Abstract
Long-term synaptic plasticity requires both gene expression in the nucleus and local protein synthesis at synapses. The effector proteins that link molecular events in the cell body with local maintenance of synaptic strength are not known. We now show that treatment with serotonin (5-HT) that produces long-term facilitation induces the Aplysia eukaryotic translation elongation factor 1α (Ap-eEF1A) as a late gene that might serve this coupling function in sensory neurons. Although the translation factor is induced, it is not transported into axon processes when the stimulation with 5-HT was restricted to the cell body. In contrast, its mRNA is transported when 5-HT was applied to both cell body and synapses. Intracellular injection of antisense oligonucleotides or antibodies that block the induction and expression of Ap-eEF1A do not affect the initial expression of long-term facilitation but do block its maintenance beyond 24 h. The transport of eEF1A protein and its mRNA to nerve terminals suggests that the translation factor plays a role in the local protein synthesis that is essential for maintaining newly formed synapses.
Long-lasting modifications in synaptic strength are thought to underlie learning and memory (1, 2). These modifications require both transcriptional activation in the nucleus and local protein synthesis at synapses (3, 4). Several lines of evidence suggest that a crucial mechanism for coupling nuclear activation and local modification of synaptic contacts is through transport of mRNAs (5-7) and their local translation at preactivated synapses (3, 4). Only select transcripts are transported: mRNAs for β-actin, cytoskeletal-associated proteins (Arc and MAP2), synaptic receptor subunits (for example, for glutamate and glycine; refs. 8 and 9) and the α-subunit of Ca/calmodulin-dependent protein kinase II (10, 11), a major component of the postsynaptic density.
Because long-term synaptic plasticity requires nuclear transcription, the products of which are available to all synapses of the neuron, we recently investigated how the distribution might be restricted to a subset of a neuron's synapses. Using an Aplysia sensory neuron-motor neuron culture system in which a single bifurcated sensory neuron establishes synaptic contacts with two spatially separated motor neurons, Martin et al. (12) found that repeated local application of serotonin (5-HT) to one set of synapses could selectively modify those synapses without altering other synaptic connections of the sensory neuron. This synapse-specific long-term facilitation requires CREB1-mediated transcription in the nucleus and local protein synthesis at synapses (12, 13). Further, Casadio et al. (14) found that mRNAs are locally translated in sensory neuron's processes when 5-HT was applied to synapses. Local protein synthesis might serve two distinct functions: first, to initiate the retrograde signal to the nucleus to activate transcription, and second, to maintain the structural changes needed for late-phase long-term facilitation at 72 h.
Application of 5-HT restricted to the cell body of an Aplysia sensory neuron induces a long-term facilitation that is cellwide, involving all of the neuron's synapses (14, 15). This cellwide facilitation, like synapse-specific facilitation, also depends on the activation of CREB1. Unlike synapse-specific modification, however, cellwide long-term facilitation occurs in the absence of local protein synthesis, does not last >48 h and is not associated with the growth of new synapses.
The proteins synthesized from the mRNAs delivered to terminals by activity-dependent transport are likely to function in the growth and stabilization of new synapses. Because the stabilization of newly grown synaptic connections occurs at least 24 h after the long-term facilitation is first induced (14), we carried out a screen for late genes induced by 5-HT that might contribute to the maintenance of long-term facilitation and found that a homolog of the eukaryotic translation elongation factor 1α (eEF1A) is up-regulated. Because the factor binds aminoacyl tRNA during the formation of the nascent polypeptide chain on ribosomes (16), the mRNA transported to neurites presumably contributes to local protein synthesis. We find that the late induction of eEF1A is needed for the maintenance of synaptic plasticity.
Materials and Methods
Cell Cultures. Cell cultures were kept for 5 days at 18°C (17). Briefly, Aplysia abdominal and pleural ganglia were incubated in type IX bacterial protease (10 mg/ml, Sigma) at 34.5°C. Sensory neurons removed from pleural ganglia of several mature animals were plated in polylysine-coated dishes with a single L7 motor neuron isolated from the abdominal ganglion of a juvenile Aplysia (18). Bifurcated sensory neuron-motor neuron cultures were prepared as described (12) for use in those experiments in which the application of 5-HT was restricted to the cell body.
