Abstract
Recent evidence indicates that cyclin-dependent kinases (CDKs, cdks) may be inappropriately activated in several neurodegenerative conditions. Here, we report that cdk5 expression and activity are elevated after administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a toxin that damages the nigrostriatal dopaminergic pathway. Supporting the pathogenic significance of the cdk5 alterations are the findings that the general cdk inhibitor, flavopiridol, or expression of dominant-negative cdk5, and to a lesser extent dominant-negative cdk2, attenuates the loss of dopaminergic neurons caused by MPTP. In addition, CDK inhibition strategies attenuate MPTP-induced hypolocomotion and markers of striatal function independent of striatal dopamine. We propose that cdk5 is a key regulator in the degeneration of dopaminergic neurons in Parkinson's disease.
Keywords: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; neurodegeneration
Parkinson's disease (PD) is a neurodegenerative disorder characterized by disabling motor abnormalities, including tremor, muscle rigidity, paucity of voluntary movements, and postural instability (1). In several mammalian species, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) produces most of the biochemical and pathological alterations seen in PD, including the loss of dopaminergic neurons of the substantia nigra pars compacta (SNc) (1). Current treatment strategies for PD consist primarily of dopamine replacement therapy with levodopa or dopamine agonists (1). Although effective in the early stages of the disease, chronic dopamine replacement therapy can cause debilitating side effects. Accordingly, concerted research efforts have been focused on developing neuroprotective strategies that will halt or slow the progression of PD.
Recent evidence implicates cyclin-dependent kinases (CDKs) in the pathogenesis of several neurodegenerative disorders. CDKs are serine/threonine kinases best characterized for their role in cell cycle progression. To be active, CDKs require binding to specific regulatory partners such as cyclins (2). Up-regulation of a variety of cell cycle-related CDKs and/or cyclins has been reported in a number of in vitro neuronal death paradigms (3-8). The importance of such observations is substantiated by reports that inappropriate activation of cell cycle-related pathways has been correlated with the pathogenesis of stroke (9, 10) and Alzheimer's disease (11). However, the identity and functional requirement of individual CDK members in neurodegeneration remain to be elucidated.
In contrast to the mitotic CDKs, cdk5 activity is predominantly, although not exclusively, associated with postmitotic neurons (12). cdk5 activation requires association with its regulatory partner, p35 (13) or p39 (14). The p35/cdk5 complex is required for proper development of the central nervous system (15, 16), process outgrowth (17), axonal migration (18, 19), cortical lamination (16, 20), cell adhesion (20), axonal transport (21), and synaptic activity (22). Just as with cell cycle-related CDKs, deregulated cdk5 activity may play a role in neurodegeneration. For example, proteolytic cleavage of the cdk5 activator p35 to the more stable p25 form has been reported in brains of patients with Alzheimer's disease (23). In addition, overexpression of p25/cdk5 complex has been shown to induce neuronal death in culture (24) and cytoskeletal abnormalities in transgenic mice (24). Deregulation of cdk5 has also been documented in a mutant superoxide dismutase (SOD) animal model of amyotrophic lateral sclerosis (25) and in a murine model of Niemann-Pick type C disease (26). With regards to PD, previous reports have suggested that cdk5 is elevated in dopamine neurons of human postmortem PD brains (27, 28). In addition, ectopic expression of cdk5 as well as cell cycle-related CDKs has been shown to occur in neonatal rat dopamine neurons undergoing programmed cell death in vivo (29-31).
From these observations, two major questions arise: (i) what is the functional relevance of CDKs in adult models of neurodegeneration, and (ii) which CDKs participate in neuronal loss in vivo. To address these questions, we examined the functional consequences of CDK inhibition in an in vivo mouse model of PD. We present evidence for the importance of cdk5, and to a lesser extent cdk2, in dopaminergic neuron loss induced by MPTP, and we suggest that cdk5 plays a key role in the pathogenesis of PD.
Materials and Methods
Animals. Eight-week-old male C57BL/6 mice (22-28 g; Charles River Laboratories) were used for all experiments. All animal experiments conformed to the guidelines set forth by the Canadian Council for the Use and Care of Animals in Research (CCAC) and the Canadian Institutes for Health Research (CIHR) and with approval from the University of Ottawa Animal Care Committee.
MPTP Administration. Mice received one i.p. injection of MPTP·HCl per day (25 mg of free base per kg of body weight per day; Sigma) for 5 consecutive days (32); control mice received an equivalent volume of 0.9% saline. Brains were extracted at indicated times and either perfused for immunohistochemical analyses or quickly removed and dissected for biochemical analyses.
Administration of the Pharmacological Inhibitor. Cannulae attached to osmotic minipumps (Alzet model 1007D) were implanted into the right lateral ventricle as previously described (33). Pumps contained 200 μl of flavopiridol [300 μM in artificial cerebroventricular fluid (aCSF)] or vehicle (aCSF). Cannulae with attached pumps were stereotaxically implanted 1 day before the initiation of MPTP treatment (as described above). Brains were extracted 14 days after MPTP treatment for immunohistochemical detection of dopamine neuron loss.
Intrastriatal Administration of Adenoviruses. The adenoviruses expressing the dominant-negative (DN) form of various cdks were engineered by using a Cre-lox adenoviral construction system (CDK constructs were generously provided by E. Harlow, Harvard Medical School, Boston). We and others have previously shown that adenoviruses can target the SNc from the striatum by retrograde transport (34). Each adenovirus was injected directly into the striatum of animals 7 days before initiation of MPTP treatment (as described above). A lacZ-containing construct was used as a control for all adenovirus experiments. A single unilateral injection of each virus (2 μl, 1 × 107 particles per μl) expressing FLAG-tagged DN forms of cdk5 (DN cdk5), cdk2 (DN cdk2), cdk4 (DN cdk4), and cdk6 (DN cdk6) was delivered to the right striatum (0.5 mm rostral, 2.2 mm right of bregma, and 3.4 mm below the skull surface). Each adenovirus injection was given at a constant rate (0.5 μl/min) by using a syringe pump system. Brains were extracted for immunohistochemistry and Western blot analysis 14 days after the first MPTP treatment.
