting with antibody specific for activated MAPK (Fig. 4) demonstrated the enhanced activation of MAPK in all of the transduced cells expressing activated H-Ras compared with that for each control cell, clearly showing that the downstream MAPK pathway was similarly activated in these cells.
Fig. 3.
Association of SV40 large T antigen with p53 and Rb. Total cell lysates were prepared from four HDF (TIG-1, TIG-3, BJ, and IMR-90) infected with retroviral vectors expressing hTERT (T), SV40 ER (S), and H-RasV12 (R), from REF infected with retroviral vectors expressing S and R, and from uninfected TIG-3. Of each total cell lysate, 250 μg was subjected to immunoprecipitation with anti-SV40 large T antibody, and the precipitates were then subjected to immunoblot analysis with the antibodies indicated on the right.
Fig. 4.
Activation of MAPK. Total cell lysates (10 μg) were subjected to immunoblot analysis with anti-phospho-MAPK antibody (Upper) or anti-MAPK antibody (Lower). Lane 1, TIG-1 infected with a retroviral vector expressing hTERT (T); lane 2, TIG-1 infected with retroviral vector expressing T, SV40 ER (S), and H-RasV12 (R); lane 3, TIG-3 infected with a retroviral vector expressing T; lane 4, TIG-3 infected with retroviral vectors expressing T, S, and R; lane 5, BJ infected with a retroviral vector expressing T; lane 6, BJ infected with retroviral vectors expressing T, S, and R; lane 7, IMR-90 infected with a retroviral vector expressing T; lane 8, IMR-90 infected with retroviral vectors expressing T, S, and R; lane 9, REF; lane 10, REF infected with retroviral vectors expressing S and R.
Characterization of HDF Expressing hTERT, SV40 ER, and H-Ras V12. After the transduction with hTERT, SV40 ER, and H-Ras V12, all HDF showed only slightly smaller and rounder morphology than the corresponding parental cells. The appearance of TIG-3 cells is shown as an example (Fig. 5). As is well known, remarkable alternations were observed with REF on introduction of SV40 ER and H-Ras V12 (Fig. 5). The expression of hTERT alone did not change the morphologies of HDF at all, and the additional introduction of SV40 ER into these hTERT-expressing HDF and REF induced only relatively minor morphological changes that could be similarly observed in both cell types (data not shown). Therefore, the presence of H-Ras V12 is responsible for the drastic differences in cell morphology between HDF and REF.
Fig. 5.
Morphological changes in fibroblasts. Cell morphologies are shown at ×40 magnification. Shown are TIG-3 (A) and TIG-3 (B) infected with retroviral vectors expressing hTERT (T), SV40 ER (S), and H-RasV12 (R). (C) REF. (D) REF infected with retroviral vectors expressing S and R.
Next, these HDF and REF were subjected to soft-agar colony formation assays to test their ability for anchorage-independent growth, one of the most reliable markers of malignant transformation. Under our experimental conditions, the human cervical carcinoma cell line HeLa, used as a positive control, formed a considerable number of colonies after 3 weeks of incubation (Table 1 and Fig. 6). As reported earlier (30), REF transduced with SV40 ER and H-Ras V12 (REF/SR) rapidly formed numerous colonies (within 1 week). In marked contrast, TIG-1 and TIG-3 transduced with SV40 ER and H-Ras V12 together with hTERT (TIG-1/TSR and TIG-3/TSR) formed very few colonies, even after a 3-week incubation (Table 1 and Fig. 6). Under the same conditions, BJ/TSR and IMR-90/TSR formed visible colonies after 3 weeks, but they were very sparse compared with those in REF/SR seen at 1 week (Table 1 and Fig. 6).