Electrophysiology. Five days after the cells were placed in culture the strength of the sensory-to-motor neuron synapse was tested with intracellular recordings of excitatory postsynaptic potentials (EPSPs) from the motor neuron evoked by extracellular stimulation of the sensory neuron (17). For short-term facilitation the cultures were treated with one 5-min pulse of 5-HT (10 μM). For long-term facilitation, cells were treated with five 5-min pulses of 10 μM 5-HT spaced at 10-min intervals. Application of 5-HT restricted to the cell body was performed as described (12, 14). Briefly, five separate episodes of 100 μM 5-HT in L15 medium containing 0.05% fast green (each episode consisting of 5-sec pulses at 10-sec intervals) were given at 10-min intervals with a perfusion micropipette connected to a picospritzer (World Precision Instruments, Sarasota, FL). After they were perfused with 5-HT, the cells were kept in 50% L15/50% Aplysia hemolymph. To measure long-term facilitation, EPSPs were recorded in L7 motor neurons 24 h (long-term facilitation) or 72 h (late-phase facilitation) after 5-HT treatment.
Intracellular Injections. Absorbed Aplysia eEF1A (Ap-eEF1A) antibody and preimmune serum were pressure-injected into sensory neurons 1 h before or 6 and 12 h after the start of the 5-HT treatment, and the EPSPs were recorded at 10 min (short-term facilitation), 24 h, or 72 h after the last pulse of 5-HT. We injected an HPLC-purified Ap-eEF1A antisense oligonucleotide (5′-GCTTGTCCTTACCCATGGTGCTAGAG-3′) complementary to nucleotides -10 to +16 of the Ap-eEF1A coding region (see Fig. 6, which is published as supporting information on the PNAS web site). A scrambled oligonucleotide (5′-TAGCTCGTCACGATCGTCGTGCGTTA-3′) served as control. Oligonucleotides were injected 4 h before or 6 h after the 5-HT treatment. Antibodies and oligonucleotides were injected in 0.5 M KCl, 10 mM Tris·HCl (pH 7.6). Data are presented as mean percentage change ± SEM in the EPSP amplitude measured after treatment as compared with the pretreatment amplitude.
Results
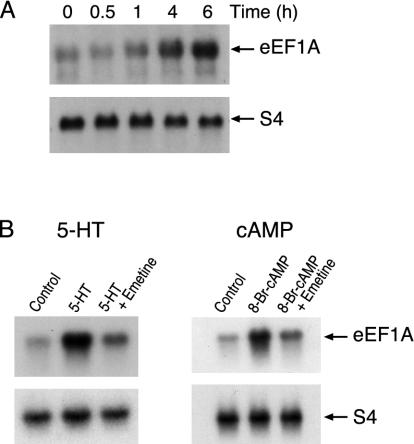
Induction of Ap-eEF1A Depends on cAMP. To identify late genes we exposed pleural ganglia to 5-HT and carried out differential hybridization screening (see Supporting Methods, which is published as supporting information on the PNAS web site). Two partial cDNA clones similar to eEF1A were isolated. The full-length cDNA obtained encoded a predicted amino acid sequence 80% similar to that of human eEF1A with an identical GTP-binding domain (see Fig. 6 A and B). The 5′ UTR of Ap-eEF1A mRNA has a tract of 11 contiguous pyrimidine residues (5′ TOP) next to the presumptive guanosine cap (see Supporting Methods and Fig. 6 C and D). Elongation factors of other animals also have this tract. Translation of mRNAs with polypyrimidine tracts is activated in proliferating cells (see ref. 19). Ap-eEF1A mRNA is induced 7-fold in sensory neurons exposed to 5-HT (Fig. 1A) or 8-Br-cAMP, peaking at 5-6 h (Fig. 1B). Ap-eEF1A is not an early response gene, as its induction is blocked by emetine (Fig. 1B).
Fig. 1.
Induction of eEF1A mRNA. (A) Time course. Total RNA was extracted from the nervous tissue from untreated Aplysia (controls) or animals treated with 5-HT and probed with 32P-labeled Ap-eEF1A cDNA. As control, the RNA samples were also probed with an S4-ribosomal cDNA that is not affected by 5-HT (21, 44). Radioactivity was quantified densitometrically, and the extent of stimulation was calculated by normalizing eEF1A values with those of S4. (B) (Left) Inhibition of induction by emetine. Total RNA was isolated 6 h after the start of the treatment with 5-HT alone or with 5-HT and emetine, and probed with 32P-labeled Ap-eEF1A cDNA (Upper) or S4 cDNA (Lower). The 7-fold (± 0.4; n = 6; P < 0.001) induction of eEF1A mRNA observed with 5-HT (Student's paired t test) was inhibited by emetine. (Right) Induction by 8-Br-cAMP. Total RNA from ganglia treated with 8-Br-cAMP and probed with 32P-labeled Ap-eEF1A cDNA (Upper) or S4 cDNA (Lower). This 5.5 (± 0.5-fold; P < 0.01; n = 6) induction was also blocked by emetine.