Immunohistochemistry. Mice were perfused transcardially and brains were fixed in paraformaldehyde and cryoprotected as previously described (33). Serial coronal sections (14-μm thickness) of the ventral midbrain and the striatum were collected either on slides or free-floating (in 0.01 M PBS/0.02% sodium azide). Sections were then incubated in primary antibody [to tyrosine hydroxylase (TH), 1:10,000, ImmunoStar; phospho-τ (AT8), 1:2,000, Innogenetics; cdk5, C-8, 1:1,000, Santa Cruz Biotechnology; and FLAG, 1:1,000, Sigma; all dilutions made in 0.3% Triton X-100/0.01 M PBS] for 48 h at 4°C. Sections were then incubated with biotinylated secondary antibody and streptavidin horseradish peroxidase-conjugated tertiary antibody and visualized by using a 3,3′-diaminobenzidine/glucose oxidase reaction as previously described (35). Alternatively, sections were visualized after incubation with CY3-conjugated secondary antibody (1:200, Jackson ImmunoResearch). To examine the colocalization of cdk5 with TH, a double-labeling immunofluorescence approach was used. After incubation with the specific primary antibody at 4°C, immunolabeling was visualized by using either CY3-conjugated anti-mouse IgG (1:200, Jackson ImmunoResearch) or biotinylated secondary antibody (1:200, Jackson ImmunoResearch) followed by streptavidin FITC antibody (1:200, Amersham Biosciences).
Quantification of Dopamine Neuron Loss. Loss of dopamine neurons was assessed by immunohistochemical analysis of TH-positive neurons in the anatomical region of the SNc corresponding to bregma - 3.08 through bregma - 3.40 (36). Sections were also stained with cresyl violet at the level of the medial terminal nucleus (MTN) as an independent measure of neuronal survival in the SNc. Total TH-positive neurons were estimated by using Abercrombie's correction (37).
For experiments using the gene delivery approach, only sections in the region containing the MTN were included. The region of the MTN has been previously shown to contain the highest level of virus-mediated expression after intrastriatal infection (34). Neurons ipsilateral and contralateral to the viral injection were assessed as described above in at least five sections per animal. The ratio of neurons ipsilateral/contralateral to the injection was then calculated accordingly.
Western Blot Analysis. Briefly, a 2-mm coronal slab of the midbrain region was isolated by using a brain matrix. SNc tissue extracts were isolated by using a 1-mm-diameter needle to dissect the SNc area. Total tissue proteins were isolated from a micro-dissection of the substantia nigra and striatum by using a biopsy needle. Samples were homogenized in lysate buffer containing 50 mM Hepes at pH 7.5, 150 mM NaCl, 1 mM EDTA, 2.5 mM EGTA, 1 mM DTT, 10% glycerol, 0.1 mM PMSF, 10 μg/ml leupeptin, 1 μg/ml aprotinin, 10 mM β-glycerophosphate, 1 mM NaF, and 0.1 mM sodium orthovanadate. Protein concentration was determined by the Bradford method (Bio-Rad). Thirty micrograms of protein was analyzed by SDS/PAGE as described previously (5), using antibodies to AT8, retinoblastoma protein (Rb) phosphorylated on Ser-795 or Ser-805/811 (Cell Signaling), and actin (Sigma).
Behavioral Analysis. Behavioral analyses were carried out by using an open field test approach to measure amphetamine-induced locomotion in a novel environment, as described previously (33).
HPLC and 1-Methyl-4-phenylpyridinium (MPP+) Analyses. Dopamine and metabolites and MPP+ were assessed by HPLC analyses as previously described (32).
cdk Kinase Assay. Tissue from the SNc was homogenized in 0.5 ml of lysate buffer containing 250 mM NaCl, 5 mM EDTA, 0.1% Nonidet P-40, 5 mM DTT, 10 mM NaF, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 mM PMSF, and 50 mM Tris·HCl at pH 7.5 (38). Samples were then centrifuged at 12,000 × g, and protein concentration was determined by the Bradford method. A 50-μg sample of protein from the supernatant was incubated overnight at 4°C with 1 μg of individual cdk antibodies (cdk5, C-8; cdk2, D-12; cdk4, H-22; and cdk6, C-21). Thirty microliters of staphylococcal protein A-Sepharose (Sigma) was added to the immunoprecipitates and samples were incubated for 2 h at 4°C. The immune complexes were then pelleted at 14,000 × g and washed in kinase buffer containing 20 mM Hepes at pH 7.6, 20 mM MgCl2, 20 mM β-glycerophosphate, 0.1 mM sodium orthovanadate, 2 mM DTT, and 20 μM unlabeled ATP. After washing, beads were incubated in kinase buffer as described above containing 2 μg of histone H1 or Rb (Cell Signaling) and 1.2 μCi (1 μCi = 37 kBq) of [γ-32P]ATP per sample in a final volume of 50 μl at 30°C for 20 min. Kinase activity was determined by SDS/PAGE and autoradiography.
