Abstract
Osmotic homeostasis is one of the most aggressively defended physiological parameters in vertebrates. However, the molecular mechanisms underlying osmotic regulation are poorly understood. The transient receptor potential channel, vanilloid subfamily (TRPV4), is an osmotically activated ion channel that is expressed in circumventricular organs in the mammalian CNS, which is an important site of osmotic sensing. We have generated trpv4-null mice and observed abnormalities of their osmotic regulation. trpv4-/- mice drank less water and became more hyperosmolar than did wild-type littermates, a finding that was seen with and without administration of hypertonic saline. In addition, plasma levels of antidiuretic hormone were significantly lower in trpv4-/- mice than in wild-type littermates after a hyperosmotic challenge. Continuous s.c. infusion of the antidiuretic hormone analogue, dDAVP, resulted in systemic hypotonicity in trpv4-/- mice, despite the fact that their renal water reabsorption capacity was normal. Thus, the response to both hyper- and hypoosmolar stimuli is impaired in trpv4-/- mice. After a hyperosmolar challenge, there was markedly reduced expression of c-FOS in the circumventricular organ, the organum vasculosum of the lamina terminalis, of trpv4-/- mice compared with wild-type mice. This finding suggests that there is an impairment of osmotic sensing in the CNS of trpv4-/- mice. These data indicate that TRPV4 is necessary for the normal response to changes in osmotic pressure and functions as an osmotic sensor in the CNS.
In vertebrate organisms, maintenance of an equilibrium of the internal environment is essential for viability, and osmotic equilibrium is aggressively defended against changes with a set-point value ranging between 280-300 milliosmol/kg in most mammals (295 in humans and rodents) (1, 2). Systemic osmotic pressure is maintained by feedback regulation in which neurons in the circumventricular organs, the organum vasculosum of the lamina terminalis (OVLT), and, perhaps, also the subfornical organ (SFO), sense osmotic pressure. These neurons in turn project to magnocellular neurons in supraoptic and paraventricular nucleus of the hypothalamus (3). Magnocellular neurons themselves possess intrinsic osmosensitivity and they synthesize antidiuretic hormone (ADH) and secrete it into the blood (1, 2, 4-9). The ADH directs free-water reabsorption in the collecting ducts of the kidney (10). With respect to the regulation of water intake behavior, CNS lesioning experiments implicate a role for the lamina terminalis that comprises the circumventricular organs, the OVLT and the SFO, and the median preoptic area (2, 5-7, 11-13).
Recently, we and others (14-17) have isolated, to our knowledge, a new vertebrate member of the transient receptor potential family, vanilloid subfamily (TRPV) of ion channels, the vanilloid receptor-related osmotically activated ion channel (VR-OAC), now named TRPV4. In heterologous expression systems, TRPV4, a nonselective cation channel, is activated by small changes in osmotic pressure (14, 15). In mammals, TRPV4 is expressed in the circumventricular organs, the OVLT and the SFO, and elsewhere (14, 17). We have found TRPV4 to be activated by hypotonicity and modulated by temperature, with peak sensitivity at the core body temperature of an organism, whereas other investigators report that TRPV4 is activated in heterologous expression systems by warm stimuli, and that this activation is augmented by hypotonicity (18, 19), as reviewed in ref. 20. In a related PNAS paper, we report that mammalian TRPV4 directs osmotic and nose-touch avoidance behaviors in the Caenorhabditis elegans TRPV mutant, osm-9, which is defective in avoidance of osmotic, nose touch, and repulsive odorant stimuli (21). The osm-9 ASH::trpv4 transgenic worms were rescued for their avoidance of hyperosmotic and mechanical stimuli but not for odorant avoidance. To add to our knowledge of the role of trpv4 in vivo, we have generated trpv4-null mice and observed abnormal osmotic regulation in these animals.
Methods
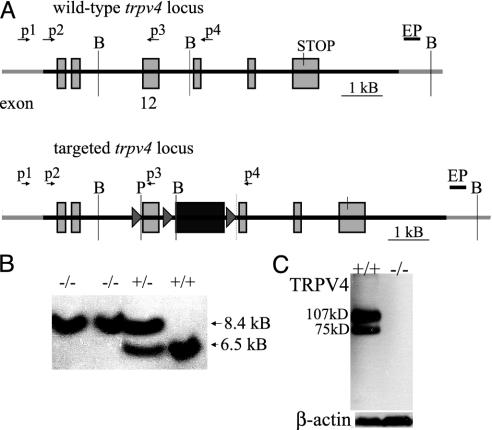
Gene Targeting and Generation of trpv4-/- Mice. Gene targeting and generation of the null allele were based on the cre-lox-mediated excision of exon 12 of the trpv4 gene, which codes for the pore-loop and adjacent transmembrane domain (17, 22). The trpv4-/- mice lacked trpv4 transcript by RT-PCR and TRPV4 protein by Western blot in kidney extract (Fig. 1 A-C). A total of 486 mice were investigated. Details are reported in Supporting Methods, which is published as supporting information on the PNAS web site.
Fig. 1.