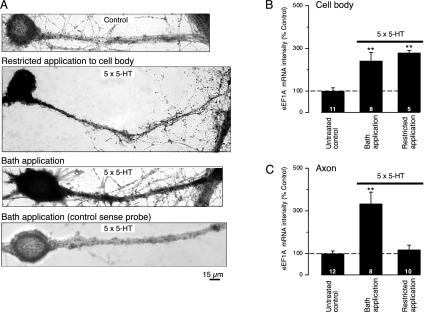
Ap-eEF1A mRNA Moves into Axons Only When 5-HT Is Applied to Both Cell Body and Axon. Casadio et al. (14) found that five pulses of 5-HT restricted to the sensory neuron's cell body induces a long-term facilitation that lasts no longer than 48 h and does not require the development of new presynaptic varicosities. Under the same conditions using in situ hybridization (Supporting Methods), we observed that Ap-eEF1A mRNA increased in the cell body and axon hillock of stimulated sensory neurons but not in axonal processes (Fig. 2A). In contrast, with bath application of 5-HT, which leads to a facilitation that persists for 72 h, there was abundant mRNA in both cell body and axon process (Fig. 2 A). The amounts of somatic mRNA were the same with either protocol of stimulation (Fig. 2B), but there was a 285.5 ± 45.7% (P < 0.01) increase in the amount of Ap-eEF1A mRNA in axons of neurons stimulated by the bath application compared with that in axons in which the stimulation had been restricted to the cell body (Fig. 2C). Thus, somatic stimulation alone induces Ap-eEF1A mRNA but fails to increase eEF1A mRNA in axons. Nocodazole (3.3 μM), an agent that disrupts microtubules, reduced the transport of the mRNA in axons; in contrast, cytochalasin B (2 μM), which depolymerizes actin filaments in Aplysia (20), had no effect (Fig. 7, which is published as supporting information on the PNAS web site). As in vertebrates, movement of mRNA into axons is mediated by microtubules and not by actin filaments (see ref. 11). Transport was selective: Ap-C/EBP mRNA, the transcript for an immediate response gene induced by 5-HT (21), did not move into axons after repeated bath applications of 5-HT (data not shown). Selective transport of other mRNAs has also been demonstrated by Sun et al. (22) and Hu et al. (23).
Fig. 2.
5-HT stimulation is necessary for transport of eEF1A mRNA into neurites. Images of cultured neurons making synaptic contact with L7 motor neurons are shown. Sensory neurons were stimulated with five pulses of 5-HT, and the amount of Ap-EF1A mRNA was measured as pixel intensity by in situ hybridization using a digoxigenin-labeled antisense mRNA probe (see Supporting Methods). (A) Neurites of the untreated (control) cell showed no staining. Local application of 5-HT to the sensory neuron's cell body resulted in the staining of the cell body and proximal axon but not of the distal portion of the axon. A strong signal for eEF1A mRNA, detected by the antisense probe, is evident in the cell body and axon of the neuron stimulated by bath application of 5-HT. Cells treated with 5-HT were not stained when hybridized with a control sense probe. Quantitation of the mean pixel intensity corresponding to the somatic (B) and axonal (C) eEF1A mRNA staining was done in sensory cells stimulated by bath-applied 5-HT and compared with cells where 5-HT stimulation was restricted to the cell body. The RNA signal in each group was normalized to the mean signal obtained for the untreated group. Bath application of 5-HT increased the amount of eEF1A mRNA in the cell body (B) and in axons (C) (**, P < 0.01, treated versus untreated controls). In contrast, application of 5-HT restricted to the cell body increased the amount of staining only in the cell body (B, **, P < 0.01, treated versus untreated controls; ANOVA and Neuman-Keul's multiple range test), and not in the axon (C).
The amounts of axonal Ap-eEF1A protein also increases after 5-HT stimulation. The distribution of Ap-eEF1A immunoreactivity was examined in sensory neuron cell bodies and dissected neurites (see Supporting Methods). Some immunoreactivity was present in both cell bodies and neurites of unstimulated neurons (Fig. 3). When sensory cells were treated with repeated pulses of 5-HT, Ap-eEF1A protein increased significantly both in the cell body and processes (Fig. 8, which is published as supporting information on the PNAS web site) 7 h after the treatment with 5-HT. Ap-eEF1A protein returns to baseline when tested at 24 h (data not shown).
Fig. 3.