Table 1. Summary of data from soft-agar colony formation assay.
| Colonies, n
|
|||
|---|---|---|---|
| Cells | Exp. 1 | Exp. 2 | Exp. 3 |
| TIG-1/TSR | 3 | 23 | 0 |
| TIG-3/TSR | 0 | 3 | 0 |
| BJ/TSR | 138 | 181 | 82 |
| IMR-90/TSR | 62 | 71 | 58 |
| REF/SR | 7,983 | 9,576 | 7,182 |
| HeLa | 1,256 | 1,681 | 1,171 |
Four HDF strains (TIG-1, TIG-3, BJ, and IMR-90) infected with retroviral vectors expressing hTERT (T), SV40 early region (S), and H-RasV12 (R), and REF infected with retroviral vectors expressing S and R, were subjected to the soft-agar colony formation assay. HeLa, a human cervical carcinoma cell line, was used as a positive control. Cells (2 × 104) were plated in soft agar as described in Materials and Methods. Colonies were stained with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, and the numbers of stained colonies were counted at 3 weeks after plating, except for those with REF/SR, which were counted at 1 week after plating. Results of three independent experiments are shown.
Fig. 6.
Photographs of soft-agar colony formation assay. One representative result of the soft-agar colony formation assay summarized in Table 1. Cells were plated and stained as described in the legend of Table 1, and photographs of stained colonies were taken.
The tumorigenicity of these cells was also examined by injecting them into nude mice. As shown in Fig. 7, REF/SR grew very aggressively and formed tumors of massive volume. We observed very similar rapid tumor growth with mouse embryo fibroblasts expressing SV40 ER and H-Ras V12 (data not shown). In HDF, BJ/TSR and IMR-90/TSR formed tumors about half the size of HeLa cell tumors. TIG-1/TSR and TIG-3/TSR cells did not make any clearly detectable tumors within the 5 weeks of observation.
Fig. 7.
Nude mouse tumorigenicity assays. The four HDF (TIG-1, TIG-3, BJ, and IMR-90) infected with retroviral vectors expressing hTERT (T), SV40 ER (S), and H-RasV12 (R), and REF infected with retroviral vectors expressing S and R, were subjected to nude mouse tumorigenicity assays. HeLa, a human cervical carcinoma cell line, was used as a control. The cells (1 × 106) were injected, and the tumor volumes were calculated as described in Materials and Methods. Each point is the average of the tumor volumes of nine inoculated sites, except for REF/SR (five inoculated sites) and HeLa (four inoculated sites).
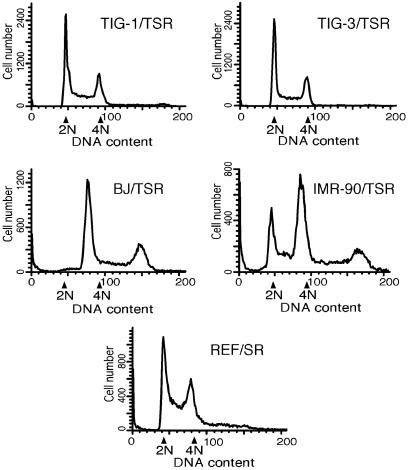
These results clearly indicate a significant heterogeneity in the susceptibility to transformation among HDF. To obtain some insight into this heterogeneity, we analyzed the cellular DNA content of these HDF by using a flow cytometer. As shown in Fig. 8, BJ/TSR and IMR-90/TSR were highly aneuploid, whereas TIG-1/TSR and TIG-3/TSR remained diploid. We confirmed that all the HDF expressing hTERT, including BJ and IMR-90, were in the diploid state before the introduction of SV40 ER and H-Ras V12 (data not shown). Intriguingly, most of the highly tumorigenic REF/SR were diploid.
Fig. 8.
Flow cytometric analysis of DNA content. Four HDF (TIG-1, TIG-3, BJ, and IMR-90) infected with retroviral vectors expressing hTERT (T), SV40 ER (S), and H-RasV12 (R), and REF infected with retroviral vectors expressing S and R, were analyzed for DNA content by using flow cytometry as described in Materials and Methods. Arrows indicate the location of 2N and 4N peaks as determined by using normal human and rat fibroblasts, where N represents the haploid genome.
