Abstract
Spermatogenesis is thought to critically depend on the high intratesticular testosterone (T) levels induced by gonadotropic hormones. Strategies for hormonal male contraception are based on disruption of this regulatory mechanism through blockage of gonadotropin secretion. Although exogenous T or T plus progestin treatments efficiently block gonadotropin secretion and suppress testicular T production, only ≈60% of treated Caucasian men reach contraceptive azoospermia. We now report that in luteinizing hormone receptor knockout mice, qualitatively full spermatogenesis, up to elongated spermatids of late stages 13-16, is achieved at the age of 12 months, despite absent luteinizing hormone action and very low intratesticular T (2% of control level). However, postmeiotic spermiogenesis was blocked by the antiandrogen flutamide, indicating a crucial role of the residual low testicular T level in this process. The persistent follicle-stimulating hormone action in luteinizing hormone receptor knockout mice apparently stimulates spermatogenesis up to postmeiotic round spermatids, as observed in gonadotropin-deficient rodent models on follicle-stimulating hormone supplementation. The finding that spermatogenesis is possible without a luteinizing hormone-stimulated high level of intratesticular T contradicts the current dogma. Extrapolated to humans, it may indicate that only total abolition of testicular androgen action will result in consistent azoospermia, which is necessary for effective male contraception.
The circulating gonadotropins and intratesticular androgens and paracrine factors form the regulatory network of development and function of the male reproductive organs and functions, including spermatogenesis (1). Spermatogenesis is a complex cyclic process, where germ cells undergo mitotic divisions (spermatogonia), meiosis (spermatocytes), and morphological differentiation (spermatids, spermiogenesis) in a delicately regulated spatiotemporal fashion in the seminiferous epithelium. The production of an appropriate number of spermatozoa is considered to depend critically on stimulation of the testes by the two pituitary gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) (1).
The mechanisms by which LH and FSH regulate spermatogenesis continue to be of interest in research on male reproductive endocrinology, infertility, and contraception. The need for LH and FSH in the maintenance of normal spermatogenesis remains a matter of debate (1-3), with different interpretations arising from different experimental models. LH receptor (LHR) is expressed in Leydig cells, and, on ligand binding, it stimulates their steroidogenesis (4). It remains a dogma that the high intratesticular level of T is indispensable for the onset, maintenance, and completion of spermatogenesis in the adult testis and for its restoration after experimentally induced azoospermia.
Numerous studies have reported that both FSH and T are required for quantitatively and qualitatively normal spermatogenesis in a variety of mammalian species and for the initiation of this process at puberty. The current contention is that FSH stimulates the early events in spermatogenesis, including spermatogonial proliferation and meiosis, but only T is able to sustain complete spermatid differentiation (2). However, the recent studies on knockout mice for FSHβ subunit and FSHR and on men with inactivating FSHR mutation indicate that spermatogenesis can occur, and fertility can be maintained, in the absence of FSH action (5). Why all three men so far identified with inactivating FSHβ mutation (5, 6) are azoospermic remains a conundrum in light of the other information. Hence, many details about the exact role of gonadotropins in spermatogenesis still remain unclear. In light of the current knowledge, it may be concluded that FSH stimulation is not obligatory for the maintenance of spermatogenesis, but it is premature to state that LH action alone is sufficient and essential. Information about the role of LH signaling in testicular function can now be obtained from genetically modified mice with the function of this hormone selectively abolished (7).
We have recently generated the LHR knockout (LuRKO) mouse by inactivating, through homologous recombination, exon 11 of the LHR gene (7). LuRKO males and females are born phenotypically normal, but postnatally their testicular growth and descent and external genital and accessory sex-organ maturation are blocked. To better understand the role of LHR signaling in male reproductive systems, we investigated spermatogenesis in LuRKO mice that were up to 12 months of age. Although spermatogenesis was arrested at round-spermatid stages 7-8 in LuRKO mice at the age of 2 months, to our surprise, at 12 months, spermatogenesis was completed up to elongating spermatids at late stages 13-16, when the spermatids are ready for spermiation. This finding is direct evidence that LHR signaling may not be essential to maintain sufficient intratesticular T level for the completion and maintenance of spermatogenesis. The finding has important implications into the development of hormonal contraceptive methods for men.