Regional distribution of Ap-eEF1A determined by immunoblotting. (A) An antipeptide antibody raised against the predicted amino acid sequence (Ser-440—Lys-459) recognized a single Mr 50,000 component in extracts of Aplysia nervous tissue (see Supporting Methods). Preimmune serum was not reactive. Immunoreactivity was abolished when the antibodies were preincubated with the peptide immunogen. (B) Immunoblotting of the extracts from the sensory neuron's cell body and neuropil show that eEF1A immunoreactivity is present in both compartments (see Supporting Methods). Reprobing the same blot with antisynaptotagmin antibodies (Santa Cruz Biotechnology) revealed the enrichment of synaptic protein in the neuropil fraction compared with that of the cell body. Cell bodies and neuropil were separated under a light microscope from ganglia treated with 2 M NaCl and 50% propylene glycol (1:1, vol/vol) and kept at -20°C for 5 h (45), which renders the solid ganglia translucent while preserving the cytological structure and thereby aids in dissection. (C) Induction of eEF1A protein. Extracts were made from sensory neurons treated with 5-HT or controls and immunoblotted (Upper). The same samples were probed with antiubiquitin antibody (Sigma) (Lower). Because the amount of ubiquitin and ubiquitin conjugates do not change after treatment with 5-HT (46), this serves as control loading control. (D) Quantification of eEF1A immunoreactivity in untreated and 5-HT-treated sensory neurons. The amount of eEF1A is significantly higher (P < 0.01; n = 6, paired t test) in 5-HT-treated sensory neurons.
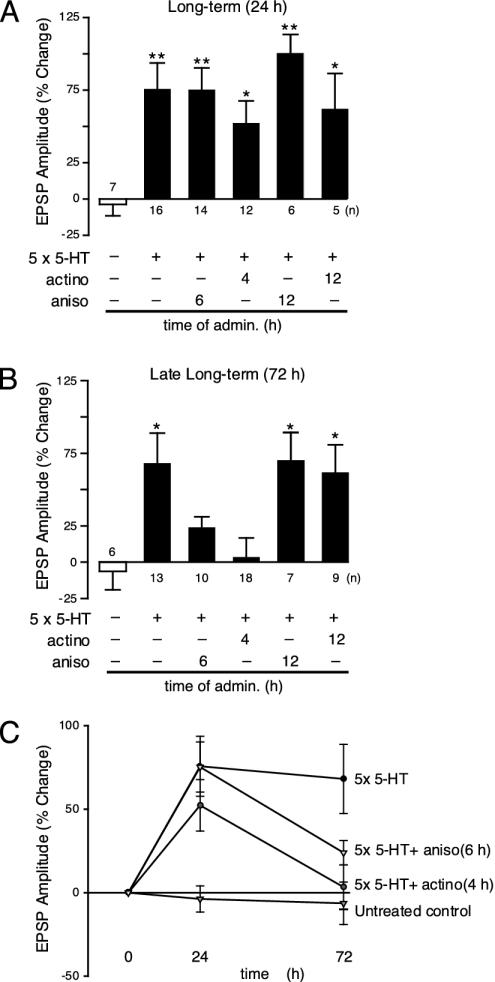
A Second Period of Protein Synthesis Critical for Late-Phase Synaptic Facilitation. Inhibitors of protein synthesis applied 1-2 h after starting the treatment with 5-HT blocked the facilitation of sensory-to-motor neuron synapses at 24 h, defining an early critical period of protein synthesis needed for facilitation at 24 h (17). Application of the protein synthesis inhibitor anisomycin (10 μM) 6 h after starting the treatment with 5-HT (Fig. 4A) did not affect the facilitation at 24 h: the EPSP amplitude of the sensory-motor neuron connection was increased at 24 h (75.3 ± 15.0%; n = 12). This facilitation did not last. At 72 h the EPSP amplitude of the treated connections did not differ from untreated controls (21.0 ± 12.6%; n = 10) (Fig. 4B), suggesting that some proteins made 6 h after the start of the treatment are required for the late phase of synaptic facilitation. These proteins are either not needed for the early phase or existing stores may have all been used up by 24 h and need to be replenished. Anisomycin applied 12 h after the 5-HT treatment did not interfere with either early-phase (88.1 ± 14.7% at 24 h; n = 6) or late-phase facilitation (70.1 ± 19.2% at 72 h; n = 7) (Fig. 4 A and B). These observations suggest that there is a second critical period of protein synthesis that starts 4-6 h after beginning the 5-HT treatment. Proteins made at 4-6 h are not needed for early long-term facilitation, but are required for the late phase measured at 72 h (Fig. 4C). Evidence for a late phase of protein synthesis has been presented (24, 25).
Fig. 4.