Generation of trpv4-null mice. (A) Targeting of exon 12 of the trpv4 gene to obtain a null allele. The figure depicts the targeted trpv4 locus on mouse chromosome 5. The trpv4 gene is comprised of at least 15 exons. Exon 12 codes for the supposed pore-loop domain and the adjacent transmembrane domains 5 and 6. This exon was flanked by loxP genetic elements, and an adjacent neomycin selection cassette was inserted, followed by another loxP site. All loxP sites were in the same orientation. A BamHI site occurring in wild type after exon 12 was eliminated in the targeting construct. The neo cassette contained an upstream BamHI site. P, PacI site; EP, external probe used in genomic Southern blotting. (B) Southern blot of mouse tail genomic DNA after mating to EIIa-cre+ mice. Depicted here are homozygous null mice, a heterozygous mouse, and a homozygous wild-type mouse. The wild-type allele measures 6.5 kb, and the targeted allele measures 8.4 kb after excision of exon 12 and the neo selection marker, as in A. DNA was digested with BamHI, and the same external probe (see A) that was used for screening of embryonic stem cells was used. Genotyping was also performed with PCR by using primers p2 and p4. Wild-type mice had a 3-kb band, null mice a 1.6-kb band, and heterozygotes had both (results not shown). (C) Absence of TRPV4 protein by Western blotting. Kidney protein preparations were examined by Western blotting for TRPV4 and for β-actin. Note the absence of TRPV4 protein in trpv4-null mice. In the wild-type mice, there is the expected 107-kDa protein, as well as a shorter 75-kDa isoform, perhaps indicative of a yet undescribed splice variant.
Testing of Mouse Behavior and Physiology. We investigated mice for their response to osmotic and somatosensory stimulation. All investigations were performed in accordance with institutional and National Institutes of Health guidelines for the ethical treatment of mice. Wild-type littermates and nulls were age- and sex-matched.
To understand better how trpv4-null mice maintain their osmotic equilibrium, we investigated spontaneous drinking behavior and response to i.p. dipsogenic stimulation with 0.5 M NaCl of single-housed mice. We also measured systemic osmotic pressure in plasma with a vapor pressure osmometer (Wescor, Logan, UT), and plasma ADH with a commercially available RIA (Alpco, Windham, NH). We tested the response of the mice to continuous s.c. infusion of the ADH analogue, dDAVP (Sigma). The immediate early gene product cFOS was determined in the CNS of trpv4-null mice by immunocytochemistry. Details are reported in Supporting Methods.
To investigate the temperature homeostasis of trpv4-null mice in response to a cold stress, animals were exposed to a constant temperature of 4°C for 150 min in a cold room. Core body temperature was determined by using a precision thermometer and a YSI 4600 rectal probe (YSI Temperature, Dayton, OH).
Somatosensory stimulation was applied to mice to determine thresholds for mechanical and temperature stimuli in keeping with standardized protocols (23). Mechanical stimuli were applied with a Randall-Sellito paw pressure analgesy meter (Ugo Basile, Varese, Italy) and a Von-Frey paw apparatus (Ugo Basile). The paw pressure analgesy meter applies an increasing pressure to the hind paw. The Von-Frey apparatus applies a mechanical stimulus with a flexible steel wire from underneath the hind paw. The force leading to withdrawal was determined for both methods. In addition, paws were stimulated with heat from underneath applied by an infrared beam (Hargreave's test apparatus; Ugo Basile), and withdrawal latencies were recorded. cFOS protein expression in the spinal cord dorsal horn was determined by immunocytochemistry. Details are reported in Supporting Methods.
Results
To test the function of TRPV4 in mice, we induced a mutation into the trpv4 gene by gene targeting (see Fig. 1, and Figs. 5 and 6, which are published as supporting information on the PNAS web site). Mice lacking TRPV4 were viable and fertile and there were no obvious behavioral or physiological abnormalities.
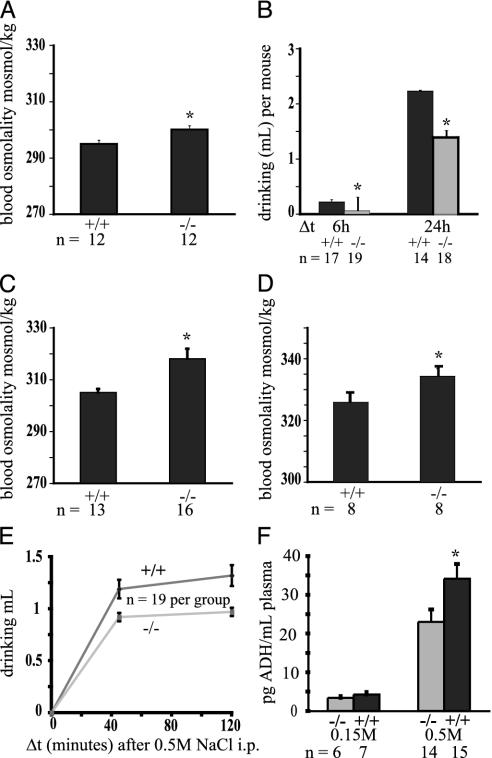
When housed in groups with free access to food and water, the osmolality and fluid intake of the mutant mice was indistinguishable from that of controls (295 vs. 295 milliosmol/kg; data not shown). However, these conditions do not allow one to specifically assess fluid balance, because under these circumstances drinking behavior is influenced by group behaviors (i.e., other animals drinking) and by the drive to drink associated with food intake. To test the response to osmotic changes more specifically, mice were single-housed without food, thus eliminating the drive to drink in groups or during feeding. Water was available from drinkometers that precisely record fluid intake. Under these conditions, after 24 h, blood osmolality was slightly, yet significantly higher in trpv4-/- mice (295 vs. 300 milliosmol/kg, P < 0.02, t test; Fig. 2A). The trpv4-/- mice drank significantly less than did wild-type littermates (Fig. 2B). This difference was more pronounced in the first 6 h of thetest (0.06 vs. 0.22 ml, P < 0.01; Fig. 2B). Fluid consumption after 24 h was <70% of the consumption of wild-type littermates (1.4 vs. 2.2 ml, P < 0.01; Fig. 2B).