Materials and Methods
Generation of LuRKO Mice. The strategy used for targeted disruption of the LHR gene (LuRKO mice) with preliminary description of their phenotype has been presented (7). In brief, exon 11 of the mouse LHR was replaced by the neo gene, which resulted in complete loss of LHR function. The embryonic stem cells were on the 129/SvEv strain, blastocycts of the C57BL/6J strain. Subsequent breedings occurred with C57BL/6J mice, and mice of generations F2 and F3, genotyped by PCR on tail DNA, were used for the experiments. All animal procedures were carried out in accordance with the institutional animal-care policy of the University of Turku.
Flutamide Treatment. Homozygous LHR-/- mice at the age of 9 months were anesthetized with 2% Avertin (2,2,2-tribromoethanol, Sigma-Aldrich), and a flutamide pellet was inserted subdermally (7 mg per pellet, 90-day release, Innovative Research of America) according to the manufacturer's instructions. Age-matched control LuRKO mice were treated with empty pellets. After 80 days of treatment, the mice were killed; the testes removed and fixed in 4% paraformaldehyde for histological analysis (see below).
Tissue Collection and Histology. LHR+/+ and LHR-/- mice were obtained from the same colony at 2 and 12 months of age and subjected to Avertin anesthesia. Blood was collected by cardiac puncture, and serum was stored at -20°C until used. The testes were dissected out and fixed in 4% paraformaldehyde at 4°C for 12 h, dehydrated, embedded in paraffin, and sectioned at a thickness of 5 μm. Sections were stained with Harris' hematoxylin and eosin (BDH). For tubular diameter measurement and mature spermatid calculation, cross-sections were cut from the middle of the testis. Tubular diameters were measured across their shortest axis from cross-sectional profiles under a ×10 objective, and mature spermatids were counted under a ×20 objective, by using a IM1000 microscope (Leica, Heerbrugg, Switzerland). Reproducibility of the morphological data was verified by similar findings in at least three different animals.
HPLC Analysis. Testis samples were weighed and homogenized in 500 μl of 0.01 M PBS. An aliquot of 420 μl of the homogenate was extracted twice with 2 ml of diethyl ether, evaporated under N2 to dryness, and reconstituted in 60 μl of acetonitrile/water (50:50, vol/vol). A 50-μl aliquot was taken for HPLC analysis (Waters) by using an acetonitrile/water (48:52, vol/vol) solution as mobile phase in a Symmetry C18 reverse-phase chromatography column (3.9 × 150 mm). The flow speed was 1 ml/min, and 1-min fractions were collected between 0 and 20 min. The acetonitrile/water solution was evaporated by SpeedVac (Hetovac, RV-1, Laurel, MD), the samples were dissolved in 60 μl of PBS, and their T levels were measured by standard RIA.
Forskolin Stimulation Test. Testicular samples of 12-month-old wild-type (wt) and LuRKO mice were decapsulated and cut into slices of 8-10 mg. The slices were then incubated in 2 ml of DMEM/F12 medium (1:1) (GIBCO/BRL), plus 10% FCS and 0.1 g/liter of gentamycin (Biological Industries, Bet-Haemek, Israel) in a shaking water bath at 32°C in 5% CO2/95% O2. After a preincubation of 30 min, the medium was replaced either by fresh medium (DMEM/F12) or a medium containing 5 μmol/liter of forskolin (Sigma). After an incubation of 4 h, the media were collected for T measurement by RIA, and the testicular slices were weighed. The concentration of T in the media was expressed per gram of tissue.
Hormone Measurements. Serum LH and FSH concentrations were determined by immunofluorometric assays (8, 9). Intratesticular T was determined by homogenizing one LHR-/- testis and a weighed aliquot of an LHR+/+ testis in 0.5 ml of PBS. The homogenates, sera, and culture media were extracted twice with 2 ml of diethyl ether, evaporated, and reconstituted in 200 μl of PBS for T measurement by RIA. Protein concentrations of the homogenates were determined by using the Bradford method.
Immunohistochemistry. Five-micrometer sections of the testicular samples were rehydrated, washed twice in 10 mM Tris·HCl (pH 8.0)/100 mM NaCl (TBS), and subjected to microwave antigen retrieval at 700 W for 15 min in 10 mM sodium citrate solution (pH 6.0). After two washes with TBS, an aliquot of 50 μl of blocking solution (TBS containing 1% BSA and 3% normal horse serum) was applied on each section and incubated for 1 h at room temperature. After blocking, 50-μl aliquots of primary antibody, diluted in TBS containing 1% BSA, were applied and incubated overnight at 4°C. Thereafter, the slides were washed in PBS and incubated with the anti-rabbit secondary antibody (Vector Laboratories); positive cells were visualized by using the Vectastain Elite kit (Vector Laboratories) as instructed by the manufacturer. The antibody against protamine was kindly donated by R. Balhorn (Lawrence Livermore National Laboratory, Livermore, CA).