Discussion
For many years, numerous attempts to transform normal human cells in vitro reproducibly have not been successful; and the minimum number of defined genetic events required for the oncogenic transformation of normal human cells has not been established. Recently, Hahn and colleagues (12, 31) reported that normal human cells could be converted into oncogenic cells when all of the following events took place: the maintenance of telomeres by hTERT, the inactivation of both Rb and p53 by SV40 large T antigen, the perturbation of PP2A by SV40 small T antigen, and the expression of constitutive active H-Ras oncoprotein. In this study, we examined the generality of this conclusion by using four different kinds of normal HDF.
Consistent with Hahn and colleagues' results (12, 31), anchorage-independent growth was observed, albeit in limited numbers, with BJ human fibroblasts transduced with hTERT, SV40 ER, and H-Ras V12. These cells also formed tumors on injection into nude mice. Similar results were obtained with IMR-90 cells. In marked contrast, however, TIG-1 and TIG-3 human fibroblasts were totally resistant to oncogenic transformation in terms of both anchorage-independent growth and tumorigenicity in nude mice. Because no significant differences occurred in the expressions and the functions of the three genetic elements introduced among all four HDF examined, the differential susceptibility to oncogenic transformation must be attributed to host cellular factors. Recently, Itahana et al. (17) reported that lung-derived HDF have a much higher tendency than skin-derived HDF, such as BJ, to express elevated levels of p16INK4A, and subsequently undergo senescence in response to environmental stress, such as oxidative stress. Such differences in the stress sensitivity depending on the origin of tissue might have contributed to the differences observed in transformability. However, the transformed IMR-90 cells were derived from fetal lung, like the untransformed TIG-1 and TIG-3, and p16INK4A was similarly induced on transduction with SV40 ER and H-Ras V12 in all the HDF used in this study (T.A., unpublished data). Therefore, this possibility seems to be unlikely.
Considerable diversities in tumor susceptibility are known among laboratory mouse strains, and many polymorphic genes modifying tumor susceptibility have been identified (32). Some of them have been shown to be genes intrinsically controlling cell growth, such as p16INK4A, c-myc, or Cdc25A (33-35). Polymorphism in coding or noncoding regulatory regions of these genes can have a significant impact on tumor susceptibility. Several human genes whose polymorphism affects biological properties have also been identified, for example, p53 and cyclin D (36, 37). Because all of the HDF used in this study were derived from different individuals (10, 21-23), the genetic backgrounds of these cells should be quite heterogeneous; and this heterogeneity is supposedly the basis for the observed differences in transformability (38). In this context, it is noteworthy that BJ and IMR-90 transduced with hTERT, SV40 ER, and H-Ras V12 were highly aneuploid, whereas TIG-1 and TIG-3 expressing the same set of genes remained diploid. The aneuploidy of a similar type of BJ fibroblasts has also been reported (19, 20). This difference in ploidy reflects some genetic variations in the machinery controlling genome stability among the HDF examined, and such chromosome abnormalities might be critically involved in the transformation of normal human fibroblasts. The possibility still remains that the expression of these three genetic elements is not sufficient, and additional genetic changes associated with the chromosome abnormalities are required for the transformation of normal human fibroblasts. At present, we are not certain whether genetic variations in HDF in terms of transformability are more extensive than we suppose, and so a wider range of HDF should thus be examined.