Results
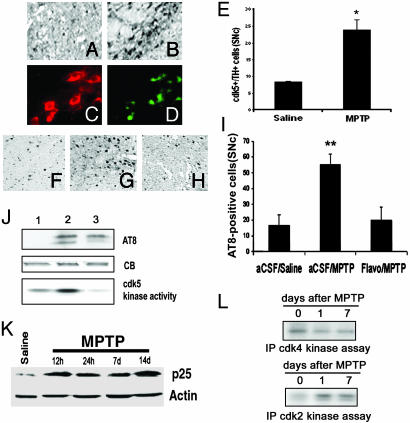
MPTP Treatment Induces Increase in Expression and Activity of cdk5. To determine whether cdk5 expression is regulated by MPTP administration, we assessed cdk5 levels in the SNc of mice treated with MPTP. We detected increased cdk5 expression (Fig. 1 A and B) in the SNc, whereas cotreatment with the general CDK inhibitor flavopiridol did not affect cdk5 induction (data not shown). This observation is consistent with the idea that flavopiridol inhibits CDK activity and does not affect induction of the CDK protein. In addition, colabeling immunofluorescence revealed that cdk5 elevation occurs in dopaminergic neurons of the SNc after MPTP treatment (Fig. 1 C and D). cdk5+/TH+ neurons were increased ≈5- to 6-fold after MPTP treatment compared with saline-treated animals (Fig. 1E). To determine whether the observed increase in cdk5 levels was associated with elevation in cdk5 kinase activity, we evaluated the phosphorylation state of τ (phospho-τ, AT8), a substrate of cdk5 (39). Immunohistochemical (Fig. 1 F-I) and Western blot (Fig. 1J) analyses revealed that AT8 was markedly increased in the SNc after MPTP treatment. Importantly, flavopiridol administration attenuated MPTP-induced phosphorylation of τ in the substantia nigra (Fig. 1I). An increase in cdk5 activity after MPTP treatment was also substantiated by an in vitro kinase assay after immunoprecipitation of cdk5 (Fig. 1J). Finally, increased levels of p25 have been suggested to contribute to increased cdk5 activity (23). Indeed, we show here that p25 levels are up-regulated after MPTP treatment (Fig. 1K). These data indicate that cdk5 activity is increased in the SNc after chronic MPTP administration. To determine whether activation of mitotic CDKs occurred in this paradigm, we investigated the phosphorylation state of Rb on Ser-795 and Ser-805/811, known cdk4/6 phosphorylation sites. We did not detect any increase in phosphorylation at these sites after MPTP treatment, by either Western blot or immunohistochemical analysis, indicating that Rb is not likely involved in the degeneration process (data not shown). Consistent with this idea, we could not detect an increase in cdk4 levels by Western blot analysis (data not shown) or cdk4 activity by in vitro immunoprecipitation kinase assay (Fig. 1L). However, cdk2 levels (data not shown) and activity were detectably increased after MPTP treatment (Fig. 1L).
Fig. 1.
Increased cdk5 expression and activity in the SNc of MPTP-treated mice. (A and B) Expression of cdk5 in the SNc of mice treated with saline (A)or MPTP (14 days) (B). (C and D) Representative photomicrographs illustrating the coexpression of cdk5 (D) with TH-positive (C) cells. (E) Quantitative analyses of TH+/cdk5+ neurons. Each bar represents mean ± SEM; * indicates statistical significance (Student's t test, P < 0.01, n = 4). (F-H) Representative photomicrographs showing AT8 expression in the SNc of mice treated with saline (F), MPTP (14 days) and vehicle (G), or MPTP and flavopiridol (H). (I) Quantitative analysis of AT8-positive neurons of the SNc. Each bar represents the mean ± SEM; ** indicates statistical significance (ANOVA, P < 0.001, n = 6-8). (J) Western blot analyses (AT8 expression, 50- to 55-kDa species) and in vitro kinase assay (histone H1 substrate) indicating cdk5 activity in SNc extracts after saline treatment (lane 1), 12 h after chronic MPTP (lane 2), and 14 days after chronic MPTP treatment (lane 3). CB, Coomassie blue stain as loading control. (K) Western blot showing the expression of the cdk5 activator p25 after saline treatment and chronic MPTP treatment at the indicated times. (L) In vitro immunoprecipitate kinase assay showing activity of cdk2 (with histone H1 as substrate) and cdk4 (with Rb as substrate) after MPTP treatment for the indicated times.
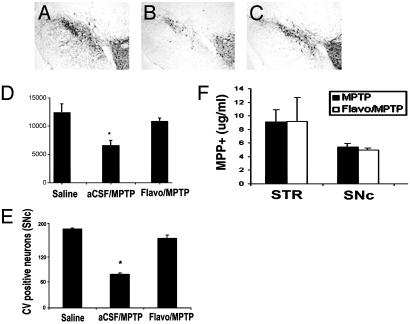
Inhibition of cdk5 Provides Neuroprotection Against MPTP-Induced Loss of Dopamine Neurons of the Nigrostriatal Dopamine Pathway. To determine whether CDK activation was related to dopaminergic neuron loss after MPTP treatment, we examined the neuroprotective effects of the general cdk inhibitor flavopiridol. As shown in Fig. 2 A-D, flavopiridol treatment attenuated MPTP-induced loss of TH-positive neurons in the SNc after chronic MPTP treatment. Because MPTP may elicit the loss of TH expression rather than death of the neuron, we used an independent histological marker (cresyl violet) to assess SNc neuron survival. (Fig. 2E). Results from the cresyl violet analyses were consistent with the TH counts. These results indicate that flavopiridol treatment is effective in protecting dopaminergic neurons from degeneration caused by MPTP. MPTP-induced dopaminergic neurotoxicity correlates linearly with the striatal content of MPTP's active metabolite, MPP+. To determine whether flavopiridol-induced neuroprotective effects were attributable to alterations in brain MPP+ accumulation, we measured striatal and ventral midbrain levels of MPP+ 90 min after injection of MPTP. We did not detect any appreciable flavopiridol-induced alteration in MPP+ levels after MPTP treatment (Fig. 2F).
