Abstract
Cereals accumulate starch in the endosperm as their major energy reserve in the grain. In most cereals the embryo, scutellum, and aleurone layer are high in oil, but these tissues constitute a very small part of the total seed weight. However, in oat (Avena sativa L.) most of the oil in kernels is deposited in the same endosperm cells that accumulate starch. Thus oat endosperm is a desirable model system to study the metabolic switches responsible for carbon partitioning between oil and starch synthesis. A prerequisite for such investigations is the development of an experimental system for oat that allows for metabolic flux analysis using stable and radioactive isotope labelling. An in vitro liquid culture system, developed for detached oat panicles and optimized to mimic kernel composition during different developmental stages in planta, is presented here. This system was subsequently used in analyses of carbon partitioning between lipids and carbohydrates by the administration of 14C-labelled sucrose to two cultivars having different amounts of kernel oil. The data presented in this study clearly show that a higher amount of oil in the high-oil cultivar compared with the medium-oil cultivar was due to a higher proportion of carbon partitioning into oil during seed filling, predominantly at the earlier stages of kernel development.
Keywords: Avena sativa, carbon partitioning, cereal, endosperm, lipid, oat, oil, starch, triacylglycerol
Introduction
Plant oils derived from oilseed crops represent an important agricultural commodity that is used primarily for food and feed purposes today (Patel et al., 2006). In recent years, the demand for plant-derived oils as renewable alternatives to fossil oil for use as biofuels and in industrial applications has increased due to the rising cost of petroleum and the increased concern about the environment. The world production of vegetable oil increased with 61% between 1997–2007 (FAOSTAT, 2008). However, the supply of vegetable oils today relies upon only a few crops; palm oil (Elaeis guineensis), soy bean (Glycine max), rape seed (Brassica napus), and sunflower (Helianthus annuus), which in 2007 accounted for 83% of total world production (FAOSTAT, 2008). The restricted supply of feedstock due to the limited amount of oil crops is now one of the biggest challenges in plant oil production (Durrett et al., 2008; Thelen and Ohlrogge, 2002b). One way to generate new oil crops that are economically viable is to redirect carbon flux within plants from starch to oil. Increased knowledge of oil biosynthesis in plants is therefore of crucial importance for the development of novel oil crops for a sustainable plant oil production in the future.
Plant seeds are designed to carry the genetic material and all the nutrients that are required to establish the next generation of the species. Degradation of the stored nutrients provides the germination process with building blocks and energy, and must last until photosynthetic activity takes over, providing further growth and development for a mature plant. During seed development, sucrose is transported from source leaves to seeds where the reduced carbon is channelled into different storage compounds. Typical storage forms of carbon in seeds are starch, protein, and oil (triacylglycerols; TAGs), the economically valuable products used for food, feed, and industrial applications. The proportions of storage compounds differ between plant species, depending upon physiological differences, and master regulators of carbon partitioning within the seed.
Cereal seeds store carbon mostly in the form of starch and protein in the endosperm, and as oil in the embryo which usually constitutes a very small part of the seed structure. However, the high oil content of some maize (Zea mays) varieties is due to an enlarged embryo (Alexander and Seif, 1963). The oil palm is another monocot that stores a large amount of oil in the kernel endosperm (Oo et al., 1985). Oil seeds like rape typically store oil in the two cotyledons that serve as the main source of energy available during germination generated by the β-oxidation cycle. The endosperm reserves in the mature non-endospermic oil seed are depleted during seed development by the embryo and then reorganized in the cotyledons (Da Silva et al., 1997; Focks and Benning, 1998). There are also many dicot oil seeds like castor bean (Ricinus communis) where the endosperm is retained as a storage tissue in the mature seed (Marriott and Northcote, 1975). Altogether, even though plants share the same metabolic pathways for energy storage and usage, there is a broad spectrum of variability in how different species partition the fixed carbon within the seed.
Oat (A. sativa) is a unique cereal, due to the storage of oil within the endosperm ranging between 3–18% oil amongst different cultivars, whereas, in other cereals (i.e. wheat; Triticum aestivum, barley; Hordeum vulgare) this range is limited to 2–3% (Price and Parsons, 1975; Peterson and Wood, 1997). Previous studies aiming to identify key regulatory steps in the oil biosynthetic pathway established that oil in oat seeds accumulates transiently and is deposited in the same endosperm cells that produce starch (Banas et al., 2007; Waheeb et al., 2008). This finding is the basis for the suitability of oat endosperm as a model system for studying carbon partitioning in cereal seeds. In contrast to oil seeds like Arabidopsis thaliana and rape where the oil is synthesized during the later developmental stages at the expense of transiently accumulated starch (Focks and Benning, 1998; Vigeolas et al., 2004), in oat seeds, a major part of the oil is accumulated at an early stage of development and starch accumulation continues throughout seed development. The ability to redirect carbon flux from starch to oil within the endosperm may enhance the oil levels in cereals to give an oil productivity exceeding that of rape since cereals are, in general, much higher yielding crops. If normal wheat yielding approximately 6 tonnes ha−1 could be converted to an oil crop with 25% oil, this oil-wheat would yield 4.5 tonnes ha−1 (the energy density of oil being approximately double compared with that of starch) giving 1.1 tonnes oil ha−1 which is comparable to oil yields of winter rape. Rape seed, which is today the only economically viable oil crop in northern Europe, requires a considerably higher input of pesticides and fertilizers and longer crop rotation periods than most cereals.
A prerequisite for such investigations is the development of an experimental system that allows metabolic flux analysis using stable and radioactive isotope labelling in oat seeds. In this study, an in vitro liquid culture system with a defined growth medium for detached oat panicles was established and optimized to mimic kernel composition during different developmental stages in planta. In order to gain a more detailed understanding of oil deposition in oat, seed filling was examined at many more developmental stages than previously reported (Banas et al., 2007). To illustrate the differences in carbon partitioning between carbohydrates and lipids in oat cultivars with differing oil concentrations, 14C-labelled sucrose was administered in an in vitro system and the accumulation of label in seeds was measured. Distribution of 14C among different lipid classes was determined to estimate differences in carbon flux between TAG and membrane lipids.