A second period of gene expression is required to maintain late-phase synaptic facilitation. EPSPs were recorded from cultured motor neurons. Changes (%) in EPSP amplitude (mean ± SEM) are shown as bar graphs. (A) Anisomycin (aniso, 10 μM applied for 1 h) did not affect the early phase of long-term facilitation (24 h) if administered at 6 or 12 h after treatment with five pulses of 5-HT (**, P < 0.01, ANOVA and Neuman-Keul's multiple range test) nor did actinomycin (actino, 50 μg/ml) administered at 4 or 12 h after treatment with 5-HT at 24 h (*, P < 0.05 and **, P < 0.01). (B) The late phase of long-term facilitation was blocked when anisomycin was applied at 6 h but not at 12 h after the stimulation with 5-HT (*, P < 0.05). Late long-term facilitation was also abolished when actinomycin D was administered 4 h after the five pulses of 5-HT, but was unaffected when transcription was inhibited at 12 h (*, P < 0.05; **, P < 0.01). (C) Effects of blocking transcription and translation on EPSP amplitude. To inhibit translation, actinomycin was added 4 h after the application of five pulses of 5-HT. To block protein synthesis, anisomycin was applied 6 h after the treatment with 5-HT.
This second wave of protein synthesis is accompanied by a second wave of transcription. We applied the transcription inhibitor actinomycin D (50 μg/ml), either 4 or 12 h after starting the 5-HT treatment. Inhibition of RNA synthesis at 4 h did not inhibit the facilitation at 24 h but did block it at 72 h (Fig. 4C). The EPSP amplitude was increased at 24 h in cells receiving five pulses of 5-HT and actinomycin (52.3 ± 15.2%; n = 14; Fig. 4A), but this increase declined to baseline at 72 h (3.3 ± 13.3%; n = 18; Fig. 4B). The necessary transcription has taken place by 12 h, however: application of actinomycin 12 h after the treatment with 5-HT did not inhibit the early (62.0 ± 24.4%; n = 5; Fig. 4A) or the late phase of synaptic facilitation (61.7 ± 19.1%; n = 9; Fig. 4B). As expected, actinomycin added 2 h after the treatment with 5-HT abolished the facilitation at 24 h (-2.7 ± 8.3%; n = 18).
Ap-eEF1A Is Essential for the Late Phase of Long-Term Facilitation. Antisense oligonucleotides injected into sensory neuron cell bodies 6 h after starting the 5-HT treatment blocked late-phase facilitation at 72 h (+15.6 ± 6.1%; n = 13; Fig. 5B) without affecting the early phase at 24 h (+83.3 ± 14.6%, n = 15; Fig. 5A). Scrambled oligonucleotides had no effect on either the late (+58.4 ± 18.7%; n = 6; Fig. 5B) or the early phase (+79.2 ± 15.2%; n = 6; Fig. 5A). Similarly, when anti-ApeEF1A antibodies were injected 6 h after exposing the cultures to five pulses of 5-HT, facilitation was also blocked at 72 h (+10.4 ± 7.9%; n = 16; Fig. 5B), but not at 24 h (+70.7 ± 13.0%; n = 17; Fig. 5A). In contrast, injection of the antibodies 1 h before the treatment with 5-HT reduced both the early (+22.3 ± 18.5%; n = 8; Fig. 5A) and the late phase (-10.3 ± 14.5%; n = 7; Fig. 5B). Injection of preimmune serum had no effect on either the late (+83.4 ± 16.4%; n = 8; Fig. 5B) or the early phase (+79.5 ± 11.5%; n = 10; Fig. 5A). Finally, injection of anti-Ap-eEF1A antibodies 12 h after the cultures were exposed to 5-HT did not influence either the early (98.1 ± 23.1%; n = 6; Fig. 5A) or the late phase (66.2 ± 16.1%; n = 6; Fig. 5B). The inhibition by antisense oligonucleotides indicates that induction of Ap-eEF1A mRNA is essential for maintaining long-term facilitation. Short-term facilitation, induced by one pulse of 5-HT, was unaffected in cells injected with antisense oligonucleotides, anti-eEF1A antibodies, preimmune serum, or scrambled oligonucleotides (Fig. 5C). Thus interference with the expression or function at 4-6 h of Ap-EF1A selectively blocks maintenance of long-term facilitation.
Fig. 5.