Even in the BJ and IMR-90 transformed by the addition of hTERT to the combined expression of SV40 ER and activated H-Ras, their transformation phenotypes were extremely more limited than those of the REF expressing SV40 ER and H-Ras V12, especially in terms of cell morphology, anchorage independence, and tumorigenicity. These results clearly indicate that the difference in the telomere biology could not fully account for the species difference in transformability. As mentioned earlier, although the effects of the expression of SV40 ER alone were not significantly different in hTERT-expressing HDF and REF, the additional expression of H-ras V12 caused pronounced differences in both cell morphology and growth properties between these cell types. To gain some insight into the molecular basis for such different consequences of the H-Ras V12 expression, we recently performed transcriptional profiling by using the DNA microarray method. We found that the additional expression of H-Ras V12 in REF expressing SV40 ER caused marked decreases in the expression of many extracellular matrix proteins (collagen, fibrillin, fibronectin, thrombospondin, and lysyl oxidase) and increases in the expression of several extracellular matrix-degrading enzymes (matrix metalloproteinase 10 and urokinase-type plasminogen activator; T.A., unpublished data). These results are consistent with the results of a study analyzing Ras-induced transformation in established rodent fibroblast cell lines (39). When the same kind of analysis was performed with TIG-3 expressing hTERT and SV40 ER, many of these genes did not exhibit any significant changes in their expression levels with or without H-Ras V12 (T.A., unpublished data). Different regulation in the expression of these genes involved in extracellular matrix remodeling could contribute to the observed differences between these cells. Besides these genes, the total number of genes differentially expressed in cells expressing SV40 ER + H-Ras V12 relative to SV40 ER alone was ≈1,200 of 8,500 genes (14.1%) in REF, and ≈1,100 of 8,400 genes (13.1%) in mouse embryo fibroblasts, but it was ≈1,080 genes of 16,400 genes (6.6%) in TIG-3 transduced with hTERT, as judged from the DNA microarray analysis (T.A., unpublished data). It is well established that Ras modulates a broad range of gene expression through several transcription factors, such as AP-1, Ets, and NF-κB (40-42). Our preliminary DNA microarray data imply that substantial differences may exist between human and rodent cells in the downstream signaling pathway of Ras, controlling the activities of these transcription factors, and that human cells are more refractory to Ras-induced alteration of gene expression. We have obtained preliminary data showing that some AP-1 and Ets family members are regulated in a different manner between REF and HDF by activated Ras (T.A., unpublished data). Hamad et al. (43) also reported differences between human and mouse cells in the signaling pathway downstream of Ras. Seeing that MAPK was similarly activated in REF and HDF expressing H-Ras V12 (Fig. 4), the point of dissociation between REF and HDF is likely located downstream of MAPK. Investigations into the molecular mechanisms underlying such species differences in the signaling pathway of Ras should shed light on the different susceptibility to oncogenic transformation by Ras between human and rat cells observed in our experimental system.
The most noteworthy finding in this study is that several HDF, such as TIG-1 and TIG-3, barely showed transformed phenotypes, even with the combined occurrence of the elongation of telomeres, the disruptions of Rb, p53, and PP2A functions, and the constitutive activation of the Ras/MAPK pathway. We also observed that TIG-1 and TIG-3 could not be transformed by the combined expression of adenovirus E1A, H-Ras V12, and MDM2, which was recently reported (13) to be effective in transformation of several kinds of HDF (T.A., unpublished data). Our results thus strongly suggest the existence of still unidentified factors that determine the transformability of normal human fibroblasts. A set of HDF showing divergent transformed phenotypes described in this study should offer a valuable experimental system suited to elucidate the molecular nature of these determining factors required for oncogenic changes in human cells.
Acknowledgments
We thank K. Shibutani for excellent technical assistance and T. Kitamura, H. Inoue, T. Shishido, P. Sharp, R. Weinberg, and S. Berberich for providing the reagents. Also, we thank M. Yutsudo for providing the facility for the experiments using retroviral vectors. This work was supported by a grant from the program Grants-in-Aid for Specially Promoted Research of the Ministry of Education, Culture, Sports, Science, and Technology of Japan and a grant from Osaka City.
Abbreviations: SV40, simian virus 40; ER, early region; REF, rat embryo fibroblasts; HDF, human diploid fibroblasts; MAPK, mitogen-activated protein kinase.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. AB086384 (pCX4bsr), AB086385 (pCX4neo), AB086386 (pCX4pur), AB086387 (pCX4hyg), AB086388 (pCX4bleo), and AB086389 (pCX4.1hisD)].