Materials and methods
Plant material, growth conditions, and seed sampling
Two oat cultivars, cv. Matilda with 10% oil in mature seeds, and cv. Freja with 6% oil (Svalöv Weibull AB, Svalöv), were grown in Conviron growth chambers (CEFa UC Davis, CA) under fluorescent light (200 μmol m−2 s−1 photosynthetically active radiation) under a 16/8 h light/dark photoperiod at 21/18 °C temperature and 70% humidity. Seeds were harvested at five developmental stages from anthesis (stage A) to maturity (stage J) defined by the size, colour, texture, and endosperm consistency of the seeds as described in Supplementary Table S1 at JXB online. The sampled developmental stages can be compared to approximately 4, 10, 14, 20, and 30 d post anthesis. Seeds were harvested, weighed, and subsequently were either frozen in N2 (l) and stored at –80 °C prior to lipid analysis, or dried at 80 °C for dry weight (DW) and starch determination. Starch concentration in seeds was determined enzymatically (Megazyme, Wicklow, Ireland). All analyses were on three biological replicates, each consisting of three seeds from one panicle.
In vitro culture of seeds on detached oat panicles
Panicles were detached from plants at anthesis. To eliminate sucrose contribution from leaf photosynthesis in order to achieve a more careful control of carbon supply, stems were cut to approximately 15 cm (panicle not included) and over the first node to exclude all leaves. Cut stems were incubated for 20 min in 0.5% chlorine for surface sterilization and transferred to 15 ml plastic tubes. All stems were cut under water to eliminate cavitation. These stems were then placed in tubes with sterile nutrient solution with various sucrose concentrations to determine the optimum concentration required for seed filling, starting from anthesis to fully matured seeds. Based on these findings, the optimum sucrose concentration was established to be 15 g l−1 sucrose. In addition to sucrose, the media contained 0.5 g l−1 2[N-morpholino] ethane sulphonic acid, 0.8 g·l−1 glutamine, and 1.47 g l−1 Murashige-Skoog medium (all chemicals from Duchefa, Harleem, The Netherlands). Glutamine was chosen as the nitrogen source since it gives higher seed filling of wheat seeds developed in vitro compared with other nitrogen sources investigated in combination with sucrose (Singh and Jenner, 1983). Tubes containing the stems were covered with parafilm and incubated at light intensities and climate conditions similar to those used for the in planta grown panicles. The nutrient solution was changed and stems were cut again up to 2–4 mm every second day. Once a week, stems were surface-sterilized using 0.5% chlorine solution. Seeds were harvested at five different developmental stages (stage C, D, E, G, and J) similar to seeds developed in planta (see above). Seeds matured in vitro germinated with the same frequency as those developed in planta (results not shown).
Radioactive isotope labelling in oat seeds
Seeds on detached oat panicles were developed from anthesis to a certain stage of development by feeding panicles with nutrient medium containing non-radioactive sucrose. At various developmental stages, panicles were fed with medium containing U-14C sucrose (Amersham, Buckinghamshire, UK) with a specific radioactivity of 30 dpm nmol−1 and were further incubated for 48 h and the seeds were then harvested for analysis. Whole seeds were sampled from four different developmental stages (stage C, D, E, and G). At stage E and G, seed samples were split into embryo+scutellum and endosperm. Subsequently, these tissues along with whole seed samples were collected.
Lipid analysis and radioactivity measurements
Total lipids were extracted from seeds according to Bligh and Dyer (1959) resulting in one chloroform phase containing the lipids, and one water/methanol phase containing the starch, sugars, cell-walls, and proteins. A small fraction of the chloroform phase was methylated using 2% sulphuric acid in methanol and analysed with gas-liquid chromatography (Schimadzu, GC-17A, BergmanLabora, Sweden) using a WCOT fused silica 50 m×0.32 mm ID capillary column coated with CP-Wax 58-CB DF=0.2 (Chrompack Inc., The Netherlands) with methyl-heptadecanoate as the internal standard to determine the total fatty acid amount.
In the case of radio-labelled seeds, an aliquot of the chloroform phase was evaporated and resuspended in the scintillation solvent (Ultima Gold F, Perkin Elmer, Shelton, USA). The water/methanol phase (including starch, sugars, cell-walls, and proteins) was vortexed and an aliquot was transferred into a scintillation solvent vial (Ultima-Flo M, Perkin Elmer, Shelton, USA). Fractions of total lipid extracts were separated using thin layer chromatography (TLC) on silica 60 plates (Merck, Darmstadt, Germany) in hexane:diethylether:acetic acid in volumes of 35 ml:15 ml:10 μl. Lipids were identified (TAGs, polar lipids, and the rest) and scraped from plates to vials with scintillation solvent (Ultima Gold F, Perkin Elmer, Shelton, USA). Radioactivity was determined using a liquid scintillation counter (PW 4700, Philips, Almelo, The Netherlands) and corrected for quenching.
Statistical analysis of data
Data were analysed by analysis of variance (ANOVA) using the general linear model at level 5% (MINITAB 14; Minitab, State College, PA, USA) in which all factors were fixed. Since interactions between parameters were the standard case in all datasets, pairwise comparisons using the method of Tukey of all treatments were made at the 5% level. All the stated differences are significant at P <0.05.
Results
Comparison of seed filling in planta and in vitro
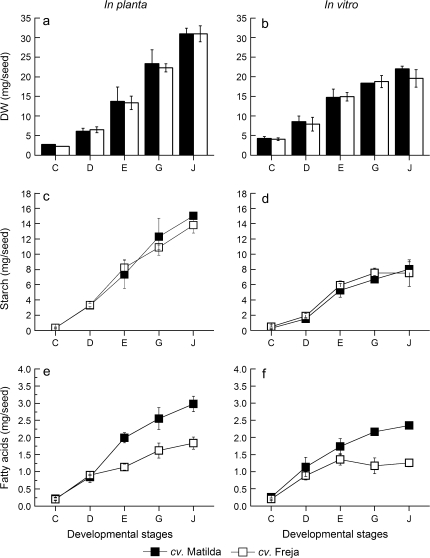
When using oat seeds developed in vitro as a model system for carbon partitioning in cereal seeds, it is of crucial importance that the kernel development reflects the normal physiological development of the seed. Therefore, dry weight, starch, and oil concentration of seeds developed in planta and on detached panicles in vitro were compared at various developmental stages from shortly after anthesis to maturity (Fig. 1; for definitions of the developmental stages see Supplementary Table S1 at JXB online). Among the sucrose concentrations tested in the in vitro system of detached oat panicles, 15 g l−1 gave the highest seed filling at maturity (approximately 60% compared to mature seeds developed in planta) and therefore this concentration was used in all experiments. At this sucrose concentration, the rate of seed development followed that of seeds in planta with approximately 30 d from anthesis to maturity. Higher sucrose concentrations in vitro induced earlier senescence of panicles as compared to in planta (results not shown), a potential contributing factor for seed filling ratios below 60% as compared with seed filling in planta.