RNA Isolation and RT-PCR Analyses of 17β-Hydroxysteroid Dehydrogenase (HSD) III and Thrombospondin (TSP) 2. Total RNA was isolated from the testicular samples by using the single-step extraction method as described (10). The quality of extracted RNA was determined by spectrophotometry. For detection of the 17β-HSD III and TSP2 mRNAs, the RT reaction and PCR were performed sequentially in the same tube with specific primer pairs: 5′-CCTGCGATCAATGGGACAATG-3′ (sense) and 5′-GCTGCGCCTGAAGAAATA-3′ (antisense) for mouse 17β-HSD III (434 bp) (11) and 5′-CTGGGCATAGGGCAAGAGCTTCT-3′ (sense) and 5′-AGGCCCACATCCTCCAGGAA-3′ (antisense) for mouse TSP2 (404 bp) (12). Two micrograms of total RNA from the mouse testes were mixed with the PCR reaction buffer [10 mmol/liter Tris·HCl (pH 8.3)/1.5 mmol/liter MgCl2/50 mmol/liter KCl/100 μmol/liter deoxy-NTPs and 1 μmol/liter each primer] and 40 units of RNase inhibitor (RNasin, Promega) in a final volume of 50 μl. After the addition of avian myeloblastosis virus reverse transcriptase (12.5 units, Promega) and Tag DNA polymerase (2.5 units, Promega), the samples were placed in a thermal cycle at 50°C for 10 min (reverse transcriptase reaction), followed by denaturation at 97°C for 3 min, then run for 40 PCR cycles (at 96°C for 1 min, 57°C for 1.5 min, and 72°C for 2 min, with final extension for 10 min at 72°C). Liquid controls were run in parallel with the testicular samples. PCR-generated DNA fragments were resolved in Tris-borate-buffered 1.5% agarose gels and visualized by ethidium bromide staining.
Statistical Analysis. The statview program (windows Version 4.57, Abacus Concepts, Berkeley, CA) was used for ANOVA and Student's t tests. Significance was set as P < 0.05. The values are presented as mean ± SEM.
Results
Testicular Phenotype of the LuRKO Mice. At birth the LuRKO and wt testes were indistinguishable (not shown). At 2 months of age, the weights of the LuRKO testes were ≈17% of those of wt animals (16.1 ± 0.5 vs. 94.5 ± 4.1 mg), but at 12 months they had increased to ≈27% of the wt controls (32.5 ± 2.0 vs. 117.3 ± 2.3 mg); the knockout mouse testes remained cryptorchid at all ages. The accessory reproductive organs, e.g., seminal vesicles, epididymides, and prostates, were invisible at all ages in the LuRKO mice.
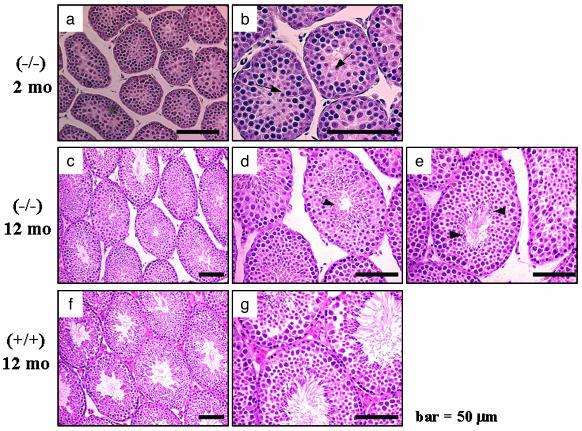
Histological examination of the LuRKO mouse testes at 2 months revealed that the number and size of Leydig cells were dramatically decreased as compared with wt mice (Fig. 1). The seminiferous tubules were narrower at each age (Table 1), and spermatogenesis was arrested at the round-spermatid stage 7-8 at 2 months (Fig. 1 a and b). No elongated spermatids were seen, suggesting that round spermatids did not progress to elongation and spermiogenesis at this age. Similarly, at the age of 12 months, the number and size of Leydig cells remained markedly decreased. In contrast, the tubular diameter was clearly widened (Table 1), and spermatogenesis progressed further, until elongated spermatids at the late stages 13-16 (Fig. 1 c-e), indicative of complete spermiogenesis.
Fig. 1.