Fig. 2.
Flavopiridol administration attenuates MPTP-induced degeneration of nigral dopamine neurons. (A-C) Representative TH immunoreactivity in the ventral midbrain of animals treated with saline (A), vehicle/MPTP (B), or flavopiridol/MPTP (C). (D) Quantitative analyses of TH-positive neurons of the SNc. (E) Quantitative analyses of cresyl violet staining at the level of the MTN. Each bar represents the mean ± SEM; * denotes significance (ANOVA, P < 0.0.05, n = 6-8). (F) MPP+ measurements, 90 min after a single-dose MPTP injection into animals treated with either vehicle or flavopiridol (given 24 h before MPTP treatment). Bar graph represents the levels of MPP+ recorded by HPLC analyses (n = 4 or 5 per group) in striatum (STR) and SNc.
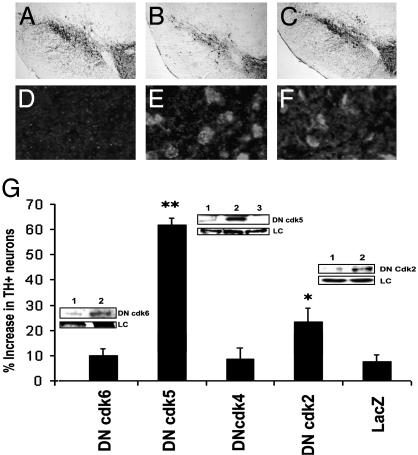
Although the above evidence suggests the importance of CDKs in this MPTP paradigm, it is important to note that flavopiridol inhibits several enzymes, including cdk2 [IC50 = 0.17 μM, (40)], cdk4 [IC50 = 0.40 μM, (41)], and cdk5 [IC50 = 0.17 μM, (42)]. Therefore, to more fully understand how flavopiridol is providing its neuroprotective effects, we used a second approach with DN, kinase-dead mutant forms of individual CDKs. Adenoviruses expressing FLAG-tagged DN forms of cdk5 (DN cdk5), cdk2 (DN cdk2), cdk4 (DN cdk4), and cdk6 (DN cdk6) or a lacZ control were individually injected unilaterally into the mouse striatum 7 days before the initiation of MPTP treatment (34). FLAG-tagged viral proteins were detected by both Western blot analyses and immunohistochemistry (Fig. 3). TH-positive neurons were analyzed 14 days after chronic MPTP administration at the level of the MTN (the level at which virus-mediated expression is highest). As shown in Fig. 3, animals expressing DN cdk5 showed a greater number of TH-positive neurons in the ipsilateral virus-injected side compared with the contralateral control side of the same animal (60% increase vs. 10% in controls). Mice expressing DN cdk2 also showed some protection (25% with DN cdk2 vs. 10% with controls), although to a much lesser extent than DN cdk5-infected brains (Fig. 3). In contrast, DN cdk4, DN cdk6, or LacZ expression did not protect SNc neurons from MPTP-induced dopaminergic neuron loss. Administration of individual viruses alone in the absence of MPTP treatment did not result in any significant degeneration of dopamine neurons compared with lacZ/saline controls (data not shown).
Fig. 3.
Adenovirus-mediated inhibition of cdk5 provides significant protection from MPTP-induced degeneration of dopaminergic neurons of the SNc. (A-C) Representative photomicrographs of TH immunoreactivity in the ventral midbrain (MTN level) of saline-treated mice (A), mice expressing lacZ and treated with MPTP 14 days earlier (B), and mice expressing DN cdk5 and MPTP (C). Mice were treated with a single unilateral virus injection into the striatum as described in Materials and Methods. (G) Quantitation of TH-positive neurons in the SNc for the indicated treatment groups. Values are described as percent increase in the number of neurons in the ipsilateral (virus-injected) vs. noninjected contralateral control side. Data are represented as mean ± SEM (n = 6 animals per group). * and ** denote significance P < 0.05 and P < 0.001, respectively (ANOVA). (Insets) Expression of DN cdk2, -5, and -6 in SNc extracts by Western blot analyses using an anti-FLAG antibody. (Insets) Lane 1, contralateral control side; lane 2, ipsilateral virus-injected side; and lane 3, noninjected control animal. LC, loading control (Coomassie blue staining). (D-F) Immunofluorescence analyses showing the expression of FLAG-tagged DN proteins in the SNc of animals treated with lacZ (D), DN cdk5 (E), or DN cdk2 (F).
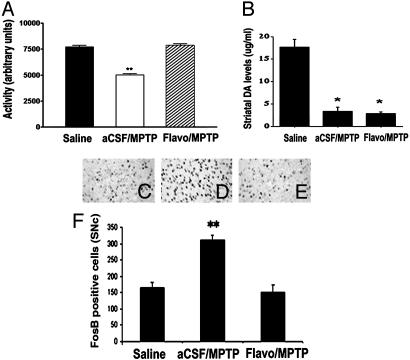
CDK Inhibition and Function. To evaluate the functional impact of CDK inhibition, we carried out behavioral analyses to detect gross motor activity of mice in an open-field test after amphetamine challenge. Locomotor activity was detected by using a computer-assisted video-tracking system that measures amphetamine-induced activity of each animal over a period of 30 min. Previous work had demonstrated that MPTP administration reduces amphetamine-induced motor activity (43). As shown in Fig. 4A, MPTP-injected mice showed significant decrease in gross activity compared with saline controls. Flavopiridol treatment significantly improved MPTP-induced hypolocomotion to a level not significantly different from that recorded for saline controls.