Fig. 1.
Oat seeds (cv. Matilda developed in planta) at developmental stages A–J. Upper row, crease side; lower row, underside.
Seed dry weight in vitro was similar to that in planta up to stage E; thereafter, however, it showed a lower seed filling rate (Fig. 2a, b). No difference in seed weight between cv. Matilda and Freja at different developmental stages was observed. This finding is in agreement with previous reports (Banas et al., 2007) and enable us to compare absolute amounts of storage products per seed between cultivars directly.
Fig. 2.
Comparison of dry weight (DW), starch, and oil concentration of oat seeds during development in planta (a, c, e) and in vitro (b, d, f) from an early stage (stage C) to mature seeds (stage J) of cv. Matilda (filled bars/symbols) and Freja (open bars/symbols). Results are the mean ±standard deviation for three samples.
The cultivar differences in oil concentration present in planta were also observed in vitro (Fig. 2e, f). This observation is of central importance for the physiological relevance of data obtained from this model system as an example of carbon partitioning between starch and oil in the cereal seed. Total fatty acid content was similar at stage C between the two cultivars, but thereafter, the differences increased throughout seed development. It should be noted that the major portion of total fatty acids at stage C were likely to be from membrane lipids and not from the TAG fraction. The final oil concentrations on a dry weight basis (calculated from the amount of fatty acids assuming that they all were TAGs) for cvs Matilda and Freja in seeds developed in planta were 10.5% and 6.4%, and in vitro were 12.0% and 7.7%, respectively.
Starch filling continued throughout seed development from anthesis to maturity in planta, whereas in vitro starch accumulation almost ceased after stage E (Fig. 2c, d). No difference in starch concentration between cultivars could be observed. The in vitro levels of starch reached 54% of the levels achieved in mature seeds in planta in both cultivars. On the other hand, oil amounts in vitro reached 65% and 78% for cvs Freja and Matilda, respectively, compared to oil levels in seeds in planta.
14C-sucrose incorporation and partitioning between lipids and carbohydrates
Detached oat panicles were fed with radioactive labelled 14C-sucrose for 48 h at four different developmental stages. The 14C accumulation in lipid fractions compared with total 14C accumulation in seeds therefore reflect the net accumulation of lipids (lipid synthesis minus degradation) at those four stages of development, assuming that the developmental stage of the seeds is not significantly altered during the 48 h incubation. It should be noted that cultivar differences found in 14C accumulation in lipids might be due to differences in either synthesis or degradation pathways. Further metabolic flux studies should include time-courses of 14C incorporation and pulse labelling of oat seeds to elucidate the turnover rates of different seed components. Moreover, the carbon contribution from glutamine has not been corrected in this study, thus the accumulation of total carbon in the non-lipid pool is underestimated in the experimental data.
It is also pertinent to note in this context that one-third of the carbons in the sucrose are lost by decarboxylation in fatty acid synthesis as reported for rape (Schwender and Ohlrogge, 2002) and sunflower (Alonso et al., 2007). Although green seeds refix much of this carbon for lipid synthesis (Ruuska et al., 2004), the oat endosperm lacks chloroplasts. Therefore, this carbon is probably lost for lipid synthesis in the endosperm, but might be refixed in the green seed coat. Thus, it is likely that the proportion of carbon channelled into lipids may be up to 30% higher than the values given here.
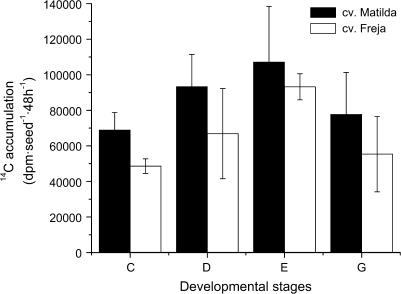
Of the two cultivars, seeds of cv. Matilda assimilated more sucrose compared with cv. Freja at all stages of development as measured by 14C accumulation from fed 14C sucrose (Fig. 3). The early stage of seed development (stage C) showed the highest incorporation of carbon to seeds on a DW basis for both cultivars, suggesting that this is one of the most active stages in the seed-filling period.
Fig. 3.
Total 14C accumulation in seeds developed in vitro on detached oat panicles of cv. Matilda (filled bars) and Freja (open bars) fed with 14C-labelled sucrose (specific activity 30 dpm nmol−1). Seeds were harvested at different developmental stages after a 48 h incubation with 14C-sucrose. Results are the mean ±standard deviation for three samples.
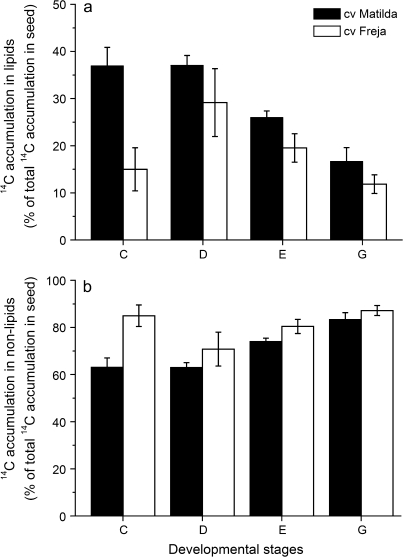
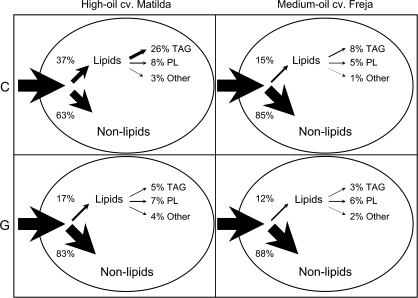
In the analysis of 14C accumulation in lipids and non-lipids (including starch, sugars, cell-walls, and proteins) of whole seeds developed in vitro, the difference between the high-oil cv. Matilda and medium-oil cv. Freja was striking. At the early stage of development (stage C), cv. Matilda incorporated 37% of total 14C accumulation into the seed as lipids, whereas cv. Freja only incorporated 15% of total 14C in lipids (Fig. 4a, b). In seeds of cv. Matilda at stage D, the proportion of 14C recovered in lipids was as high as in stage C, whereas in seeds of cv. Freja the proportion increased from 15% in stage C to approximately 29% in stage D (Fig. 4a). The proportion of 14C recovered in lipids gradually decreased from stage E to stage G where both cultivars accumulated approximately 15% of total 14C in lipids.