Representative light micrographs of testis sections from homozygous LHR-/- (-/-) and wt control (+/+) mice. Samples were taken at the ages of 2 months (a and b) and 12 months (c-e) and from control LHR+/+ mice at 12 months (f and g). b, d, e, and g are views of a, c, and f at higher magnification. Arrows and arrowheads indicate round spermatids and elongated spermatids, respectively. mo, month.
Table 1. Tubular diameters and mature spermatids in 2- and 12-month-old LHR+/+ and LHR−/− mice.
| Age (mice) | Tubular diameter, μm | Mature spermatids, % of total tubules | Tubules calculated per specimen, n |
|---|---|---|---|
| 2 mo (LHR−/−) | 93.6 ± 0.44* | 600-650 | |
| 2 mo (LHR+/+) | 158.6 ± 0.83 | 500-550 | |
| 12 mo (LHR−/−) | 124.4 ± 0.78* | 58.7 ± 4.0** | 580-650 |
| 12 mo (LHR+/+) | 168.1 ± 0.82 | 97.6 ± 0.3 | 500-550 |
P < 0.05
P < 0.001 (compared with the same age of LHR+/+ group). n = 3-4 mice per group.
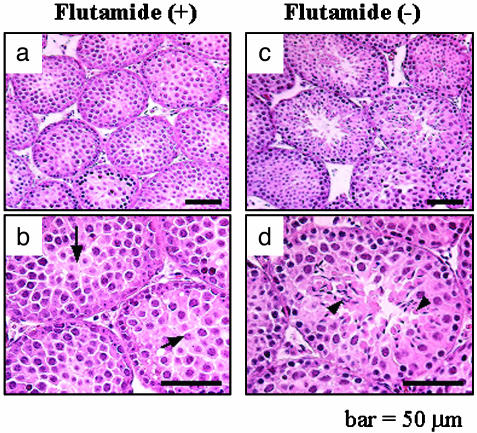
Testicular histology of LuRKO mice treated with flutamide between 9 and 12 months of age showed that spermatogenesis was developed until round spermatids (Fig. 2 a and b), in comparison with control LHR-/- mice of the same age in which spermatogenesis had developed until elongated spermatids (Fig. 2 c and d), indicating that T plays a vital role in the late stage of spermatogenesis, from round to elongated spermatids, and the low T levels in LHR-/- mice are sufficient for spermiogenesis.
Fig. 2.
Representative light micrographs of testis sections from LuRKO mice treated with flutamide. (a and b) Testicular histology from LuRKO mice treated with flutamide for 80 days at the age of 12 months. (c and d) Testicular histology from age-matched, nontreated LuRKO mice. b and d are similar to a and c but at a higher magnification. Arrows and arrowhead indicate round spermatids and elongated spermatids, respectively.
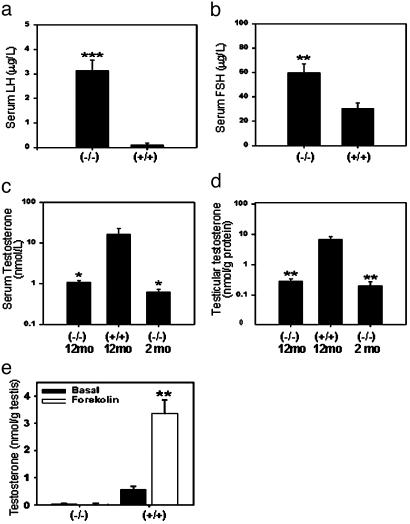
Hormone Levels. The elevation in serum LH and FSH levels of male LuRKO mice has been reported at the age of 2 months (10), and the animals in the current study exhibited similar alterations in these levels. At 12 months, serum LH was dramatically increased in LHR-/- males, 3.14 μg/liter vs. 0.10 μg/liter in wt mice (Fig. 3a). The FSH levels were increased ≈2-fold in the LHR-/- mice (Fig. 3b). Serum and testicular T levels of the LHR-/- mice were similarly decreased, by >90%, at both ages (Fig. 3 c and d).
Fig. 3.
Serum gonadotropins, serum and intratesticular T, and in vitro production of T on forskolin stimulation in testis slices of control (LHR+/+) and LuRKO (LHR-/-) male mice at the age of 12 months (plus T levels in 2-month-old LuRKO mice). (a) Serum LH levels. (b) Serum FSH levels. (c) Serum T levels. (d) Testicular T level. (e) Basal and forskolin-stimulated T production in vitro. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 [compared with (+/+) groups or basal level (b)]. mo, month; n = 7-10 per group.