Fig. 4.
Flavopiridol administration reduces behavioral impairments and δ FosB (a marker for postsynaptic changes in the denervated striatum levels) after MPTP treatment in mice but does not prevent loss of striatal dopamine. (A) Locomotor activity 14 days after chronic MPTP treatment. Bar graph represents total activity over a 30-min period immediately after amphetamine challenge in a novel open-field test. Each bar represents the mean ± SEM (n = 6-8 animals per group); * indicates statistical significance (P < 0.001, ANOVA). (B) Striatal dopamine content. The striatum was extracted from mice treated with either vehicle/MPTP (14 days) or flavopiridol/MPTP (14 days) and analyzed for dopamine levels by HPLC. Values are expressed as mean ± SEM; * denotes statistical significance (Student's t test, P < 0.01). (C-E) Expression of
δ FosB in the striatum of mice treated with saline (C), vehicle and MPTP (14 days) (D), or flavopiridol and MPTP (14 days) (E). (F) Quantitation of δ FosB-positive cells; each bar represents mean ± SEM; * indicates statistical significance (P < 0.05, ANOVA, n = 6 animals per group).
With evidence indicating that CDK inhibition attenuates MPTP-induced loss of dopaminergic cell bodies (Fig. 2) and improves an aspect of motor behavior, we next evaluated the protective effects of CDK inhibition on the integrity of striatal function. Although PD is characterized predominantly by the progressive loss of dopamine neurons in the SNc, the main manifestations of the disorder are thought to be mediated by striatal dysfunction, a consequence of cell body loss in the SNc (44). Importantly, neuroprotection did not lead to an improvement in striatal dopamine levels as assessed by HPLC analyses (Fig. 4B). In addition, there was no significant difference in dopamine turnover as measured by the ratio of 3,4-dihydroxy-phenylacetic acid to dopamine in any treatment group (data not shown). Taken together, these data indicate that although there was histological preservation of cell bodies in the SNc, striatal dopamine fibers were functionally impaired with respect to dopamine content.
Because there appeared to be indications of improved locomotor activity without normalized dopamine levels in the striatum, we hypothesized that flavopiridol-mediated neuroprotection of dopamine neuron cell bodies might indirectly modulate postsynaptic striatal function. Expression of the immediate early gene encoding δ FosB is dramatically up-regulated in the dopaminergically denervated striatum after MPTP treatment (45). FosB up-regulation is associated with neural plasticity during addiction and with hypersensitization of striatal dopamine receptors after denervation (46). Accordingly, we surmised that if the striatal circuitry were normalized with flavopiridol treatment, then delta FosB induction would also be attenuated. The results indicate that MPTP-induced FosB expression was significantly attenuated by flavopiridol treatment (Fig. 4 C-F). These results suggest that cdk inhibition provides functional adaptation of the basal ganglion (BG) circuitry through inhibition of postsynaptic changes in the denervated striatum.
Discussion
CDKs and Dopaminergic Neuron Death. Although abnormal levels/activities of both cell cycle-related CDKs and cdk5 have been implicated in a variety of neurodegenerative conditions, the fundamental questions of functional importance as well as identity of individual CDK members required for neuronal death is unclear. Our present results clarify some of these important issues. First, we demonstrate that CDK family members play a required role in nigral degeneration. Second, of the CDK members examined, cdk5 plays the major role in MPTP-induced dopaminergic loss. This conclusion is supported by our observations that cdk5 levels and activity are increased after MPTP treatment and that DN cdk5 expression inhibits death. This observation also indicates the requirement of cdk5 as a major signal in neuronal death pathways in vivo. Third, select cell cycle-related CDK members such as cdk2 may also play a minor role in the neurodegenerative process initiated by MPTP. Finally, the cyclin D1/cdk4/6/Rb pathway does not play a role in MPTP-induced death. In support of this conclusion, expression of DN cdk4 or DN cdk6 did not prevent dopaminergic loss and Rb phosphorylation could not be detected after MPTP treatment. The latter observation is particularly intriguing, considering observations that the cyclin D1, cdk4, and cdk6 activities have been shown to be required in in vitro models of neuronal loss (6, 8) and up-regulated in a variety of in vivo contexts, including ischemia (9, 10). Why cdk2 (at least in a minor fashion) and not cdk4/6 and Rb appear to be involved in MPTP-induced neuronal loss is unclear. However, cdk2 has roles that diverge from those of cdk4/6 (47). For example, cdk2 is known to regulate p53 (48), a required signal for MPTP-induced neuronal loss (49). It is important to emphasize that our understanding of the relative contributions of the CDK members is specific to the present degenerative paradigm, and CDK involvement in other neuropathological conditions will have to be determined empirically.
The manner by which cdk5 is activated after MPTP treatment remains to be fully clarified, but it may include multiple mechanisms. An increase in cdk5 levels, as we have shown, may contribute to the death activation process, whereas posttranslational modifications of cdk5 may be an alternative mechanism to control activation. In fact, stimulatory phosphorylation of cdk5 on Tyr-15 by c-Abl has been previously reported (50). Finally, conversion of the cdk5 activator p35 to a more stable p25 form may lead to increased cdk5 activity. Consistent with this possibility, we have shown an increase in p25 levels after MPTP treatment. This latter possibility is particularly intriguing. Several reports suggest that the p35-to-p25 conversion is mediated through calpains (23, 51). Interestingly, we have previously demonstrated that calpains are activated and required for death in the chronic MPTP paradigm of PD and that calpain activation also occurs in postmortem PD brains (33).