Fig. 2.
Impaired response to hypertonicity in trpv4-/- mice. (A) Impaired osmotic regulation in trpv4-/- mice when they are single-housed. Normal systemic osmotic pressure was apparent in both genotypes when mice were group-housed with freely available food and water (data not shown). When mice were single-housed without food and free access to water, a slight, yet significant, rise in systemic tonicity in trpv4-/- mice was seen. (B) Impaired drinking of trpv4-/- mice without challenge. Single-housing, no access to food as described in A. Drinking volumes were adjusted per 20 g of body weight. A significantly reduced water intake was measured in trpv4-/- mice, which was at its most pronounced in the first 6 h of the experiment. (C) Greater hyperosmolality in trpv4-/- mice in response to dehydration. Mice were single-housed without food and water, and systemic tonicity was determined after 48 h. A significant rise of blood osmolality in trpv4-/- mice was observed. (D) Impaired defense against hypertonicity in trpv4-/- mice after systemic osmotic challenge with 0.5 M NaCl (i.p. administration). Mice were single-housed without food and water during the experiment. Blood osmolality was determined 90 min after administration of 0.5 M NaCl. A significant increase in systemic osmotic tonicity was observed in trpv4-/- mice. (E) Impaired drinking of trpv4-/- mice after systemic osmotic challenge with 0.5 M NaCl (i.p. administration). Water intake is shown here. The setting was the same as in D. A significantly reduced amount of water intake in trpv4-/- mice is apparent. (F) ADH response after systemic hypertonic challenge. Depicted is the amount of plasma ADH after i.p. administration of 0.15 and 0.5M NaCl. A significantly lower amount of ADH could be noticed in trpv4-/- mice in response to hypertonic saline administration.
We next challenged mice osmotically with hypertonicity. First, single-housed trpv4-/- and wild-type littermates were deprived of water for 48 h. Systemic osmotic pressure was significantly higher in trpv4-/- mice with a plasma osmolality of 318 vs. 305 milliosmol/kg in wild-type littermates (P < 0.01; Fig. 2C). To further evaluate the response to hyperosmotic stimuli, we challenged mice with an i.p. injection of a hyperosmotic solution (0.5 M NaCl, 0.8 ml per 20 g of body weight). Systemic osmotic pressure was significantly higher in trpv4-/- mice (334.3 vs. 325.9 milliosmol/kg, P < 0.05; Fig. 2D). The fluid intake in trpv4-/- mice was significantly lower than in wild-type littermate controls (0.92 vs. 1.19 ml at 45 min, P < 0.02; 0.97 vs. 1.32 ml at 120 min, P < 0.01; Fig. 2E). Furthermore, latency of drinking was increased in the absence of TRPV4 (5.0 vs. 3.2 min, P = 0.01; Fig. 6). Finally, ADH secretion in response to osmotic stimulation was also impaired in trpv4-/- animals. The ADH levels of trpv4-/- mice were significantly lower than in wild-type littermates (4.2 vs. 3.4 pg/ml for 0.15 M NaCl; 34.1 vs. 23 pg/ml for 0.5 M NaCl, P < 0.05; Fig. 2F). These findings demonstrate a reduced ability of trpv4-/- mice to sense systemic osmotic pressure in circumventricular organs and/or an impaired capability to synthesize ADH in magnocellular neurons of the supraoptic and paraventricular nucleus of the hypothalamus. In aggregate, these results establish that there is an impaired response to a hypertonic stimulus in the absence of TRPV4.