The testicular extracts were fractionated by HPLC, and individual fractions were analyzed for T immunoreactivity. The majority of the immunoreactivity was eluted at the elution time of T standard, also from the LuRKO testes (data not shown). Therefore, the T levels measured in the ether-extracted but unfractionated samples represent genuine T immunoreactivity.
Forskolin (FK) Stimulation of T Production in Testis Slices. The basal and FK-stimulated rates of T production were measured at 4 h of incubation of the testis slices of LHR-/- and wt mice at the age of 12 months (Fig. 3e). The basal and FK-stimulated T levels were very low, or even undetectable, in the LHR-/- testes as compared with wt animals, producing basal and FK-stimulated T levels of 0.56 and 3.47 nmol/g testis tissue, respectively.
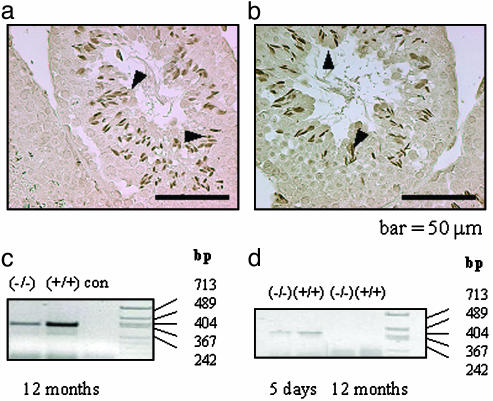
Immunohistochemical Staining for Protamine. To further verify the presence of mature elongated spermatids in the LuRKO mouse testes at the age of 12 months, immunohistochemical staining for protamine was carried out. Positive immunolabeling was detected in the elongated spermatids of both (+/+) and (-/-) testes at the age of 12 months (Fig. 4 a and b). This finding further confirmed the advancement of spermatogenesis to the elongated-spermatid stage in 12-month-old LuRKO mice.
Fig. 4.
Protamine protein and 17β-HSD III and TSP2 mRNA expression in the in 12-month-old LHR-/- and LHR+/+ testes (plus TSP2 at 5 days). Representative light micrographs of immunohistochemical staining for protamine are shown in the LHR-/- (a) and LHR+/+ (b) mouse testes at the age of 12 months. Arrowheads indicate elongated spermatids. (c) A representative RT-PCR analysis of 17β-HSD III mRNA expression at 12 months. (d) RT-PCR analysis of TSP2 mRNA at 5 days and 12 months in LHR-/- and LHR+/+ testes. con, liquid control; bp, lengths of molecular size markers.
The Expression of TSP2 and 17β-HSD III in Mouse Testes. The type of the Leydig cells in the 12-month-old testes was studied by RT-PCR, by assessing the expression of the fetal and adult Leydig cell markers, TSP2 and 17β-HSD III, respectively (13). The results clearly demonstrated that 17β-HSD III mRNA was expressed both in the LHR+/+ and LHR-/- testes at the age of 12 months (Fig. 4c). TSP2 mRNA was detected in both LHR+/+ and LHR-/- testes at 5 days of age, but not in the 12-month-old LHR+/+ or LHR-/- testes (Fig. 4d), indicating that the Leydig cells at the latter age were of the adult type both in LHR+/+ and LHR-/- testes.
Discussion
Spermatogenesis is a complex process by which the spermatogonia mature gradually to spermatozoa through a series of events involving mitoses, meiosis, and cellular differentiation. The existing literature has indicated for decades that the two gonadotropins, LH and FSH, along with the high intratesticular T concentration, are necessary in the regulation of this progress (2, 3). Acute T withdrawal depletes the testes of germ cells at specific stages of the seminiferous epithelial cycle, in particular, pachytene spermatocytes and spermatids at stages 7 and 8. The conversion of spermatids from stage 7 to 8 is considered intimately dependent on T (1, 14).
To understand better the hormonal regulation of spermatogenesis, it is essential to identify the specific effects of the hormones involved. The experimental approaches include classical animal models such as gonadotropin ablation by hypophysectomy, inhibition of gonadotropin-releasing hormone (GnRH) secretion or action by sex steroids (androgens, estrogens, and progestins) or GnRH analogs, or gonadotropin immunoneutralization (1-3). Other methods include Leydig cell destruction by the selective toxin, ethylene dimethanesulfonate, and the use of GnRH-deficient mutant mice (hpg) (15). The drawback of these methods is that they seldom bring about complete hormone abolition, and they equally affect both gonadotropins, thus making it impossible to study the functions of one of them in isolation. Moreover, the older hormonal replacement studies often remain inconclusive because of insufficient purity of the biological gonadotropin preparations used. Genetically modified mouse models are now available to address the numerous controversies and uncertainties that still remain about the hormonal control of spermatogenesis. The specific role of FSH can be studied by using FSHβ subunit (16) or FSHR knockout mice (17) and that of LH by using LHR knockout mice (7). Also numerous human mutations of the same genes have been described recently (5). The key role of LH as the stimulus of testicular production of T has not been challenged even in light of the newest information.