Inhibition of Ap-eEF1A prevents the late but not the early phase of long-term facilitation. EPSPs were recorded from cultured motor neurons. Sensory neurons were injected with antibodies or oligonucleotides or were uninjected. Changes (%) in EPSP amplitude (mean ± SEM) are shown as bar graphs. (A) EPSPs were recorded at 24 h after five 5-min pulses of 5-HT. Anti-eEF1A antibody (Ab) inhibited the early phase if injected 1 h before 5-HT treatment but not at 6 or 12 h after treatment with 5-HT. Injection of preimmune (Pre) serum had no effect. eEF1A antisense oligonucleotide (AS) also had no effect on the induction of long-term facilitation at 24 h if injected 6 h after the 5-HT treatment (**, P < 0.01, ANOVA and Neuman-Keul's multiple range test). (B) EPSPs were recorded at 72 h after five 5-min pulses of 5-HT. Late long-term facilitation was completely blocked when anti-eEF1A antibody was injected 1 h before and 6 h after the 5-HT application. Fully expressed late long-term facilitation was seen with anti-eEF1A antibody injected 12 h after the 5-HT treatment. Injection of preimmune serum had no effect. Injection of eEF1A antisense oligonucleotides at 6 h after the 5-HT treatment also completely blocked late long-term facilitation (*, P < 0.05). (C) EPSPs were recorded at 10 min (short-term facilitation) after one 5-min pulse of 5-HT. Neither the anti-eEF1A antibody nor the antisense oligonucleotide had any effect on short-term facilitation (1 × 5-HT). Preimmune serum and scrambled oligonucleotide (Scr) also had no effect (**, P < 0.01).
Discussion
The long-term synaptic plasticity required for memory storage depends on synthesis of new gene products. Most of the genes related to long-term memory so far identified encode two general classes of regulatory proteins: protein kinases (Ca/calmodulin-dependent protein kinase II, protein kinase A, mitogen-activated protein kinase, PKC) and transcription factors (CREB, C/EBP, FOS) (26, 27). Here we show that induction of eEF1A is essential for late-phase long-term facilitation. The transport of eEF1A mRNA to nerve terminals suggests that the local expression of the translation factor is essential for maintaining newly grown synapses.
Ap-eEF1A is induced by 5-HT as a late gene. Because it can also be stimulated by 8-Br-cAMP, this induction is likely to be part of the cascade of gene expression activated by CREB1 (28). Induction of eEF1A occurs during a second wave of transcription and translation that takes place 6-12 h after the stimulation by 5-HT. We showed that proteins synthesized during this period are crucial for maintaining, but not for inducing, long-term facilitation. Thus late gene expression is needed for consolidating the facilitation and stabilizing new synapses. Consistent with this idea, inhibiting eEF1A blocked the late phase of long-term facilitation (measured at 72 h) but not the early phase (measured at 24 h). In other types of cells, increased expression of eEF1A is characteristic of growth and proliferation (29, 30). Casadio et al. (14) found that late-phase synaptic facilitation and formation of new synapses requires local protein synthesis at terminals of sensory neurons.
Ap-eEF1A is an abundant protein. Why then would it need to be induced further? During long-term facilitation of sensory-motor neuron synapses the number of presynaptic varicosities almost doubles (31). The newly synthesized eEF1A could operate locally to produce the substantial amounts of actin and other cytoskeletal proteins needed for new synapses (32). It is possible that eEF1A may also serve more than one function. In addition to promoting the elongation of nascent polypeptide chains, eEF1A possesses actin filament binding and bundling activities (16); it also interacts with CAP (adenylyl cyclase-associated protein) (33), a protein identified in yeast (34), Drosophila, and mammals (35). A local increase in eEF1A might also modulate the activity-dependent alteration of the actin cytoskeleton in synapses after 5-HT stimulation (36).
An important observation is that Ap-eEF1A mRNA is induced when 5-HT is applied to the cell body but that the mRNA moves into neurites only if the synaptic sites present on these neurites are also stimulated. This activity-dependent transport of Ap-eEF1A mRNA might explain why the changes related to long-term plasticity at synapses disappear after 24 h if the activation is restricted to the cell body. This selective targeting would permit transport of mRNAs to activated synapses for translation as needed to form new synapses. Steward et al. (37) showed that Arc is accumulated selectively only in areas of hippocampus containing previously activated dendrites of granule cells. Transport of Arc depends on the activation of synaptic N-methyl-d-aspartate glutamate receptors (38). Similarly, Thomas et al. (39) showed that transcripts of Ca/calmodulin-dependent protein kinase II accumulate in distal dendrites after the induction of long-term potentiation.