Although our results validate a role for cdk5 in PD, the precise mechanism(s) by which cdk5 modulates downstream effectors remains to be elucidated. Cdk5 may modify cytoskeletal structures as has been proposed for Alzheimer's disease (18, 23). These cytoskeletal abnormalities could lead to activation of downstream death effectors such as Bax (32) and caspases (52), which have been implicated in MPTP-induced nigral loss. In addition, cdk5 may more directly activate other death-related proteins. In particular, cdk5 is reported to activate p53 (53). However, cdk5 may also inhibit prosurvival signals. In this regard, a recent report has indicated that nuclear cdk5 activity leads to phosphorylation and inactivation of MEF2, a transcription factor thought to be required for neuronal survival under select conditions (54).
A final intriguing possibility is that cdk5 may affect function of cell cycle-related CDKs. Indeed, the functional role of cell cycle-related cdks is supported by our present results indicating protection with DN cdk2 expression. Consistent with the results presented here, cyclin E, one activator of cdk2, has recently been shown to be up-regulated in response to a deficiency of parkin, which is encoded by a gene linked to familial PD (55). In addition, cdc2 (a cell cycle CDK) expression has been reported in a neonatal model of induced dopaminergic neuron loss (31). Although the exact relationship between cell cycle-related CDKs and cdk5 is presently unknown, cell cycle CDK up-regulation in an amyotrophic lateral sclerosis (ALS) model is alleviated by expression of neurofilament H, a proposed phosphorylation sink for cdk5 (56). A similar relationship might exist with PD. Taking these findings together, an attractive model of dopaminergic death is one in which cdk5 acts concertedly on multiple substrates, including cytoskeletal components and death-related signaling components such as p53, MEF2, and cell cycle CDKs. These signals act coordinately to regulate a Bax-mediated death process. The potential convergent actions of cdk5 may also explain why inhibition of cdk5 is more effective at neuroprotection than cdk2. However, it will be important in future experiments to definitively elucidate any potential downstream target(s) of cdk5 as it relates to dopaminergic cell loss.
CDK5 and Striatal Function. An important aspect of our study critical to development of treatment strategies for PD is the observation that cdk5 inhibition leads to nigral protection, normalized markers of BG circuitry, and improved aspects of behavior. These improvements were not associated with preservation of striatal dopamine levels or by compensatory increases in dopamine turnover. These observations are consistent with increasing evidence that the response of the BG to striatal denervation is not solely dependent upon the loss of striatal dopamine and that preservation of nigral function may also be of critical importance. In this regard, it is important to note that nigral dopaminergic neurons release dopamine not only from their axons projecting to the striatum but also from their dendrites (57, 58). This dendrodendritic release of dopamine by SNc neurons may modulate the BG and explain the behavioral improvements observed presently (33, 59). Alternatively, CDK inhibition may directly affect BG neurotransmitter systems at either postsynaptic striatal sites or other basal ganglia nuclei. In support of this possibility, cdk5 is reported to play a role in synaptic transport and dopamine signaling (60). It may therefore be possible that modulation of cdk5 activity, independent of its role in nigral death, may affect BG function.
In conclusion, our results represent an important demonstration that inhibition of CDKs, in a model of PD, not only retards neurodegeneration but also ameliorates dopamine-related functional impairment in mice. While caution must be exercised in directly relating the MPTP animal model to the human condition, we propose that these findings hold potential for the development of novel PD therapeutic strategies.
Acknowledgments
We thank R. Lee and R. Marcelissen for technical assistance. This work was supported by grants from the Canadian Institutes of Health Research (to D.S.P. and H.A.), the Parkinson's Society Canada, and the Parkinson's Disease Foundation (to S.P., V.J.-L., and D.S.P.), the National Institute of Neurological Disorders and Stroke, the U.S. Department of Defense, the Lowenstein Foundation, the Lillian Goldman Charitable Trust, the Muscular Dystrophy Association, and the ALS Association (to S.P.), and the Canadian Stroke Network (to D.S.P.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PD, Parkinson's disease; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; SNc, substantia nigra pars compacta; CDK, cyclin-dependent kinase; cdk, postmitotic CDK; DN, dominant-negative; TH, tyrosine hydroxylase; MTN, medial terminal nucleus; MPP+, 1-methyl-4-phenylpyridinium; Rb, retinoblastoma protein; BG, basal ganglion.