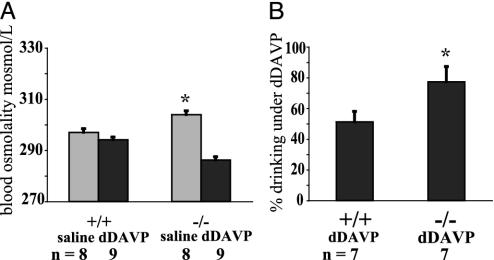
We next tested the response to systemic hypotonicity by infusing dDAVP, an analogue of ADH (10), by using an s.c. minipump. dDAVP is a strong antidiuretic, but it has negligible effects on cardiovascular function (24, 25). This experiment also allowed us to test the reabsorptive capacity of the kidney in trpv4-/- mice. TRPV4 is expressed in tubular epithelial cells in the kidney of wild-type mice (14, 15, 17). The experiment was performed by using a crossover design where animals were treated first with saline, then with dDAVP. Consistent with the above results, baseline osmotic pressure was higher in null mice compared with controls (304 in vs. 297 milliosmol/kg in wild-type littermates, P < 0.01, t test; Fig. 3A). After the dDAVP infusion, wild-type littermates remained normotonic, whereas trpv4-/- mice became significantly hypotonic (294 vs. 286 milliosmol/kg, P < 0.001, t test; Fig. 3A). Continuous infusion of dDAVP reduced the amounts of drinking by 49% in wild-type (1.22 ml per 30 h) vs. 23% (1.61 ml per 30 h) in trpv4-/- mice (P < 0.05, t test; Fig. 3B). This difference is noteworthy, in light of the fact that the null mice were more hypoosmolar than controls, and thus should have been drinking less. In contrast, urine osmolality did not differ significantly between both groups (3,829 milliosmol/kg in wild-type littermates and 3,517 milliosmol/kg in nulls, P = 0.26, t test; Fig. 7, which is published as supporting information on the PNAS web site, www.pnas.org). The capability of trpv4-/- mice to concentrate their urine to >3,500 milliosmol/kg is significant, in view of the expression of TRPV4 in tubular epithelial cells. Of note, during the control saline infusion, both genotypes drank similar amounts (2.4 vs. 2.4 ml, results not shown). This outcome is slightly different from that depicted in Fig. 2B, where a similar experiment was carried out without implanted minipumps. This result, perhaps, may have resulted from the stress due to the implantation of the pumps, wound healing, and associated inflammation. In this respect, the much larger variation in drinking in trpv4-/- mice with osmotic pumps is noteworthy (SEM = 0.53 for the group vs. SEM < 0.1 in Fig. 2C). Taken together, these studies show that trpv4-/- mice have an impaired response to hypoosmolar stimulation and that their renal response to ADH is intact. Thus, the defect in trpv4-/- mice is likely to be localized in the osmoregulatory regions of the CNS. In addition, our results suggest the existence of an inhibitory system within the osmoregulatory circuits of the CNS that reduces drinking under hypoosmolar conditions. This inhibitory system is compromised in trpv4-/- mice, and they are drinking proportionately more than are wild-type controls. This finding thus suggests a substantial role for the ion channel, TRPV4, in the inhibitory response to systemic hypotonicity by the CNS.
Fig. 3.
Response to dDAVP in trpv4-/- mice. (A) Response to chronic systemic administration of the ADH analogue, dDAVP. Blood osmolality is shown here. trpv4-/- mice and wild-type littermate controls were implanted with osmotic minipumps loaded first with saline, then pumps were switched to chronic infusion of dDAVP (1 μg per 20 g of body weight in 100 μl, chronic infusion over 3 days). Systemic osmotic pressure was slightly increased in trpv4-/- mice while receiving saline. Chronic infusion of dDAVP led to systemic hypotonicity in trpv4-/- mice, whereas wild-type littermates remained normotonic. During the entire experiment, mice were single-housed, water was freely available, and food was withheld for periods of 24 h, after which blood osmolality and drinking amounts were recorded. (B) Response to chronic systemic administration of the ADH analogue, dDAVP. Shown here is fluid intake (percent of saline amount). The average percentage of fluid intake while receiving dDAVP is shown. One hundred percent is the amount drunk by each individual mouse while receiving saline. After dDAVP treatment, each animal drank only a percentage of this baseline amount. The average percentage was calculated. The difference between genotypes is statistically significant (P < 0.05, t test). Whereas the kidneys of trpv4-/- mice responded to dDAVP treatment (as in C), their increased drinking in comparison to wild-type littermates (77% as compared with 51% reduction of drinking) led to systemic hypotonicity (as in A).