An important question in the molecular studies of memory storage is: How can enduring changes in synaptic strength be achieved at some nerve terminals but not at others if transcription is cellwide? One way of achieving this selectivity would be to synthesize proteins only at those synapses that have been stimulated (see refs. 3 and 7). mRNAs encoding structural (N-actin, tubulin, Arc) and functional components (Ca/calmodulin-dependent protein kinase II, mGluR1) of synapses have been found both in dendrites of mouse hippocampal neurons (5, 11, 40) and neurites of sensory neurons of Aplysia (12, 41). The presence of a polypyrimidine tract (5′ TOP) in the 5′ UTR of Ap-eEF1A mRNA provides support for the idea that 5-HT might activate the synthesis of Ap-eEF1A protein at synapses. 5′ TOP mRNAs can be regulated by the rapamycin-sensitive S6 kinase signaling pathway, which is activated by 5-HT (42). This mechanism of regulating local protein synthesis is also consistent with the observation that rapamycin-sensitive local protein synthesis is required to maintain late-phase facilitation in Aplysia (14) and for the late phase, but not the early phase, of long-term potentiation in hippocampal slices (43). eEF1A may therefore be critical in determining the synapse specificity of memory storage.
Supplementary Material
Acknowledgments
We thank Kelsey Martin for help with immunocytochemistry and Christopher Bonsignore, Mireille Valbrun, and Huixiang Zhu for technical assistance. This work was supported by Compagnia di San Paolo (M.G.), a Human Frontier Science Program long-term fellowship (to M.G.), the Howard Hughes Medical Institute (E.R.K.), and National Institute of Mental Health Grant MH48850 (to J.H.S.).
Abbreviations: eEF1A, eukaryotic translation elongation factor 1α; Ap-eEFIA, Aplysia eEF1A; 5-HT, serotonin; EPSP, excitatory postsynaptic potential.
References
- 1.Kandel, E. R. & Schwartz, J. H. (1982) Science 218, 433-443. [DOI] [PubMed] [Google Scholar]
- 2.Kandel, E. R. (2001) Science 294, 1030-1038. [DOI] [PubMed] [Google Scholar]
- 3.Martin, K. C., Barad, M. & Kandel, E. R. (2000) Curr. Opin. Neurobiol. 10, 587-592. [DOI] [PubMed] [Google Scholar]
- 4.Jiang, C. & Schuman, E. M. (2002) Trends Biochem. Sci. 27, 506-513. [DOI] [PubMed] [Google Scholar]
- 5.Kiebler, M. A. & DesGroseillers, L. (2000) Neuron 25, 19-28. [DOI] [PubMed] [Google Scholar]
- 6.Mohr, E. & Richter, D. (2000) J. Neurocytol. 29, 783-791. [DOI] [PubMed] [Google Scholar]
- 7.Steward, O. & Schuman, E. M. (2001) Annu. Rev. Neurosci. 24, 299-325. [DOI] [PubMed] [Google Scholar]
- 8.Miyashiro, K., Dichter, M. & Eberwine, J. (1994) Proc. Natl. Acad. Sci. USA 91, 10800-10804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Racca, C., Gardiol, A. & Triller, A. (1997) J. Neurosci. 17, 1691-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace, C. S., Lyford, G. L., Worley, P. F. & Steward, O. (1998) J. Neurosci. 18, 26-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Job, C. & Eberwine, J. (2001) Nat. Rev. Neurosci. 2, 889-898. [DOI] [PubMed] [Google Scholar]
- 12.Martin, K. C., Casadio, A., Zhu, H., Yaping, E., Rose, J. C., Chen, M., Bailey, C. H. & Kandel, E. R. (1997) Cell 91, 927-938. [DOI] [PubMed] [Google Scholar]
- 13.Sherff, C. M. & Carew, T. J. (1999) Science 285, 1911-1914. [DOI] [PubMed] [Google Scholar]
- 14.Casadio, A., Martin, K. C., Giustetto, M., Zhu, H., Chen, M., Bartsch, D., Bailey, C. H. & Kandel, E. R. (1999) Cell 99, 221-237. [DOI] [PubMed] [Google Scholar]
- 15.Sun, Z. Y. & Schacher, S. (1998) J. Neurosci. 18, 3991-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Condeelis, J. (1995) Trends Biochem. Sci. 20, 169-170. [DOI] [PubMed] [Google Scholar]
- 17.Montarolo, P. G., Goelet, P., Castellucci, V. F., Morgan, J., Kandel, E. R. & Schacher, S. (1986) Science 234, 1249-1254. [DOI] [PubMed] [Google Scholar]
- 18.Schacher, S. & Proshansky, E. (1983) J. Neurosci. 3, 2403-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyuhas, O. & Hornstein, E. (2000) in Translational Control of Gene Expression, eds. Sonenberg, N., Hershey, J. W. B. & Mathews, M. B. (Cold Spring Harbor Lab. Press, Plainview, NY), pp. 671-693.