References
- 1.Przedborski, S., Jackson-Lewis, V., Naini, A. B., Jakowec, M., Petzinger, G., Miller, R. & Akram, M. (2001) J. Neurochem. 76, 1265-1274. [DOI] [PubMed] [Google Scholar]
- 2.Pines, J. (1993) Biochem. Soc. Trans. 21, 921-925. [DOI] [PubMed] [Google Scholar]
- 3.Freeman, R. S., Estus, S. & Johnson, E. M., Jr. (1994) Neuron 12, 343-355. [DOI] [PubMed] [Google Scholar]
- 4.Gao, C. Y. & Zelenka, P. S. (1995) Exp. Cell Res. 219, 612-618. [DOI] [PubMed] [Google Scholar]
- 5.O'Hare, M. J., Hou, S. T., Morris, E. J., Cregan, S. P., Xu, Q., Slack, R. S. & Park, D. S. (2000) J. Biol. Chem. 275, 25358-25364. [DOI] [PubMed] [Google Scholar]
- 6.Park, D. S., Morris, E. J., Padmanabhan, J., Shelanski, M. L., Geller, H. M. & Greene, L. A. (1998) J. Cell Biol. 143, 457-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giovanni, A., Keramaris, E., Morris, E. J., Hou, S. T., O'Hare, M., Dyson, N., Robertson, G. S., Slack, R. S. & Park, D. S. (2000) J. Biol. Chem. 275, 11553-11560. [DOI] [PubMed] [Google Scholar]
- 8.Park, D. S., Levine, B., Ferrari, G. & Greene, L. A. (1997) J. Neurosci. 17, 8975-8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osuga, H., Osuga, S., Wang, F., Fetni, R., Hogan, M. J., Slack, R. S., Hakim, A. M., Ikeda, J. E. & Park, D. S. (2000) Proc. Natl. Acad. Sci. USA 97, 10254-10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang, F., Corbett, D., Osuga, H., Osuga, S., Ikeda, J. E., Slack, R. S., Hogan, M. J., Hakim, A. M. & Park, D. S. (2002) J. Cereb. Blood Flow Metab. 22, 171-182. [DOI] [PubMed] [Google Scholar]
- 11.McShea, A., Harris, P. L., Webster, K. R., Wahl, A. F. & Smith, M. A. (1997) Am. J. Pathol. 150, 1933-1939. [PMC free article] [PubMed] [Google Scholar]
- 12.Nikolic, M., Dudek, H., Kwon, Y. T., Ramos, Y. F. & Tsai, L. H. (1996) Genes Dev. 10, 816-825. [DOI] [PubMed] [Google Scholar]
- 13.Tsai, L. H., Delalle, I., Caviness, V. S., Jr., Chae, T. & Harlow, E. (1994) Nature 371, 419-423. [DOI] [PubMed] [Google Scholar]
- 14.Ko, J., Humbert, S., Bronson, R. T., Takahashi, S., Kulkarni, A. B., Li, E. & Tsai, L. H. (2001) J. Neurosci. 21, 6758-6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohshima, T., Ward, J. M., Huh, C. G., Longenecker, G., Veeranna, Pant, H. C., Brady, R. O., Martin, L. J. & Kulkarni, A. B. (1996) Proc. Natl. Acad. Sci. USA 93, 11173-11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chae, T., Kwon, Y. T., Bronson, R., Dikkes, P., Li, E. & Tsai, L. H. (1997) Neuron 18, 29-42. [DOI] [PubMed] [Google Scholar]
- 17.Paglini, G., Peris, L., Diez-Guerra, J., Quiroga, S. & Caceres, A. (2001) EMBO Rep. 2, 1139-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikolic, M., Chou, M. M., Lu, W., Mayer, B. J. & Tsai, L. H. (1998) Nature 395, 194-198. [DOI] [PubMed] [Google Scholar]
- 19.Rashid, T., Banerjee, M. & Nikolic, M. (2001) J. Biol. Chem. 276, 49043-49052. [DOI] [PubMed] [Google Scholar]
- 20.Kwon, Y. T. & Tsai, L. H. (1998) J. Comp Neurol. 395, 510-522. [DOI] [PubMed] [Google Scholar]
- 21.Julien, J. P. & Mushynski, W. E. (1998) Prog. Nucleic Acid Res. Mol. Biol. 61, 1-23. [DOI] [PubMed] [Google Scholar]
- 22.Rosales, J. L., Nodwell, M. J., Johnston, R. N. & Lee, K. Y. (2000) J. Cell Biochem. 78, 151-159. [DOI] [PubMed] [Google Scholar]
- 23.Patrick, G. N., Zukerberg, L., Nikolic, M., de la Monte, S., Dikkes, P. & Tsai, L. H. (1999) Nature 402, 615-622. [DOI] [PubMed] [Google Scholar]
- 24.Ahlijanian, M. K., Barrezueta, N. X., Williams, R. D., Jakowski, A., Kowsz, K. P., McCarthy, S., Coskran, T., Carlo, A., Seymour, P. A., Burkhardt, J. E., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 2910-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen, M. D., Lariviere, R. C. & Julien, J. P. (2001) Neuron 30, 135-147. [DOI] [PubMed] [Google Scholar]
- 26.Bu, B., Li, J., Davies, P. & Vincent, I. (2002) J. Neurosci. 22, 6515-6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura, S., Kawamoto, Y., Nakano, S., Akiguchi, I. & Kimura, J. (1997) Acta Neuropathol. (Berlin) 94, 153-157. [DOI] [PubMed] [Google Scholar]
- 28.Brion, J. P. & Couck, A. M. (1995) Am J. Pathol. 147, 1465-1476. [PMC free article] [PubMed] [Google Scholar]
- 29.Henchcliffe, C. & Burke, R. E. (1997) Neurosci. Lett. 230, 41-44. [DOI] [PubMed] [Google Scholar]
- 30.Neystat, M., Rzhetskaya, M., Oo, T. F., Kholodilov, N., Yarygina, O., Wilson, A., El-Khodor, B. F. & Burke, R. E. (2001) J. Neurochem. 77, 1611-1625. [DOI] [PubMed] [Google Scholar]
- 31.El-Khodor, B. F., Oo, T. F., Kholodilov, N. & Burke, R. E. (2003) Exp. Neurol. 179, 17-27. [DOI] [PubMed] [Google Scholar]
- 32.Vila, M., Jackson-Lewis, V., Vukosavic, S., Djaldetti, R., Liberatore, G., Offen, D., Korsmeyer, S. J. & Przedborski, S. (2001) Proc. Natl. Acad. Sci. USA 98, 2837-2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crocker, S. J., Smith, P. D., Jackson-Lewis, V., Lamba, W. R., Hayley, S. P., Grimm, E., Callaghan, S. M., Slack, R. S., Melloni, E., Przedborski, S., et al. (2003) J. Neurosci. 23, 4081-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crocker, S. J., Lamba, W. R., Smith, P. D., Callaghan, S. M., Slack, R. S., Anisman, H. & Park, D. S. (2001) Proc. Natl. Acad. Sci. USA 98, 13385-13390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crocker, S. J., Morelli, M., Wigle, N., Nakabeppu, Y. & Robertson, G. S. (1998) Brain Res. Mol. Brain Res. 53, 69-77. [DOI] [PubMed] [Google Scholar]
- 36.Franklin, K. B. & Paxinos, G. (2001) The Mouse Brain in Stereotaxic Coordinates (Academic, New York).