Fig. 4.
Proportions of 14C accumulation in lipids (a) and non-lipids (b). Seeds were developed in vitro on detached oat panicles of cv. Matilda (filled bars) and cv. Freja (open bars) and harvested at different developmental stages after a 48 h incubation with 14C-sucrose. Non-lipid fractions include starch, sugar, cell-wall, and protein. Results (mean ±standard deviation for three samples) are percentages of the total 14C accumulation in the whole seed.
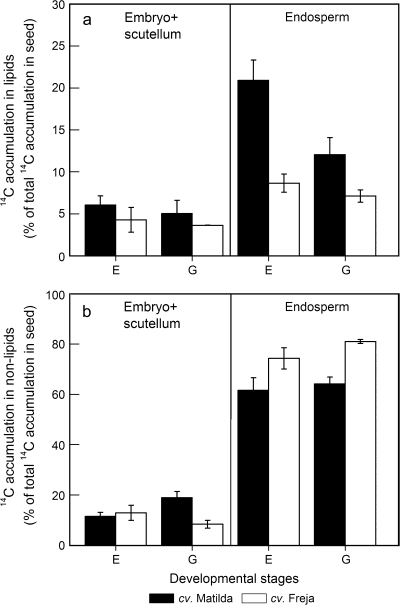
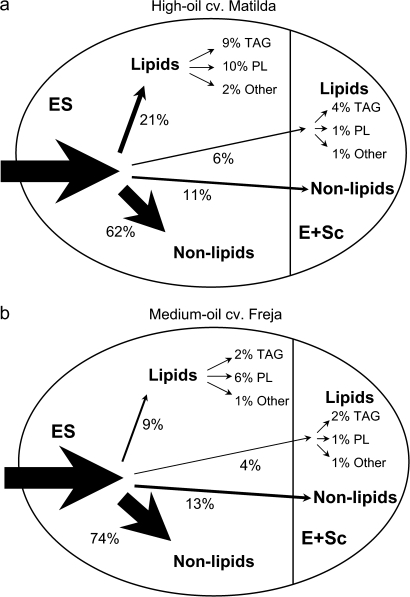
To determine whether total seed lipid accumulation during the later stages of development is as a result of lipid accumulation in the embryo+scutellum (which is an oil-dense tissue in all cereals) or in the endosperm, 14C accumulation in lipids and non-lipids of total 14C label at stages E and G was measured in those parts of the seeds (Fig. 5). Of the total 14C incorporation in seeds at stage E, 21% and 9% was found in endosperm lipids in cvs Matilda and Freja, respectively (Fig. 5a). At stage G, when seeds were almost totally yellow and endosperm had started to solidify, the proportion of 14C found in endosperm lipids of cv. Matilda had decreased to approximately 12% of total 14C incorporation in the seed. However, this proportion was still 5.4% higher than in cv. Freja endosperm. In the embryo+scutellum, no cultivar differences in the proportions of 14C accumulation were found, neither in lipids (approximately 5% of total 14C incorporation in the seed, Fig. 5a) nor in non-lipid metabolites (approximately 10% of total 14C incorporation in the seed, Fig. 5b). There were no differences in 14C accumulation in the embryo+scutellum between development stages E and G.
Fig. 5.
Proportions of 14C accumulation into lipids (a) and non-lipids (b) in embryo+scutellum and endosperm of seeds developed in vitro on detached oat panicles of cv. Matilda (filled bars) and Freja (open bars). Seeds were harvested at different developmental stages after a 48 h incubation with 14C-labelled sucrose. Non-lipid fractions include starch, sugar, cell-wall, and protein. Results (mean ±standard deviation for three samples) are percentages of the total 14C accumulation in the whole seed.
Carbon partitioning to TAG in oat seeds
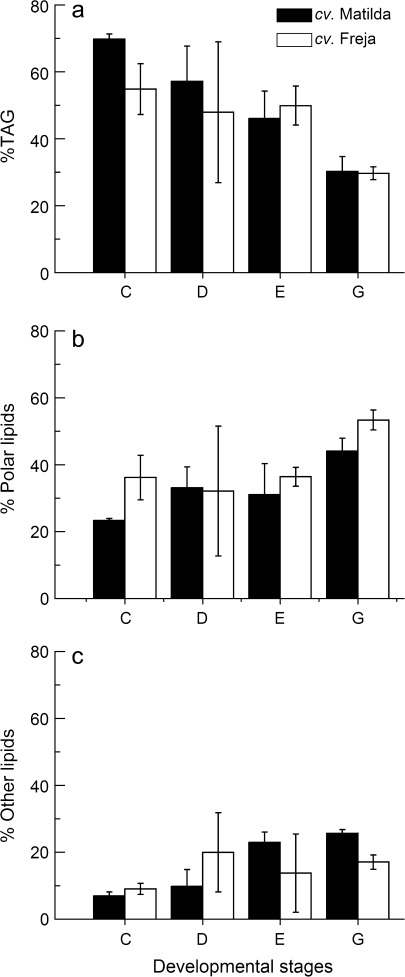
To investigate the channelling of carbon into various seed lipid species, 14C incorporation into TAG, polar lipids, and ‘other lipids’ (non-polar lipids other than TAGs, including diacylglycerol and free fatty acids) was measured. Of 14C accumulation in total seed lipids at stage C, 70% and 55% was found in TAG in cvs Matilda and Freja, respectively (Fig. 6a). The proportion of 14C recovered in TAG relative to total lipids decreased gradually throughout seed development, reaching 30% at stage G for both cultivars, with concomitant increased proportions of 14C found in other non-polar lipids as well as polar lipids (Fig. 6b, c).
Fig. 6.
Proportions of 14C accumulation in different lipid classes in seeds developed in vitro on detached oat panicles of cv. Matilda (filled bars) and cv. Freja (open bars) at different developmental stages. Results (mean ±standard deviation for three samples) are percentages of the 14C accumulation in total lipids of the whole seed. TAG, triacylglycerol; other lipids, non-polar lipids other than TAG.