The aim of the present study was to revisit the role and importance of LHR signaling and the high intratesticular level of T in spermatogenesis by using the LuRKO mouse model. Our results indicate that spermatogenesis is arrested at the round-spermatids stages 7-8 in these mice at the age of 2-3 months. Compared with the hpg mice (15), in which spermatogenesis is arrested at the diplotene stage (before meiosis), the process in LuRKO mice was more advanced, i.e., up to completion of meiosis. A very similar finding was made recently with a transgenic mouse expressing FSH at the hpg background (18); these animals had very low LH and, like LuRKO mice, very low T but somewhat elevated FSH levels. Hence, FSH alone is apparently able to drive spermatogenesis through meiosis but not further. Full spermatogenesis appears in hpg mice when they are subjected to high-dose androgen replacement (15). Hence, the current data indicate that meiosis can be stimulated by either FSH or androgen, but the postmeiotic maturation is strictly androgen-dependent.
To our surprise, the analysis of spermatogenesis in LuRKO testes at the age of 12 months showed that it had developed further, until elongated spermatids at late stages 13-16, indicating completion of spermiogenesis even without the LH/LHR signaling and without high interatesticular T. In addition to morphological criteria, we used protamine as a marker of spermatid maturation in the LuRKO testes. The mRNA of protamine is stored as translationally repressed free-mRNAs in round spermatids and is actively translated on polysomes of elongated spermatids (19). Its transcription is thus restricted to meiotic and/or postmeiotic cells (20). Similar positive immunostaining for protamine in the elongated spermatids of wt and LuRKO testes at 12 months of age provided further evidence for the completion of spermatogenesis in the LuRKO mouse testes, even in the presence of dramatically reduced serum and intratesticular levels of T (≈2% of control level).
We next wanted to clarify whether the completion of spermiogenesis in the 12-month-old LuRKO testes was totally independent of androgens or driven by their low residual levels. The mice were treated with flutamide between 9 and 12 months of age to eliminate the remaining androgen stimulus. Testicular histology of the flutamide-treated LuRKO mice showed spermatogenesis that was arrested at round spermatids in contrast to elongating spermatids in nontreated LuRKO mice. This finding prompted the surprising conclusion that very low gonadotropin-independent T production may be sufficient to stimulate spermatogenesis. Maintenance of spermatogenesis has been shown with low doses of exogenous T in gonadotropin-depleted animals (21). However, the current data are the first demonstration that the very low endogenous level of T that is produced without LH stimulation is sufficient for the completion of spermatogenesis. Because serum FSH is about 2-fold elevated in the LuRKO mice, it is possible that FSH sensitizes spermatogenesis to the low androgen levels, but the completion of this process is clearly not an FSH effect, because it was abolished by antiandrogen. The role of FSH priming in this response remains unclear, because treatment of gonadotropin-deficient hpg mice with high doses of T alone can induce normal spermatogenesis (15).
Both morphological and endocrine analyses showed that the adult LuRKO testes have only rudimentary Leydig cells in the interstitial compartment. These cells do express low levels of steroidogenic enzymes (F.-P.Z., T.P., F. Zhu, M.P., and I.H., unpublished data) and maintain low T concentration in the testis tissue. However, the in vitro incubations with forskolin stimulation, to bypass the missing LH action, showed that the steroidogenic capacity is very low. We then asked whether the remaining Leydig cells represented persistence of cells of the fetal population, known to be able to develop and function without LH action (22), or whether they were poorly differentiated adult Leydig cells. A recent study by O'Shaughnessy et al. (13) indicated that TSP2 gene is a marker of fetal Leydig cells, and 17β-HSD III is exclusively expressed in adult-type Leydig cells. Our data demonstrated that the Leydig cells present in 12-month-old LuRKO testes expressed 17β-HSD III but not TSP2, indicating that they are of the adult type. Hence, our findings provide evidence that the initiation of development and partial differentiation of adult Leydig cells is possible in the absence of LH/LHR signaling.