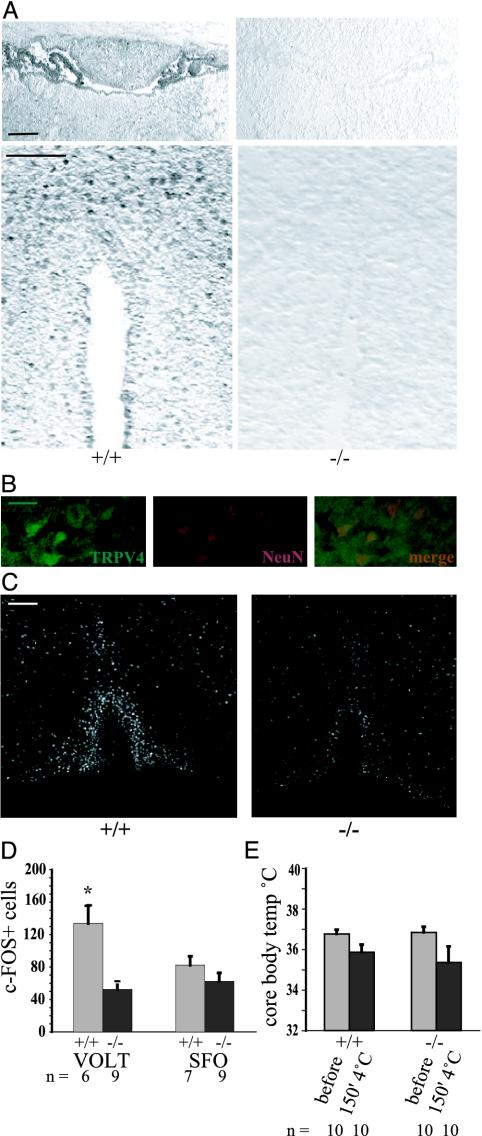
The observation that trpv4-/- mice showed reduced fluid intake and an impaired ADH response to a hyperosmolar stimulus suggests that the defect in these mice is upstream or at the level of ADH secretion and is likely to be localized in the CNS. The abnormal drinking response to administration of dDAVP with a normal renal response is also consistent with this possibility. Immunohistochemistry confirmed the expression of TRPV4 in the circumventricular organs (Fig. 4A; colabeling of TRPV4+ cells from the OVLT with neuronal marker NeuN in Fig. 4B). To further evaluate the possibility that the mutant mice have an impaired CNS response to altered osmotic pressure, we analyzed the expression of the immediate early response gene c-FOS in osmotically responsive cells of the CNS after a hyperosmolar stimulus, according to well established protocols (26, 27). Fig. 4C depicts representative micrographs of the lamina terminalis, where c-FOS was present in nuclei of the OVLT circumventricular organ in both genotypes. After a hyperosmolar stimulus, staining intensity and the number of stained nuclei in wild-type littermates was significantly higher than in trpv4-/- mice. Morphometric analysis confirmed a significantly reduced number of stained cells in the OVLT in trpv4-/- mice (52 vs. 134 cells, P < 0.02; Fig. 4D). In contrast, the number of stained cells in the SFO was not significantly different (82.3 vs. 62.3 cells, P = 0.21; Fig. 4E). With respect to c-FOS activation in regions of the brain that are not involved in osmotic regulation, c-FOS+ cells were equally abundant in the piriform cortex of both genotypes (29 vs. 29.2 cells, P = 0.78; results not shown), thus rendering a general loss of activation of c-FOS in trpv4-/- mice unlikely. This difference in c-FOS staining between the OVLT and SFO is consistent with the suggested role for the OVLT in osmotic sensing and for the SFO in sodium sensing and angiotensin and relaxin signaling (6, 8, 28-30). Further studies of the response of these cells in wild-type and mutant mice can be performed using mice in which the responding cells are labeled in vivo with fluorescent proteins being driven by the trpv4 promoter.
Fig. 4.
Specific impairment of CNS sensing of systemic osmotic pressure in trpv4-/- mice. (A) Immunohistochemistry for TRPV4 in the lamina terminalis. (Upper) Depicted is the circumventricular organ, SFO, and adjacent to it is the choroid plexus. The SFO is prominent in the rostral part of the third ventricle and is located caudally to the ventral hippocampal commissure. Note the absence of specific staining in the nulls. (Lower) Depicted is the circumventricular organ, the OVLT. The rostral portion of the anterior end of the third ventricle is sectioned; note specific staining in the OVLT in wild-type littermate mice, but not in trpv4-null mice. (Bar, 50 μm.) (B) Colabeling of TRPV4+ cells in the OVLT (Left, green fluorescence) with neuronal marker NeuN (Center, red fluorescence). Merged images are on the right. (Bar, 10 μm.) (C) Micrograph of the circumventricular organ, the OVLT, in the lamina terminalis. Shown here is immunohistochemistry for the c-FOS immediate early response gene. Representative micrographs of the lamina terminalis in a trpv4-/- mouse and in a wild-type littermate control mouse are shown. Note activation of the OVLT (inverted-U shape) and the rostrally positioned median preoptic area, MnPO. Mice were injected i.p. with 0.5 M NaCl, and their brains were fixated in formalin 90 min later. (Bar, 50 μm.) (D) Morphometry of c-FOS+ cells in circumventricular organs, the OVLT and the SFO. A significantly lower count of cells was noted in the OVLT of trpv4-/- mice, whereas the lower count in the SFO did not reach statistical significance. (E) Response to cold stress. Core body temperature was recorded by a rectal probe at 0 and 150 min. Mice were single-housed without food and water and exposed to 4°C for 150 min. Core body temperature dropped <36°C in both genotypes. Mean body temperature after the cold stress test was slightly lower in trpv4-/- mice, but the difference did not reach statistical significance.