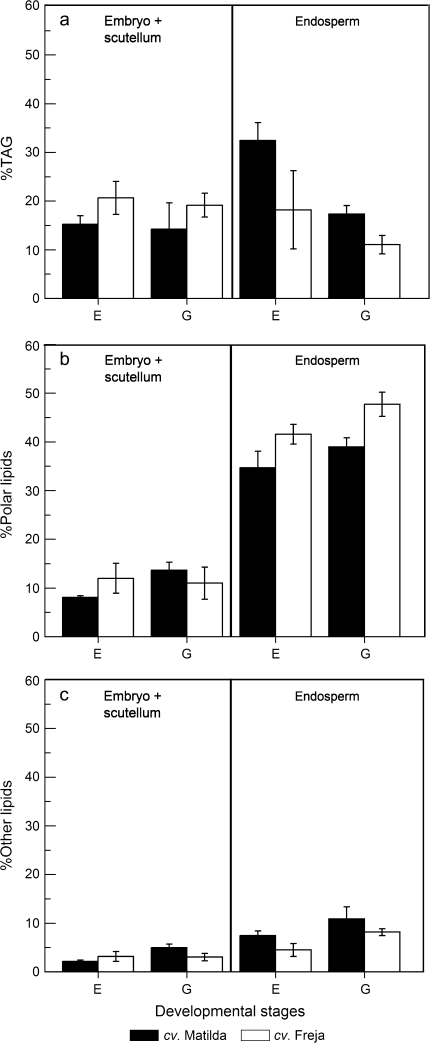
In the embryo+scutellum, approximately 18% of 14C accumulation in total lipids in seeds was found in TAG with no significant differences between cultivars or developmental stages (Fig. 7a). In contrast to the embryo+scutellum, in the endosperm nearly twice the proportion (32%) of 14C was recovered in TAG relative to total lipids in seeds of cv. Matilda compared to cv. Freja (18%) at stage E. This proportion decreased in the endosperm from stage E to stage G in both cultivars to a level that was comparable to the proportion of 14C accumulation in TAG in the embryo+scutellum at stage G (Fig. 7).
Fig. 7.
Proportions of 14C accumulation in different lipid classes in embryo+scutellum and endosperm of seeds developed in vitro on detached oat panicles of cv. Matilda (filled bars) and Freja (open bars) at different developmental stages. Results (mean ±standard deviation for three samples) are percentages of the 14C accumulation in total lipids of the whole seed. TAG, triacylglycerol; other lipids, non-polar lipids other than TAG.
Overview on carbon incorporation into oat seed components
Data sets are displayed as overview maps to illustrate the differences found in carbon partitioning into different components of oat seeds. The first map comparing 14C accumulation in whole seeds of the two cultivars at the very early and at the late stage of development (Fig. 8) demonstrates that the biggest differences were found at the early stage, when a much higher proportion of 14C accumulated in lipids as well as in TAG in cv. Matilda compared with cv. Freja. The second map comparing 14C accumulation in different parts of the seeds at stage E represents the latest stage of development where the two cultivars still show differences in 14C accumulation in endosperm lipids before maturation (Fig. 9).
Fig. 8.
Relative incorporation of 14C-sucrose into components of oat seeds of high-oil cv. Matilda and medium-oil cv. Freja at early (C) and late (G) stages of development. Thicknesses of arrows represent sizes of incorporation. The first arrow to the seed represents 100% of total 14C-sucrose that is incorporated into the seed. Non-lipid fractions include starch, sugar, cell-wall, and protein. TAG, triacylglycerol; other lipids, non-polar lipids other than TAG. Values are mean values for three samples.
Fig. 9.
Relative incorporation of 14C-sucrose into components of oat seeds of high-oil cv. Matilda (a) and medium-oil cv. Freja (b) at development stage E. Thicknesses of arrows represent sizes of incorporation. The first arrow to the seed represents 100% of total 14C-sucrose that is incorporated into the seed. Non-lipid fractions include starch, sugar, cell-wall, and protein. TAG, triacylglycerol; other lipids, non-polar lipids other than TAG. Values are mean values for three samples.
Discussion
Oat seeds developed on detached panicles as a model system for carbon partitioning in cereal seeds
Redirection of carbon flux from starch to oil in the cereal seed can provide for new high-yielding oil crops. Using oat as a model system, we would enhance our understanding of carbon partitioning from starch to oil in a cereal endosperm. Such studies require a method of studying these metabolic fluxes with radioactive and stable isotope labelling. This research details the successful implementation of an in vitro culture system for oat seeds that allows the examination of metabolic flux throughout development.
The in vitro system used in this study utilizes detached oat panicles fed with sucrose and nutrient solution through the transpiration stream. Maximum filling of oat seeds in vitro in this study (60% compared with in planta) was achieved at 15 g l−1 sucrose (44 mM) and 0.8 g l−1 glutamine. As a comparison, the in vivo sucrose concentration in the crease phloem of developing wheat grains is approximately 150–300 mM (Fisher and Wang, 1995). The seed filling was somewhat less than that reported previously in similar growth systems for Oryza sativa (Lee et al., 2000) and Triticum aestivum (Singh and Jenner, 1983). However, and more importantly, oat seeds in vitro displayed comparable developmental growth rates and cultivar differences in oil accumulation as those in planta. Oil concentrations, both in vitro and in planta, were also comparable to the levels detected under greenhouse and field conditions (Banas et al., 2007; www.ffe.slu.se).
Cultivar differences in starch concentrations were not observed under our growth conditions in planta or on detached panicles. This is in contrast to previous data, where cv. Freja accumulated a higher starch level as compared to cv. Matilda under greenhouse (Banas et al., 2007) and field growth conditions (Å Ekman, unpublished data). This inverse relationship between starch and oil levels in oat seeds was also shown in the field in a selection of 25 oat lines with elevated oil levels (Peterson and Wood, 1997). It is probable that the environmental conditions used in this study promoted a shift in sucrose import rate into the cv. Matilda seeds compared to greenhouse or field conditions. However, the weight ratios between starch and oil in cvs Matilda and Freja are only marginally changed depending on growth condition (cv. Freja; field 8:1, growth chamber 8:1, in vitro 5:1 and for cv. Matilda, field 4:1, growth chamber 5:1, in vitro 3:1). This finding clearly illustrates that the cultivar differences in partitioning of carbon between starch and oil are present under our experimental conditions.
A potential explanation on why the in vitro-grown seeds did not reach seed weight levels of those achieved in planta may be due to decreased starch deposition during the later stages of the in vitro development. Oil accumulation that peaks during the early stages of development was unchanged in vitro as compared to in planta conditions. However, the in vitro system can be regarded as a reasonable qualitative, though not quantitative, model system for storage filling throughout seed development.
Carbon partitioning between lipids and carbohydrates in oat seeds
The in vitro system of oat seed development on detached oat panicles was used for the first time in analyses of carbon partitioning between lipids and non-lipids in a cereal seed.