In conclusion, our study provides evidence that LH/LHR signaling and a high intratesticular T level are not obligatory for qualitatively complete spermatogenesis. However, the very low residual level of T in the absence of LH signaling is crucial for the postmeiotic stage of spermatogenesis, from round to elongated spermatids. The persistent FSH action in the LuRKO mice is not able to compensate for the low level of T that seems to be critical for the completion of spermatogenesis, as demonstrated by the flutamide-induced block of this process. On the basis of these findings, a constitutive component of spermatogenesis seems to exist that appears to be independent of the stimulating effect of high physiological levels of gonadotropins and T. This maturational process apparently needs long priming, because it was observed only in relatively old mice. The spermatogenesis observed is merely a “proof of principle,” because the aged LuRKO males remain infertile because of the low circulating androgen levels and permanent underdevelopment of their accessory sex glands and ducts (7).
Conspicuously, the LuRKO testes remained cryptorchid throughout the whole life span. Cryptorchid mice with normal gonadotropin and intratesticular T levels show complete absence of postmeiotic germ cells (23). Why the cryptorchid LuRKO testes showed more advanced spermatogenesis is an intriguing question. The normal intratesticular T in the presence of the testicular insult caused by the elevated intra-abdominal temperature may in fact be harmful for the spermatogenesis in cryptorchidism. The situation would be analogous to the one where the suppression of testicular T level clearly improves the recovery of spermatogenesis after cytotoxic or irradiation injury (24). Similar effects of lowering intratesticular T levels by GnRH agonist treatment have been found in cryptorchid rats after orchiopexy (25).
Our finding provides an important clinical perspective, because it may explain why it is difficult to achieve total azoospermia in men treated with T for contraceptive purposes (3). The approach of the contraceptive effect has been to suppress gonadotropin secretion by treatment with T alone or combined with other gonadotropin-suppressing agents. This leads to suppression of testicular T production, which then blocks spermatogenesis. The outcome of the hormonal contraceptive trials usually leaves 30-40% of the men oligozoospermic and potentially fertile, for an as yet unknown reason. The normal intratesticular T level decreases in men during antigonadotropic treatments (GnRH agonist or T) by ≈98%, but it still remains at about 50 nmol/liter (26, 27). This level is higher than the normal peripheral concentration and well within the range of stimulating the androgen receptor and possibly spermatogenesis. The latter effect is even likely, because the T levels in the LuRKO mouse testes, still capable of stimulating spermatogenesis, were >10-fold lower (2 nmol/liter). Regarding the men remaining oligozoospermic during contraceptive trials, either the residual intratesticular T level remains high enough to stimulate spermatogenesis, or individual variation occurs in the T sensitivity. Nevertheless, our findings indicate that spermatogenesis can be maintained by T levels that are clearly below the physiological range and produced constitutively without LH stimulation. For this very reason, the best contraceptive efficacy has been achieved in experimental and clinical trials by combining antigonadotropic treatment with antiandrogen, apparently to block effects of the residual intratesticular T (reviewed in ref. 3). Therefore, uniform suppression to azoospermia in male contraceptive trials may only be successful if total elimination of intratesticular T is achieved, and this goal may not be reached only by suppressing gonadotropin secretion.
Acknowledgments
We thank F. Zhu, T. Laiho, J. Vesa, N. Messner, and K. Huhtinen for skillful technical assistance. This work was supported by grants from the Academy of Finland, the Sigrid Jusélius Foundation, and the Wellcome Trust.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; HSD, hydroxysteroid dehydrogenase; LH, luteinizing hormone; LHR, LH receptor; LuRKO, LHR knockout; T, testosterone; TSP, thrombospondin; wt, wild-type.
References
- 1.Sharpe, R. (1994) in The Physiology of Reproduction, eds. Knobil, E. & Neill, J. (Raven, New York), 2nd Ed., pp. 1364-1434.