TRP ion channels have also been suggested to sense thermal and mechanical stimuli (31-33). With respect to thermal sensing, we evaluated the response of the trpv4-/- and wild-type littermates to a cold stress test at 4°C. Basal body temperature recordings did not differ between genotypes (36.76 vs. 36.84°C in wild-type vs. null, Fig. 4E), and there was no difference in core temperature after cold stress (35.86 vs. 35.36°C in wild-type vs. null, P = 0.58; Fig. 4D). Thus, the altered response to osmotic stimulation in trpv4-/- mice represents a specific homeostatic deficit of the CNS. With respect to thermal nociceptive stimulation of the somatosensory system, withdrawal latencies for noxious heat was not significantly different between trpv4-/- and wild-type littermates (3.8 vs. 3.78 s for controls vs. nulls; see Fig. 7). Thus, we could not detect significant differences between trpv4-/- and wild-type littermates for thermal sensing. These results are consistent with our findings in osm-9 ASH::trpv4 transgenic worms (21). With respect to inner ear function, both trpv4-/- mice and wild-type littermates responded similarly to an acoustic startle and did not reveal any gross abnormalities indicative of vestibular dysfunction (results not shown). These findings do not exclude the possibility that abnormalities in these or other systems will be found.
In contrast to temperature sensing and hearing, the trpv4-/- mice did show a reduced response to noxious mechanical stimuli (Fig. 7), a finding confirming and extending the results of recent reports (34, 35). Our results are also consistent with the suggestion that changes in osmotic pressure are sensed by altered mechanical forces through changes in membrane tension (15, 36-41). Furthermore, in a recent investigation (42), it was reported that TRPV4 conferred a response to shear stress in TRPV4-expressing mammalian cells. This response was absent at room temperature and was strikingly present at 37°C (42). The mechanism of the impaired response to mechanical stimulation in trpv4-/- mice, and the relationship between mechanical and osmotic sensing, will be the subject of future studies.
Discussion
In aggregate, our findings indicate that TRPV4 plays an important role in regulating fluid homeostasis in mice by sensing osmotic pressure and activating osmotically responsive neurons in the OVLT. In a related investigation (21) on the role of trpv4 in vivo, we have studied the effects of TRPV4 expression in ASH sensory neurons of the C. elegans TRPV mutant osm9, a worm mutant lacking osmotic avoidance to hypertonic stimuli. Our results in worms suggest that TRPV4 rescues avoidance of hypertonicity and is a component of the osmotic sensing apparatus (21).
In the mouse, osmotically activated sensory cells in the OVLT and SFO then activate a well established adaptive response that includes alterations in fluid intake and ADH secretion (1, 4, 7). There is likely to be redundancy in this system because systemic osmotic pressure is only partially dysregulated in trpv4-/- mice, and there is still residual c-FOS staining in the OVLT in response to hyperosmolar stimulation. The identity of the molecules that still sense osmotic pressure in the trpv4-/- mice are unknown, but are amenable to further studies. Given the paramount importance of osmotic homeostasis for a species' survival in evolution, it is not surprising that there is redundancy of the systems that control osmotic homeostasis in trpv4-/- mice. It may also be that, similar to thermal sensing, no single molecule can sample osmotic pressure over the entire range that needs to be sensed. Another not mutually exclusive possibility that could explain the redundancy evident in trpv4-/- mice is that parallel systems that respond to changes in Na+ levels and alterations of circulating volume also respond to the osmotic stimuli that we have used. Systemically applied osmotic solutions always induce changes in volume and Na+ concentration, and the respective homeostatic systems could have provided powerful compensation for a greatly diminished osmotic regulation. In an attempt to address this issue, we have infused a hypertonic solution directly into the third ventricle of the CNS. However, this procedure did not result in a reliable increase of drinking in wild-type littermate controls when using hypertonic artificial cerebrospinal fluid (350 milliosmol/kg) (W.L., unpublished observations). Future studies using double knockouts of trpv4 and functionally related genes, pharmacological modulation of other homeostatic systems, and a CNS-specific knockout of trpv4 may help to elucidate the underlying mechanisms of homeostasis.
The impaired osmotic regulation in trpv4-null mice reported here differs from that published in a recent report (43). Whereas our experiments show that trpv4-null mice secrete reduced amounts of ADH in response to hypertonic stimulation, the results by Mizuno et al. (43) suggest that there is an accentuated ADH response to water depravation and subsequent systemic administration of propylene glycol. Their results suggest that the absence of TRPV4 is associated with an accentuated ADH response, whereas our results indicate that absence of TRPV4 blunts ADH secretion in response to a hypertonic stimulus. The reasons for this discrepancy are not clear.
The in vivo data reported here thus indicate that TRPV4 plays a role in sensing both hypo- and hyperosmolar stimuli in mice. This finding is in contrast to the properties of TRPV4 in transfected cells, where it is only activated by reduced osmotic pressure. In mammals, it is possible that other proteins that directly interact with TRPV4 are essential for the response of a multiprotein complex to hyperosmolar stimuli. Such a hypothetical multiprotein complex without TPRV4 could still function in the residual sensing of TRPV4 mutant mice. In keeping with this hypothesis, TRPV4 rescues the escape response to hypertonic stimuli of the osmoinsensitive C. elegans TRPV mutant osm-9 (21). The rescue appears to depend on direct protein-protein interaction with OCR-2, another C. elegans TRPV channel (44), and with other, yet unknown binding partners, is required for this response. It is also possible that the ability of TRPV4 to respond to hyperosmolar stimuli is determined by the lipid composition of the adjacent plasma membrane (45).