Cultivar differences clearly show that the high-oil cv. Matilda accumulated a much higher proportion of total 14C incorporation in the seed into lipids (37%) than the medium-oil cv. Freja (15%) at the very early stage of seed development. The proportion of 14C recovered in lipids relative to total seed incorporation decreased in both cultivars with increasing maturity. This is in agreement with a study where oat plants were fed 14CO2 at different developmental stages (Beringer, 1971). If a possible turnover of lipids is disregarded, the data suggest that the proportion of sugar channelled towards lipid synthesis contributes to the double amount of lipids observed in the mature seeds of cv. Matilda as compared to cv. Freja. Whether cultivar differences in the turnover of endosperm- specific lipids also play a role in the final levels of oil stored in the endosperm remains to be elucidated, although turnover of lipids in oat seeds during development has been suggested (Banas et al., 2007). Degradation of fatty acids through β-oxidation in the endosperm may be possible in premature monocot seeds when endosperm cells have yet to undergo programmed cell death (Young and Gallie, 2000). The turnover of lipids has been characterized in dicot oil seeds during normal seed development (Poirier et al., 1999; Sarmiento et al., 1998).
Despite the reduction in 14C accumulation in lipids throughout seed development, seeds accumulate rather high 14C at later stages of development, potentially due to lipid synthesis in the oil-dense tissues of the embryo+scutellum which expands after the midstage of seed development (Banas et al., 2007). Nevertheless, the main part of 14C accumulation in lipids is still carried out in the endosperm, but with differences between cultivars: after the midstage of development, the high-oil cultivar was accumulating a significantly higher proportion of total 14C in the seed into endosperm lipids compared to the medium-oil cultivar.
Carbon partitioning to TAG in oat seeds
Differences in lipid accumulation between cultivars were not only found at the level of carbon partitioning to total lipids, but also at the level of carbon partitioning to different lipid classes. In fact, the basis for the different oil content between the two cultivars, if not taking turnover of lipids into account, is because cv. Matilda incorporates more carbon into the seeds and also channels a substantially higher proportion of that carbon into lipids. At the very early developmental stage cv. Matilda accumulated sucrose up to 850 nmol seed−1 48 h−1 in TAG whereas cv. Freja accumulated only up to 250 nmol seed−1 48 h−1 (estimated from 14C accumulation). The ratio of carbon accumulation in TAG relative to other lipids decreased in both cultivars at the later stages of development, establishing altered oil metabolism in matured seeds.
Contrary to the embryo+scutellum, the major part of the lipids accumulating in the endosperm during the later stages of development (stage E and G) was not TAG but polar lipids, i.e. membrane lipids. However, proportions of carbon accumulation in TAG relative to other lipids in the endosperm still differed between cultivars at the earlier stage of development when seeds were still green. These differences were minimized to almost equal levels at the stage when seeds were almost yellow. The embryo+scutellum showed no differences in the ratio of carbon accumulation in TAG relative to other lipids after the midstage of development and these proportions were the same for both cultivars, suggesting that differences in lipid metabolism are most probably explained predominantly by differences in the endosperm tissue. It should be noted that the proportion of 14C found in TAG compared to other lipids was considerably less than what was found by mass analysis at all stages (Banas et al., 2007). The most plausible explanation for this is that the 48 h labelling may not be long enough to generate a steady-state labelling pattern since there is a flux of fatty acids to TAG via polar lipids (Stymne and Stobart, 1987).
Putative reasons for higher carbon partitioning to oil
The regulation of oil synthesis in developing seeds can occur at multiple levels in the biochemical conversion of photosynthetically fixed carbon into TAG. Sucrose transport in conjunction with the regulatory mechanisms in the endosperm play a central role in determining the final amount of oil in the endosperm. Sucrose from source organs is imported into the seed and degraded to pyruvate through glycolysis, both in the cytosol and in the plastid (Plaxton and Podesta, 2006). Pyruvate subsequently enters de novo fatty acid synthesis in the plastid and finally is acylated to the glycerol backbone in the endoplasmatic reticulum to give rise to TAG. Starch is synthesized in the plastid from hexose sugars upstream in glycolysis with less metabolic cost for the plant as compared to oil synthesis. This raises the following questions: does the high-oil oat cultivar have a lower starch synthesizing capacity, or does the higher oil result from a more efficient oil synthesis in the two competing pathways?
The key force behind ‘sink strength’ (the capacity to attract sucrose) is the combined activity of different isoforms of invertases and sucrose synthase that cleaves sucrose in sink tissues (Sturm and Tang, 1999). Since these enzymes also regulate the sucrose/hexose ratio, they have been implicated as important factors in carbon partitioning into different biochemical pathways (Weber et al., 1996; Cheng and Chourey, 1999; Sturm and Tang, 1999; Weschke et al., 2003). The high-oil cv. Matilda exhibited increased sink strength under our experimental conditions as suggested by the higher sucrose incorporation together with the higher energy content contained in the accumulated oil plus starch as compared to cv. Freja. This may be, in part, due to alterations in enzyme activities involved with sucrose cleavage in the endosperm. However, this difference in total energy content of oil plus starch is not seen in these cultivars grown under greenhouse conditions, regardless of those differences seen in oil content (Banas et al., 2007). It is therefore likely that the most upstream biochemical component responsible for the difference in oil content between the cultivars is in the competition in sugar utilization between glycolysis and synthesis of starch and other sugar polymers in the endosperm cells.
Attempts to increase oil production of oil seeds include the following alternative approaches: (i) increasing fatty acid synthesis by modifying the activity of acetyl-CoA carboxylase (ACC) in Arabidopsis thaliana (Thelen and Ohlrogge, 2002a), (ii) increasing the supply of glycerol-3-phosphate through the overexpression of G3P dehydrogenase in rape seeds (Vigeolas et al., 2007), or (iii) increasing the transfer of acyl groups to the glycerol backbone through the overexpression of lysophosphatidate acyl transferase (Taylor et al., 2002) and diacylgycerol acyltransferase (Weselake et al., 2008) in rape seed. A major quantitative trait locus (QTL) for oil content in oat has been linked to an ACC gene (Kianian et al., 1999). ACC catalysis is the first committed step in fatty acid biosynthesis. However, this step is far downstream from the utilization of glucose for starch synthesis, which makes it unlikely to be the single determining enzyme for the switch from starch to oil synthesis in the endosperm cells.