- 2.Plant, T. M. & Marshall, G. R. (2001) Endocr. Rev. 22, 764-786. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, R. A. & Baird, D. T. (2002) Endocr. Rev. 23, 735-762. [DOI] [PubMed] [Google Scholar]
- 4.Ascoli, M., Fanelli, F. & Segaloff, D. L. (2002) Endocr. Rev. 23, 141-174. [DOI] [PubMed] [Google Scholar]
- 5.Themmen, A. P. N. & Huhtaniemi, I. T. (2000) Endocr. Rev. 21, 551-583. [DOI] [PubMed] [Google Scholar]
- 6.Layman, L. C., Porto, A. L., Xie, J., da Motta, L. A., da Motta, L. D, Weiser, W. & Sluss, P. M. (2002) J. Clin. Endocrinol. Metab. 87, 3702-3707. [DOI] [PubMed] [Google Scholar]
- 7.Zhang, F.-P., Poutanen. M., Wilbertz, J. & Huhtaniemi, I. (2001) Mol. Endocrinol. 15, 172-183. [DOI] [PubMed] [Google Scholar]
- 8.Haavisto, A.-M., Pettersson, K., Bergendahl, M., Perheentupa, A., Roser J. & Huhtaniemi, I. (1993) Endocrinology 132, 1687-1691. [DOI] [PubMed] [Google Scholar]
- 9.van Casteren, J. I., Schoonen, W. G. & Kloosterboer, H. J. (2000) Biol. Reprod. 62, 886-894. [DOI] [PubMed] [Google Scholar]
- 10.Zhang, F.-P., Hämäläinen, T., Kaipia, A., Pakarinen, P. & Huhtaniemi, I. (1994) Endocrinology 134, 2206-2213. [DOI] [PubMed] [Google Scholar]
- 11.Mustonen, M. V., Poutanen, M. H., Isomaa, V. V., Vihko, P. T. & Vihko, R. K. (1997) Biochem. J. 325, 199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bornstein, P., Devarayalu, S., Li, P., Disteche, C. M. & Framson, P. (1991) Proc. Natl. Acad. Sci. USA. 88, 8636-8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Shaughnessy, P. J., Willerton, L. & Baker, P. J. (2002) Biol. Reprod. 66, 966-975. [DOI] [PubMed] [Google Scholar]
- 14.Parvinen, M., Vihko, K. K. & Toppari, J. (1986) Int. Rev. Cytol. 104, 115-151. [DOI] [PubMed] [Google Scholar]
- 15.Singh, J., O'Neill, C. & Handelsman, D. J. (1995) Endocrinology 136, 5311-5321. [DOI] [PubMed] [Google Scholar]
- 16.Kumar, T. R., Wang, Y., Lu, N. & Matzuk, M. M. (1997) Nat. Genet. 15, 201-204. [DOI] [PubMed] [Google Scholar]
- 17.Dierich, A., Sairam, M. R., Monaco, L., Fimia, G. M., Gansmuller, A., LeMeur, M. & Sassone-Corsi, P. (1998) Proc. Natl. Acad. Sci. USA 95, 13612-13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allan, C. M., Haywood, M., Swaraj, S., Spalviero, J., Koch, A., Jimenez, M., Poutanen, M., Levallet, J., Huhtaniemi, I., Illingworth, P. & Handelsman, D. J. (2001) Endocrinology 142, 2213-2220. [DOI] [PubMed] [Google Scholar]
- 19.Kleene, K. C. (2001) Mech. Dev. 106, 3-23. [DOI] [PubMed] [Google Scholar]
- 20.Lee, K., Fajardo, M. A. & Braun, R. E. (1996) Mol. Cell. Biol. 16, 3023-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLachlan, R. I., Wreford, N. G., Tsonis, C., De Kretser, D. M. & Robertson, D. M. (1994) Biol. Reprod. 50, 271-280. [DOI] [PubMed] [Google Scholar]
- 22.Pakarinen, P., Kimura, S., El-Gehani, F., Pelliniemi, L. J. & Huhtaniemi, I. (2002) Endocrinology 143, 4477-4482. [DOI] [PubMed] [Google Scholar]
- 23.Zimmermann, S., Steding, G., Emmen, J. M., Brinkmann, A. O., Nayernia, K., Holstein, A. F., Engel, W. & Adham, I. M. (1999) Mol. Endocrinol. 13, 681-691. [DOI] [PubMed] [Google Scholar]
- 24.Meistrich, M. L. & Shetty, G. (2003) J. Androl. 24, 135-148. [DOI] [PubMed] [Google Scholar]
- 25.Udagawa, K., Takeda, M., Hosaka, M., Kubota, Y. & Ogawa, T. (2002) J. Urol. 168, 1279-1283. [DOI] [PubMed] [Google Scholar]
- 26.Huhtaniemi, I., Parvinen, M., Nikula, H. & Rannikko, S. (1987) J. Androl. 8, 363-373. [DOI] [PubMed] [Google Scholar]
- 27.McLachlan, R. I., O'Donnell, L., Stanton, P. G., Balourdos, G., Frydenberg, M., de Kretser, D. M. & Robertson, D. M. (2002) J. Clin. Endocrinol. Metab. 87, 546-556. [DOI] [PubMed] [Google Scholar]