In summary, both osmotic and mechanical sensing are impaired in trpv4-/- mice. In a related PNAS paper, in C. elegans, mammalian TRPV4 restores osmotic and mechanical sensing behaviors in the C. elegans TRPV mutant osm-9. Taken together, the results strongly suggest that TRPV4 acts as a molecular component of the osmotic sensor. These studies provide a basis for a fuller understanding of the molecular mechanisms operative within the CNS centers that maintain osmotic equilibrium and evoke thirst.
Supplementary Material
Acknowledgments
We thank Chingwen Yang (The Rockefeller University Gene Targeting Facility), who provided invaluable assistance with gene targeting; Jim Hudspeth (The Rockefeller University), Stefan Heller (Harvard University, Boston), Alan Kim Johnson (University of Iowa, Iowa City), Cornelia Bargmann (University of California, San Francisco), Michael McKinley, and Derek Denton (Howard Florey Institute, University of Melbourne, Melbourne), who provided suggestions and support; Josh Boyce (The Rockefeller University), who assisted with genotyping; and Esra Asilmaz (The Rockefeller University), who assisted with mouse procedures. W.L. and J.M.F. were supported by the National Institutes of Health. J.M.F. is an Investigator with the Howard Hughes Medical Institute.
Abbreviations: TRPV, transient receptor potential channel, vanilloid subfamily; ADH, antidiuretic hormone; OVLT, organum vasculosum of the lamina terminalis; SFO, subfornical organ.
References
- 1.Bourque, C. W. & Oliet, S. H. (1997) Annu. Rev. Physiol. 59, 601-619. [DOI] [PubMed] [Google Scholar]
- 2.Denton, D. A., McKinley, M. J. & Weisinger, R. S. (1996) Proc. Natl. Acad. Sci. USA 93, 7397-7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oldfield, B. J., Badoer, E., Hards, D. K. & McKinley, M. J. (1994) Neuroscience 60, 255-262. [DOI] [PubMed] [Google Scholar]
- 4.Verney, E. B. (1947) Proc. R. Soc. London 135, 25-26. [PubMed] [Google Scholar]
- 5.Fitts, D. A., Starbuck, E. M. & Ruhf, A. (2000) Am. J. Physiol. 279, R2277-R2286. [DOI] [PubMed] [Google Scholar]
- 6.Johnson, A. K. & Loewy, A. D. (1990) in Central Regulation of Autonomic Function, eds. Loewy A. D. & Spyer, K. (Oxford Univ. Press, Oxford), pp. 247-267.
- 7.McKinley, M. J., Bicknell, R. J., Hards, D., McAllen, R. M., Vivas, L., Weisinger, R. S. & Oldfield, B. J. (1992) Prog. Brain Res. 91, 395-402. [DOI] [PubMed] [Google Scholar]
- 8.McKinley, M. J. & Oldfield, B. J. (1990) in The Human Nervous System, ed. Paxinos, G. (Academic, San Diego), pp. 415-438.
- 9.Bourque, C. W. (1998) Prog. Brain Res. 119, 59-76. [DOI] [PubMed] [Google Scholar]
- 10.Singer, I., Oster, J. R. & Fishman, L. M. (1997) Arch. Intern. Med. (Moscow) 157, 1293-1301. [PubMed] [Google Scholar]
- 11.Johnson, A. K. & Thunhorst, R. L. (1995) Prog. Psychobiol. Physiol. Psychol. 16, 145-176. [PubMed] [Google Scholar]
- 12.McKinley, M. J., Allen, A. M., Burns, P., Colvill, L. M. & Oldfield, B. J. (1998) Clin. Exp. Pharmacol. Physiol. Suppl. 25, S61-S67. [DOI] [PubMed] [Google Scholar]
- 13.McKinley, M. J., Allen, A. M., Chai, S. Y., Hards, D. K., Mendelsohn, F. A. & Oldfield, B. J. (1989) Acta Physiol. Scand. Suppl. 583, 113-118. [PubMed] [Google Scholar]
- 14.Liedtke, W., Choe, Y., Marti-Renom, M. A., Bell, A. M., Denis, C. S., Sali, A., Hudspeth, A. J., Friedman, J. M. & Heller, S. (2000) Cell 103, 525-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strotmann, R., Harteneck, C., Nunnenmacher, K., Schultz, G. & Plant, T. D. (2000) Nat. Cell Biol. 2, 695-702. [DOI] [PubMed] [Google Scholar]
- 16.Wissenbach, U., Bodding, M., Freichel, M. & Flockerzi, V. (2000) FEBS Lett. 485, 127-134. [DOI] [PubMed] [Google Scholar]
- 17.Delany, N. S., Hurle, M., Facer, P., Alnadaf, T., Plumpton, C., Kinghorn, I., See, C. G., Costigan, M., Anand, P., Woolf, C. J., et al. (2001) Physiol. Genomics 4, 165-174. [DOI] [PubMed] [Google Scholar]