References
- 1.DiPaolo, J. A. (1983) J. Natl. Cancer Inst. 70, 3-8. [PubMed] [Google Scholar]
- 2.Holliday, R. (1996) Cancer Surv. 28, 103-115. [PubMed] [Google Scholar]
- 3.Kuroki, T. & Huh, N. H. (1993) Jpn. J. Cancer Res. 84, 1091-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Land, H., Parada, L. F. & Weinberg, R. A. (1983) Nature 304, 596-602. [DOI] [PubMed] [Google Scholar]
- 5.Ruley, H. E. (1983) Nature 304, 602-606. [DOI] [PubMed] [Google Scholar]
- 6.Balmain, A. & Harris, C. C. (2000) Carcinogenesis 21, 371-377. [DOI] [PubMed] [Google Scholar]
- 7.Kim, N. W., Piatyszek, M. A., Prowse, K. R., Harley, C. B., West, M. D., Ho, P. L., Coviello, G. M., Wright, W. E., Weinrich, S. L. & Shay, J. W. (1994) Science 266, 2011-2015. [DOI] [PubMed] [Google Scholar]
- 8.Prowse, K. R. & Greider, C. W. (1995) Proc. Natl. Acad. Sci. USA 92, 4818-4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hahn, W. C. & Weinberg, R. A. (2002) Nat. Rev. Cancer 2, 331-341. [DOI] [PubMed] [Google Scholar]
- 10.Bodnar, A. G., Ouellette, M., Frolkis, M., Holt, S. E., Chiu, C. P., Morin, G. B., Harley, C. B., Shay, J. W., Lichtsteiner, S. & Wright, W. E. (1998) Science 279, 349-352. [DOI] [PubMed] [Google Scholar]
- 11.Vaziri, H. & Benchimol, S. (1998) Curr. Biol. 8, 279-282. [DOI] [PubMed] [Google Scholar]
- 12.Hahn, W. C., Counter, C. M., Lundberg, A. S., Beijersbergen, R. L., Brooks, M. W. & Weinberg, R. A. (1999) Nature 400, 464-468. [DOI] [PubMed] [Google Scholar]
- 13.Seger, Y. R., Garcia-Cao, M., Piccinin, S., Cunsolo, C. L., Doglioni, C., Blasco, M. A., Hannon, G. J. & Maestro, R. (2002) Cancer Cell 2, 401-413. [DOI] [PubMed] [Google Scholar]
- 14.Jove, R. & Hanafusa, H. (1987) Annu. Rev. Cell Biol. 3, 31-56. [DOI] [PubMed] [Google Scholar]
- 15.Kim, H., You, S., Farris, J., Kong, B. W., Christman, S. A., Foster, L. K. & Foster, D. N. (2002) Exp. Cell Res. 272, 199-208. [DOI] [PubMed] [Google Scholar]
- 16.Venkatesan, R. N. & Price, C. (1998) Proc. Natl. Acad. Sci. USA 95, 14763-14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itahana, K., Zou, Y., Itahana, Y., Martinez, J. L., Beausejour, C., Jacobs, J. J., Van Lohuizen, M., Band, V., Campisi, J. & Dimri, G. P. (2003) Mol. Cell. Biol. 23, 389-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorenz, M., Saretzki, G., Sitte, N., Metzkow, S. & von Zglinicki, T. (2001) Free Radical Biol. Med. 31, 824-831. [DOI] [PubMed] [Google Scholar]
- 19.Zimonjic, D., Brooks, M. W., Popescu, N., Weinberg, R. A. & Hahn, W. C. (2001) Cancer Res. 61, 8838-8844. [PubMed] [Google Scholar]
- 20.Li, R., Sonik, A., Stindl, R., Rasnick, D. & Duesberg, P. (2000) Proc. Natl. Acad. Sci. USA 97, 3236-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohashi, M., Aizawa, S., Ooka, H., Ohsawa, T., Kaji, K., Kondo, H., Kobayashi, T., Noumura, T., Matsuo, M., Mitsui, Y., et al. (1980) Exp. Gerontol. 15, 121-133. [DOI] [PubMed] [Google Scholar]