Transcription factors regulating oil synthesis in plants are promising targets to engineer crops to produce higher oil yields. Candidate transcription factors that regulate oil synthesis that have been characterized in plants include WRINKLED1 in Arabidopsis thaliana (Cernac and Benning, 2004; Masaki et al., 2005; Baud et al., 2007). Mutants lacking this gene have a deficiency in oil biosynthesis of up to 80% due to reduced glycolytic enzyme activity (Focks and Benning, 1998). Similar results have been reported for A. thaliana overexpressing the LEC2 gene (Santos-Mendoza et al., 2008), a gene proposed to act upstream of WRI1 (Baud et al., 2007). Other examples are the dof-type transcription factors from soybean (Glycince max) GmDof4 and GmDof11, that by overexpression in A. thaliana induce the expression of one of the subunits of acetyl CoA carboxylase (Wang et al., 2007).
In this study, the high accumulation of carbon in oil found in the high-oil oat cv. Matilda compared to the medium-oil oat cv. Freja during the very early stages of development suggests that the regulation of genes or proteins involved with oil biosynthesis and/or breakdown is different in the endosperms of these cultivars. Global gene expression studies using novel methods for ultra-deep EST sequencing of oat endosperm (Schuster, 2008) is likely to identify key events regarding gene expression as well as to identify enzymatic pathways regulating the carbon flux into oil in oat endosperm. These studies, in combination with metabolic flux analyses and the silencing of identified genes through antisense ODN (Sun et al., 2007), will improve our understanding of those factors which contribute to the accumulation of starch and oil in oat endosperm. It is this understanding that will enable us to engineer enhanced pathways into plants that have the potential to redirect fixed carbon from photosynthesis into oil.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Definitions of stages A-J of seed development in planta.
Supplementary Material
Acknowledgments
This work was supported by Chevron (Award nr CHV1KD4), LTJ-faculty grant for LTH-SLU collaborations, and the Swedish Research Council for Environment, Agricultural Sciences, and Spatial Planning (Formas). We would also like to thank Mr Hassan Hartman for initial studies of the in vitro method for detached oat panicles.
Glossary
Abbreviations
- DW
dry weight
- TAG
triacylglycerol
References
- Alexander DE, Seif RD. Relation of kernel oil content to some agronomic traits in maize. Crop Science. 1963;3:354–355. [Google Scholar]
- Alonso AP, Goffman FD, Ohlrogge JB, Shachar-Hill Y. Carbon conversion efficiency and central metabolic fluxes in developing sunflower (Helianthus annuus L.) embryos. The Plant Journal. 2007;52:296–308. doi: 10.1111/j.1365-313X.2007.03235.x. [DOI] [PubMed] [Google Scholar]
- Banas A, Debski H, Banas W, et al. Lipids in grain tissues of oat (Avena sativa): differences in content, time of deposition, and fatty acid composition. Journal of Experimental Botany. 2007;58:2463–2470. doi: 10.1093/jxb/erm125. [DOI] [PubMed] [Google Scholar]
- Baud S, Mendoza MS, To A, Harscoet E, Lepiniec L, Dubreucq B. WRINKLED1 specifies the regulatory action of LEAFY COTYLEDON2 towards fatty acid metabolism during seed maturation in Arabidopsis. The Plant Journal. 2007;50:825–838. doi: 10.1111/j.1365-313X.2007.03092.x. [DOI] [PubMed] [Google Scholar]
- Beringer H. Influence of temperature and seed ripening on in vivo incorporation of 14CO2 into lipids of oat grains (Avena sativa L.) Plant Physiology. 1971;48:433–436. doi: 10.1104/pp.48.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Cernac A, Benning C. WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. The Plant Journal. 2004;40:575–585. doi: 10.1111/j.1365-313X.2004.02235.x. [DOI] [PubMed] [Google Scholar]
- Cheng WH, Chourey PS. Genetic evidence that invertase-mediated release of hexoses is critical for appropriate carbon partitioning and normal seed development in maize. Theoretical and Applied Genetics. 1999;98:485–495. [Google Scholar]
- Da Silva PMFR, Eastmond PJ, Hill LM, Smith AM, Rawsthorne S. Starch metabolism in developing embryos of oilseed rape. Planta. 1997;203:480–487. [Google Scholar]
- Durrett TP, Benning C, Ohlrogge J. Plant triacylglycerols as feedstocks for the production of biofuels. The Plant Journal. 2008;54:593–607. doi: 10.1111/j.1365-313X.2008.03442.x. [DOI] [PubMed] [Google Scholar]
- FAOSTAT. ProdSTAT, crops processed. Rome: Food and Agriculture Organization of the United Nations; 2008. [Google Scholar]
- Fisher DB, Wang N. Sucrose concentration gradients along the post-phloem transport pathway in the maternal tissues of developing wheat grains. Plant Physiology. 1995;109:587–592. doi: 10.1104/pp.109.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focks N, Benning C. wrinkled1: a novel, low-seed-oil mutant of Arabidopsis with a deficiency in the seed-specific regulation of carbohydrate metabolism. Plant Physiology. 1998;118:91–101. doi: 10.1104/pp.118.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kianian SF, Egli MA, Phillips RL, et al. Association of a major groat oil content QTL and an acetyl-CoA carboxylase gene in oat. Theoretical and Applied Genetics. 1999;98:884–894. [Google Scholar]
- Lee HJ, Seebauer JR, Below FE. An improved technique for culture of rice panicles. Plant Cell Tissue and Organ Culture. 2000;60:55–60. [Google Scholar]
- Marriott KM, Northcote DH. Breakdown of lipid reserves in endosperm of germinating castor beans. Biochemical Journal. 1975;148:139–144. doi: 10.1042/bj1480139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki T, Mitsui N, Tsukagoshi H, Nishii T, Morikami A, Nakamura K. ACTIVATOR of Spo(min)::LUC1/WRINKLED1 of Arabidopsis thaliana transactivates sugar-inducible promoters. Plant and Cell Physiology. 2005;46:547–556. doi: 10.1093/pcp/pci072. [DOI] [PubMed] [Google Scholar]
- Oo KC, Teh SK, Khor HT, Ong ASH. Fatty-acid synthesis in the oil palm (Elaeis guineensis): incorporation of acetate by tissue slices of the developing fruit. Lipids. 1985;20:205–210. [Google Scholar]