- 18.Guler, A. D., Lee, H., Iida, T., Shimizu, I., Tominaga, M. & Caterina, M. (2002) J. Neurosci. 22, 6408-6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe, H., Vriens, J., Suh, S. H., Benham, C. D., Droogmans, G. & Nilius, B. (2002) J. Biol. Chem. 277, 47044-47051. [DOI] [PubMed] [Google Scholar]
- 20.Nilius, B., Watanabe, H. & Vriens, J. (2003) Pflügers Arch. 446, 298-303. [DOI] [PubMed] [Google Scholar]
- 21.Liedtke, W., Tobin, D. M., Bargmann, C. I. & Friedman, J. M. (2003) Proc. Natl. Acad. Sci. USA. 100, 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed]
- 22.Lakso, M., Pichel, J. G., Gorman, J. R., Sauer, B., Okamoto, Y., Lee, E., Alt, F. W. & Westphal, H. (1996) Proc. Natl. Acad. Sci. USA 93, 5860-5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen, C. C., Zimmer, A., Sun, W. H., Hall, J. & Brownstein, M. J. (2002) Proc. Natl. Acad. Sci. USA 99, 8992-8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robertson, G. L. (1995) Endocrinol. Metab. Clin. North Am. 24, 549-572. [PubMed] [Google Scholar]
- 25.Burrow, G. N., Wassenaar, W., Robertson, G. L. & Sehl, H. (1981) Acta Endocrinol. 97, 23-25. [DOI] [PubMed] [Google Scholar]
- 26.McKinley, M. J., Badoer, E., Vivas, L. & Oldfield, B. J. (1995) Brain Res. Bull. 37, 131-137. [DOI] [PubMed] [Google Scholar]
- 27.Wilson, Y., Nag, N., Davern, P., Oldfield, B. J., McKinley, M. J., Greferath, U. & Murphy, M. (2002) Proc. Natl. Acad. Sci. USA 99, 3252-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Starbuck, E. M. & Fitts, D. A. (1998) Appetite 31, 309-331. [DOI] [PubMed] [Google Scholar]
- 29.Thrasher, T. N. & Keil, L. C. (1987) Am. J. Physiol. 253, R108-R120. [DOI] [PubMed] [Google Scholar]
- 30.Sunn, N., Egli, M., Burazin, T. C., Burns, P., Colvill, L., Davern, P., Denton, D. A., Oldfield, B. J., Weisinger, R. S., Rauch, M., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 1701-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clapham, D. E., Runnels, L. W. & Strubing, C. (2001) Nat. Rev. Neurosci. 2, 387-396. [DOI] [PubMed] [Google Scholar]
- 32.Gunthorpe, M. J., Benham, C. D., Randall, A. & Davis, J. B. (2002) Trends Pharmacol. Sci. 23, 183-191. [DOI] [PubMed] [Google Scholar]
- 33.Montell, C., Birnbaumer, L., Flockerzi, V., Bindels, R. J., Bruford, E. A., Caterina, M. J., Clapham, D. E., Harteneck, C., Heller, S., Julius, D., et al. (2002) Mol. Cell 9, 229-231. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki, M., Mizuno, A., Kodaira, K. & Imai, M. (2003) J. Biol. Chem. 278, 22664-22668. [DOI] [PubMed] [Google Scholar]
- 35.Allessandri-Haber, N., Yeh, J., Boyd, A. E., Parada, C. A., Chen, X., Reichling, D. B. & Levine, J. D. (2003) Neuron 39, 497-511. [DOI] [PubMed] [Google Scholar]
- 36.Blount, P. & Moe, P. C. (1999) Trends Microbiol. 7, 420-424. [DOI] [PubMed] [Google Scholar]
- 37.French, A. S. (1992) Annu. Rev. Physiol. 54, 135-152. [DOI] [PubMed] [Google Scholar]
- 38.Gillespie, P. G. & Walker, R. G. (2001) Nature 413, 194-202. [DOI] [PubMed] [Google Scholar]
- 39.Hamill, O. P. & Martinac, B. (2001) Physiol. Rev. 81, 685-740. [DOI] [PubMed] [Google Scholar]
- 40.Sackin, H. (1995) Annu. Rev. Physiol. 57, 333-353. [DOI] [PubMed] [Google Scholar]
- 41.Corey, D. P. (2003) Neuron 39, 585-588. [DOI] [PubMed] [Google Scholar]
- 42.Gao, X., Wu, L. & O'Neil, R. G. (2003) J. Biol. Chem. 278, 27129-27137. [DOI] [PubMed] [Google Scholar]
- 43.Mizuno, A., Matsumoto, N., Imai, M. & Suzuki, M. (2003) Am. J. Physiol. 285, C96-C101. [DOI] [PubMed] [Google Scholar]
- 44.Tobin, D., Madsen, D. M., Kahn-Kirby, A., Peckol, E., Moulder, G., Barstead, R., Maricq, A. V. & Bargmann, C. I. (2002) Neuron 35, 307-318. [DOI] [PubMed] [Google Scholar]
- 45.Martinac, B. & Hamill, O. P. (2002) Proc. Natl. Acad. Sci. USA 99, 4308-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.