- 22.Matsuo, M., Kaji, K., Utakoji, T. & Hosoda, K. (1982) J. Gerontol. 37, 33-37. [DOI] [PubMed] [Google Scholar]
- 23.Nichols, W. W., Murphy, D. G., Cristofalo, V. J., Toji, L. H., Greene, A. E. & Dwight, S. A. (1977) Science 196, 60-63. [DOI] [PubMed] [Google Scholar]
- 24.Morita, S., Kojima, T. & Kitamura, T. (2000) Gene Ther. 7, 1063-1066. [DOI] [PubMed] [Google Scholar]
- 25.Akagi, T., Shishido, T., Murata, K. & Hanafusa, H. (2000) Proc. Natl. Acad. Sci. USA 97, 7290-7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jat, P. S., Cepko, C. L., Mulligan, R. C. & Sharp, P. A. (1986) Mol. Cell. Biol. 6, 1204-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson, M. W. & Berberich, S. J. (2000) Mol. Cell. Biol. 20, 1001-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akagi, T., Murata, K., Shishido, T. & Hanafusa, H. (2002) Mol. Cell. Biol. 22, 7015-7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saenz-Robles, M. T., Sullivan, C. S. & Pipas, J. M. (2001) Oncogene 20, 7899-7907. [DOI] [PubMed] [Google Scholar]
- 30.Michalovitz, D., Fischer-Fantuzzi, L., Vesco, C., Pipas, J. M. & Oren, M. (1987) J. Virol. 61, 2648-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hahn, W. C., Dessain, S. K., Brooks, M. W., King, J. E., Elenbaas, B., Sabatini, D. M., DeCaprio, J. A. & Weinberg, R. A. (2002) Mol. Cell. Biol. 22, 2111-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balmain, A. & Nagase, H. (1998) Trends Genet. 14, 139-144. [DOI] [PubMed] [Google Scholar]
- 33.Cozma, D., Lukes, L., Rouse, J., Qiu, T. H., Liu, E. T. & Hunter, K. W. (2002) Genome Res. 12, 969-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang, S., Qian, X., Redman, C., Bliskovski, V., Ramsay, E. S., Lowy, D. R. & Mock, B. A. (2003) Oncogene 22, 2285-2295. [DOI] [PubMed] [Google Scholar]
- 35.Zhang, S., Ramsay, E. S. & Mock, B. A. (1998) Proc. Natl. Acad. Sci. USA 95, 2429-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dumont, P., Leu, J. I., Della Pietra, A. C., III, George, D. L. & Murphy, M. (2003) Nat. Genet. 33, 357-365. [DOI] [PubMed] [Google Scholar]
- 37.Kong, S., Amos, C. I., Luthra, R., Lynch, P. M., Levin, B. & Frazier, M. L. (2000) Cancer Res. 60, 249-252. [PubMed] [Google Scholar]
- 38.Ponder, B. A. (2001) Nature 411, 336-341. [DOI] [PubMed] [Google Scholar]
- 39.Zuber, J., Tchernitsa, O. I., Hinzmann, B., Schmitz, A. C., Grips, M., Hellriegel, M., Sers, C., Rosenthal, A. & Schafer, R. (2000) Nat. Genet. 24, 144-152. [DOI] [PubMed] [Google Scholar]
- 40.Treisman, R. (1996) Curr. Opin. Cell Biol. 8, 205-215. [DOI] [PubMed] [Google Scholar]
- 41.Galang, C. K., Der, C. J. & Hauser, C. A. (1994) Oncogene 9, 2913-2921. [PubMed] [Google Scholar]
- 42.Downward, J. (2003) Nat. Rev. Cancer 3, 11-22. [DOI] [PubMed] [Google Scholar]
- 43.Hamad, N. M., Elconin, J. H., Karnoub, A. E., Bai, W., Rich, J. N., Abraham, R. T., Der, C. J. & Counter, C. M. (2002) Genes Dev. 16, 2045-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]