- Patel M, Crank M, Dornburg V, Hermann B, Roes L, Hüsing B, Overbeek L, Terragni F, Recchia E. 2006. Medium and long-term opportunities and risks of the biotechnological production of bulk chemicals from renewable resources: the potential of white biotechnology. Utrecht University, http://www.chem.uu.nl/brew/BREW_Final_Report_September_2006.pdf. [Google Scholar]
- Peterson DM, Wood DF. Composition and structure of high-oil oat. Journal of Cereal Science. 1997;26:121–128. [Google Scholar]
- Plaxton WC, Podesta FE. The functional organization and control of plant respiration. Critical Reviews in Plant Sciences. 2006;25:159–198. [Google Scholar]
- Poirier Y, Ventre G, Caldelari D. Increased flow of fatty acids toward beta-oxidation in developing seeds of Arabidopsis deficient in diacylglycerol acyltransferase activity or synthesizing medium-chain-length fatty acids. Plant Physiology. 1999;121:1359–1366. doi: 10.1104/pp.121.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price PB, Parsons JG. Lipids of seven cereal grains. Journal of the American Oil Chemists Society. 1975;52:490–493. doi: 10.1007/BF02640738. [DOI] [PubMed] [Google Scholar]
- Ruuska SA, Schwender J, Ohlrogge JB. The capacity of green oilseeds to utilize photosynthesis to drive biosynthetic processes. Plant Physiology. 2004;136:2700–2709. doi: 10.1104/pp.104.047977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Mendoza M, Dubreucq B, Baud S, Parcy F, Caboche M, Lepiniec L. Deciphering gene regulatory networks that control seed development and maturation in Arabidopsis. The Plant Journal. 2008;54:608–620. doi: 10.1111/j.1365-313X.2008.03461.x. [DOI] [PubMed] [Google Scholar]
- Sarmiento C, Garces R, Mancha M. Oleate desaturation and acyl turnover in sunflower (Helianthus annuus L.) seed lipids during rapid temperature adaptation. Planta. 1998;205:595–600. [Google Scholar]
- Schuster SC. Next-generation sequencing transforms today's biology. Nature Methods. 2008;5:16–18. doi: 10.1038/nmeth1156. [DOI] [PubMed] [Google Scholar]
- Schwender J, Ohlrogge JB. Probing in vivo metabolism by stable isotope labeling of storage lipids and proteins in developing Brassica napus embryos. Plant Physiology. 2002;130:347–361. doi: 10.1104/pp.004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh BK, Jenner CF. Culture of detached ears of wheat in liquid culture: modification and extension of the method. Australian Journal of Plant Physiology. 1983;10:227–236. [Google Scholar]
- Sturm A, Tang GQ. The sucrose-cleaving enzymes of plants are crucial for development, growth and carbon partitioning. Trends in Plant Science. 1999;4:401–407. doi: 10.1016/s1360-1385(99)01470-3. [DOI] [PubMed] [Google Scholar]
- Stymne S, Stobart AK. Triacylglycerol biosynthesis. Biochemistry of Plants. 1987;9:175–214. [Google Scholar]
- Sun CX, Ridderstrale K, Hoglund AS, Larsson LG, Jansson C. Sweet delivery: sugar translocators as ports of entry for antisense oligodeoxynucleotides in plant cells. The Plant Journal. 2007;52:1192–1198. doi: 10.1111/j.1365-313X.2007.03287.x. [DOI] [PubMed] [Google Scholar]
- Taylor DC, Katavic V, Zou JT, et al. Field testing of transgenic rapeseed cv. Hero transformed with a yeast sn-2 acyltransferase results in increased oil content, erucic acid content and seed yield. Molecular Breeding. 2002;8:317–322. [Google Scholar]
- Thelen JJ, Ohlrogge JB. Both antisense and sense expression of biotin carboxyl carrier protein isoform 2 inactivates the plastid acetyl-coenzyme A carboxylase in Arabidopsis thaliana. The Plant Journal. 2002a;32:419–431. doi: 10.1046/j.1365-313x.2002.01435.x. [DOI] [PubMed] [Google Scholar]
- Thelen JJ, Ohlrogge JB. Metabolic engineering of fatty acid biosynthesis in plants. Metabolic Engineering. 2002b;4:12–21. doi: 10.1006/mben.2001.0204. [DOI] [PubMed] [Google Scholar]
- Waheeb KH, Karlsson G, Brismar K, et al. Fusion of oil bodies in endosperm of oat grains. Planta. 2008;228:589–599. doi: 10.1007/s00425-008-0761-x. [DOI] [PubMed] [Google Scholar]
- Wang HW, Zhang B, Hao YJ, Huang J, Tian AG, Liao Y, Zhang JS, Chen SY. The soybean Dof-type transcription factor genes, GmDof4 and GmDof11, enhance lipid content in the seeds of transgenic Arabidopsis plants. The Plant Journal. 2007;52:716–729. doi: 10.1111/j.1365-313X.2007.03268.x. [DOI] [PubMed] [Google Scholar]
- Weber H, Borisjuk L, Wobus U. Controlling seed development and seed size in Vicia faba: a role for seed coat-associated invertases and carbohydrate state. The Plant Journal. 1996;10:823–834. [Google Scholar]
- Weschke W, Panitz R, Gubatz S, Wang Q, Radchuk R, Weber H, Wobus U. The role of invertases and hexose transporters in controlling sugar ratios in maternal and filial tissues of barley caryopses during early development. The Plant Journal. 2003;33:395–411. doi: 10.1046/j.1365-313x.2003.01633.x. [DOI] [PubMed] [Google Scholar]
- Weselake RJ, Shah S, Tang M, et al. Metabolic control analysis is helpful for informed genetic manipulation of oilseed rape (Brassica napus) to increase seed oil content. Journal of Experimental Botany. 2008;59:3543–3549. doi: 10.1093/jxb/ern206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigeolas H, Mohlmann T, Martini N, Neuhaus HE, Geigenberger P. Embryo-specific reduction of ADP-Glc pyrophosphorylase leads to an inhibition of starch synthesis and a delay in oil accumulation in developing seeds of oilseed rape. Plant Physiology. 2004;136:2676–2686. doi: 10.1104/pp.104.046854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigeolas H, Waldeck P, Zank T, Geigenberger P. Increasing seed oil content in oil-seed rape (Brassica napus L.) by over-expression of a yeast glycerol-3-phosphate dehydrogenase under the control of a seed-specific promoter. Plant Biotechnology Journal. 2007;5:431–441. doi: 10.1111/j.1467-7652.2007.00252.x. [DOI] [PubMed] [Google Scholar]
- Young TE, Gallie DR. Regulation of programmed cell death in maize endosperm by abscisic acid. Plant Molecular Biology. 2000;42:397–414. doi: 10.1023/a:1006333103342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.