- 37.Abercrombie, M. (1946) Anat. Rec. 94, 239-247. [DOI] [PubMed] [Google Scholar]
- 38.Terada, M., Yasuda, H., Kogawa, S., Maeda, K., Haneda, M., Hidaka, H., Kashiwagi, A. & Kikkawa, R. (1998) J. Neurochem. 71, 2600-2606. [DOI] [PubMed] [Google Scholar]
- 39.Paudel, H. K., Lew, J., Ali, Z. & Wang, J. H. (1993) J. Biol. Chem. 268, 23512-23518. [PubMed] [Google Scholar]
- 40.Kim, K. S., Sack, J. S., Tokarski, J. S., Qian, L., Chao, S. T., Leith, L., Kelly, Y. F., Misra, R. N., Hunt, J. T., Kimball, S. D., et al. (2000) J. Med. Chem. 43, 4126-4134. [DOI] [PubMed] [Google Scholar]
- 41.Knockaert, M., Greengard, P. & Meijer, L. (2002) Trends Pharmacol. Sci. 23, 417-425. [DOI] [PubMed] [Google Scholar]
- 42.Leclerc, S., Garnier, M., Hoessel, R., Marko, D., Bibb, J. A., Snyder, G. L., Greengard, P., Biernat, J., Wu, Y. Z., Mandelkow, E. M., et al. (2001) J. Biol. Chem. 276, 251-260. [DOI] [PubMed] [Google Scholar]
- 43.Schroeder, U., Kreutz, M. R., Schroeder, H. & Sabel, B. A. (1997) Pharmacol. Biochem. Behav. 56, 281-285. [DOI] [PubMed] [Google Scholar]
- 44.Graybiel, A. M. (1990) Trends Neurosci. 13, 244-254. [DOI] [PubMed] [Google Scholar]
- 45.Doucet, J. P., Nakabeppu, Y., Bedard, P. J., Hope, B. T., Nestler, E. J., Jasmin, B. J., Chen, J. S., Iadarola, M. J., St-Jean, M., Wigle, N., et al. (1996) Eur. J. Neurosci. 8, 365-381. [DOI] [PubMed] [Google Scholar]
- 46.Fasano, S. & Brambilla, R. (2002) Curr. Mol. Med. 2, 649-665. [DOI] [PubMed] [Google Scholar]
- 47.Kitagawa, M., Higashi, H., Jung, H. K., Suzuki-Takahashi, I., Ikeda, M., Tamai, K., Kato, J., Segawa, K., Yoshida, E., Nishimura, S. & Taya, Y. (1996) EMBO J. 15, 7060-7069. [PMC free article] [PubMed] [Google Scholar]
- 48.Price, B. D., Hughes-Davies, L. & Park, S. J. (1995) Oncogene 11, 73-80. [PubMed] [Google Scholar]
- 49.Trimmer, P. A., Smith, T. S., Jung, A. B. & Bennett, J. P., Jr. (1996) Neurodegeneration 5, 233-239. [DOI] [PubMed] [Google Scholar]
- 50.Zukerberg, L. R., Patrick, G. N., Nikolic, M., Humbert, S., Wu, C. L., Lanier, L. M., Gertler, F. B., Vidal, M., Van Etten, R. A. & Tsai, L. H. (2000) Neuron 26, 633-646. [DOI] [PubMed] [Google Scholar]
- 51.Kusakawa, G., Saito, T., Onuki, R., Ishiguro, K., Kishimoto, T. & Hisanaga, S. (2000) J. Biol. Chem. 275, 17166-17172. [DOI] [PubMed] [Google Scholar]
- 52.Eberhardt, O. & Schulz, J. B. (2003) Toxicol. Lett. 139, 135-151. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, J., Krishnamurthy, P. K. & Johnson, G. V. (2002) J. Neurochem. 81, 307-313. [DOI] [PubMed] [Google Scholar]
- 54.Gong, X., Tang, X., Wiedmann, M., Wang, X., Peng, J., Zheng, D., Blair, L. A., Marshall, J. & Mao, Z. (2003) Neuron 38, 33-46. [DOI] [PubMed] [Google Scholar]
- 55.Staropoli, J. F., McDermott, C., Martinat, C., Schulman, B., Demireva, E. & Abeliovich, A. (2003) Neuron 37, 735-749. [DOI] [PubMed] [Google Scholar]
- 56.Nguyen, M. D., Boudreau, M., Kriz, J., Couillard-Despres, S., Kaplan, D. R. & Julien, J. P. (2003) J. Neurosci. 23, 2131-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bjorklund, A. & Lindvall, O. (1975) Brain Res. 83, 531-537. [DOI] [PubMed] [Google Scholar]
- 58.Cheramy, A., Leviel, V. & Glowinski, J. (1981) Nature 289, 537-542. [DOI] [PubMed] [Google Scholar]
- 59.Robertson, G. S. & Robertson, H. A. (1989) J. Neurosci. 9, 3326-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bibb, J. A., Snyder, G. L., Nishi, A., Yan, Z., Meijer, L., Fienberg, A. A., Tsai, L. H., Kwon, Y. T., Girault, J. A., Czernik, A. J., et al. (1999) Nature 402, 669-671. [DOI] [PubMed] [Google Scholar]