- 20.Forscher, P. & Smith, S. J. (1988) J. Cell. Biol. 107, 1505-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alberini, C. M., Ghirardi, M., Metz, R. & Kandel, E. R. (1994) Cell 76, 1099-1114. [DOI] [PubMed] [Google Scholar]
- 22.Sun, Z. Y., Wu, F. & Schacher, S. (2001) J. Neurobiol. 46, 41-47. [DOI] [PubMed] [Google Scholar]
- 23.Hu, J. Y., Meng, Y. & Schacher, S. (2003) J. Neurosci. 23, 1804-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barzilai, A., Kennedy, T. E., Sweatt, J. D. & Kandel, E. R. (1989) Neuron 2, 1577-1586. [DOI] [PubMed] [Google Scholar]
- 25.Yanow, S. K., Manseau, F., Hislop, J., Castellucci, V. F. & Sossin, W. S. (1998) J. Neurochem. 70, 572-583. [DOI] [PubMed] [Google Scholar]
- 26.Alberini, C. M. (1999) J. Exp. Biol. 202, 2887-2891. [DOI] [PubMed] [Google Scholar]
- 27.Elgersma, Y. & Silva, A. J. (1999) Curr. Opin. Neurobiol. 9, 209-213. [DOI] [PubMed] [Google Scholar]
- 28.Bartsch, D., Ghirardi, M., Casadio, A., Giustetto, M., Karl, K. A., Zhu, H. & Kandel, E. R. (2000) Cell 103, 595-608. [DOI] [PubMed] [Google Scholar]
- 29.Merrick, W. C. & Nyborg, J. (2000) in Translational Control of Gene Expression, eds. Sonenberg, N., Hershey, J. W. B. & Mathews, M. B. (Cold Spring Harbor Lab. Press, Plainview, NY), pp. 89-125.
- 30.Proud, C. (2000) in Translational Control of Gene Expression, eds. Sonenberg, N., Hershey, J. W. B. & Mathews, M. B. (Cold Spring Harbor Lab. Press, Plainview, NY), pp. 719-739.
- 31.Bailey, C. H., Bartsch, D. & Kandel, E. R. (1996) Proc. Natl. Acad. Sci. USA 93, 13445-13452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noel, F., Nunez-Regueiro, M., Cook, R., Byrne, J. H. & Eskin, A. (1993) Brain Res. Mol. Brain Res. 19, 203-210. [DOI] [PubMed] [Google Scholar]
- 33.Yanagihara, C., Shinkai, M., Kariya, K., Yamawaki-Kataoka, Y., Hu, C. D., Masuda, T. & Kataoka, T. (1997) Biochem. Biophys. Res. Commun. 232, 503-507. [DOI] [PubMed] [Google Scholar]
- 34.Zelicof, A., Gatica, J. & Gerst, J. E. (1993) J. Biol. Chem. 268, 13448-13453. [PubMed] [Google Scholar]
- 35.Baum, B., Li, W. & Perrimon, N. (2000) Curr. Biol. 10, 964-973. [DOI] [PubMed] [Google Scholar]
- 36.Hatada, Y., Wu, F., Sun, Z. Y., Schacher, S. & Goldberg, D. J. (2000) J. Neurosci. 20, RC82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steward, O., Wallace, C. S., Lyford, G. L. & Worley, P. F. (1998) Neuron 21, 741-751. [DOI] [PubMed] [Google Scholar]
- 38.Steward, O. & Worley, R. F. (2001) Neuron 30, 227-240. [DOI] [PubMed] [Google Scholar]
- 39.Thomas, K. L., Laroche, S., Errington, M. L., Bliss, T. V. & Hunt, S. P. (1994) Neuron 13, 737-745. [DOI] [PubMed] [Google Scholar]
- 40.Kuhl, D. & Skehel, P. (1998) Curr. Opin. Neurobiol. 8, 600-606. [DOI] [PubMed] [Google Scholar]
- 41.Schacher, S., Wu, F., Panyko, J. D., Sun, Z. Y. & Wang, D. (1999) J. Neurosci. 19, 6338-6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan, A., Pepio, A. M. & Sossin, W. S. (2001) J. Neurosci. 21, 382-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang, S. J., Reis, G., Kang, H., Gingras, A. C., Sonenberg, N. & Schuman, E. M. (2002) Proc. Natl. Acad. Sci. USA 99, 467-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hegde, A. N., Inokuchi, K., Pei, W., Casadio, A., Ghirardi, M., Chain, D. G., Martin, K. C., Kandel, E. R. & Schwartz, J. H. (1997) Cell 89, 115-126. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz, J. H. & Swanson, M. E. (1987) Methods Enzymol. 139, 277-290. [DOI] [PubMed] [Google Scholar]
- 46.Hegde, A. N., Broome, B. M., Qiang, M. & Schwartz, J. H. (2000) Brain Res. Mol. Brain Res. 76, 424-428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.