Abstract
Bone morphogenetic proteins (BMPs) have an emerging role in human cancers. Here we demonstrate that the BMP-signaling pathway is intact and functional in human pancreatic cancer cells, with several BMP signaling components and transcriptional targets upregulated in human pancreatic cancer specimens compared with normal pancreatic tissue. Functionally, multiple BMP family members, including BMP-2, BMP-4 and BMP-7, induce an epithelial to mesenchymal transition (EMT) in the human pancreatic cancer cell line Panc-1, as demonstrated by morphological alterations and loss of E-cadherin expression. BMP-mediated EMT results in an increase in invasiveness of Panc-1 cells, in part through increased expression and activity of matrix metalloproteinase (MMP)-2, a known mediator of pancreatic cancer cell invasiveness. Accompanying EMT, BMP reduces expression of the transforming growth factor (TGF)-β superfamily receptor, transforming growth factor-β type III receptor (TβRIII), for which we have previously demonstrated loss of expression during pancreatic cancer progression. Maintaining TβRIII expression inhibits BMP-mediated invasion and suppresses Smad1 activation. Further, Smad1 is required for BMP-induced invasiveness and partially responsible for BMP-mediated increases in MMP-2 activity. These data suggest that BMP signaling, through Smad1 induction and upregulation of MMP-2, is an important mediator of pancreatic cancer invasiveness and a potential therapeutic target for treating this deadly disease.
Introduction
Pancreatic cancer is the fourth leading cause of cancer death in the USA. Patients rarely exhibit symptoms before the cancer has become locally advanced or metastatic (1). Epithelial-derived cancer cells within a primary tumor become metastatic, in part, by undergoing epithelial to mesenchymal transition (EMT), enabling these cells to penetrate the basement membrane and access the bloodstream. EMT is characterized by loss of epithelial cell markers, including E-cadherin, and concomitant gain of mesenchymal cell markers, including Slug. EMT is also associated with an increase in enzymes that remodel the extracellular matrix, including matrix metalloproteinases (MMPs). MMPs have been implicated in facilitating the invasion and metastasis of breast, colon and pancreatic cancers (2,3). The signaling pathways that regulate MMP expression in these cancers have not been fully characterized. Better understanding of the molecular processes that facilitate the invasiveness and metastasis of pancreatic cancer cells is required to develop effective prevention and treatment strategies for this highly metastatic and deadly disease.
Bone morphogenetic proteins (BMPs) regulate developmental EMT, including neural crest migration and cardiac morphogenesis (2,4), and a role for BMP signaling in promoting the metastatic cascade is emerging (5–8). BMPs signal by binding an oligomeric receptor complex consisting of a type I receptor [activin-receptor like kinase (ALK)-2, ALK3 or ALK6] and a type II receptor [activin type II receptor, activin type II receptor B or BMP type II receptor (BMPR2)]. The constitutively active type II receptor transphosphorylates and activates the kinase activity of the type I receptor. Once active, the type I receptor phosphorylates the intracellular effector proteins, Smad1, 5 and 8, which complex with Smad4 to accumulate in the nucleus and mediate target gene transcription (9).
The expression of a number of BMP family members increases during tumorigenesis (5,6); however, the molecular and cellular consequences of this increased expression in cancer cells is not well understood. Overexpression of BMP in colon, prostate and ovarian cancer cells results in changes in cell morphology and increased cell motility and invasiveness, characteristic of EMT (5,7,10). While the contribution of BMP family members to EMT and its associated invasiveness has not been extensively characterized in pancreatic cancer cells, one recent study implicated BMP-4 in contributing to pancreatic carcinogenesis. BMP-4 induced EMT in the pancreatic cancer cell line, Panc-1, and this was associated with increased motility (8). BMP-4 expression has also been demonstrated to be increased in preinvasive pancreatic ductal lesions and in invasive pancreatic ductal adenocarcinoma as compared with normal pancreatic tissue in a murine model of pancreatic cancer (11). In human pancreatic adenocarcinoma, BMP-2, BMPR2 and ALK3 messenger RNA (mRNA) levels were increased and there was a significant correlation between BMP-2 expression and reduced post-operative survival of pancreatic cancer patients (12). These observations suggest that alterations in BMP signaling may have important consequences on human pancreatic cancer progression. In this study, we evaluate the BMP-signaling pathway and determine the functional consequences of BMP signaling in pancreatic cancer cells.
Materials and methods
Cell culture and reagents
All cell lines were obtained from American Type Culture Collection and maintained in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum. Transforming growth factor (TGF)-β1, BMP-2, BMP-4 and BMP-7 were obtained from R&D Systems (Minneapolis, MN).
Transforming growth factor-β type III receptor protein expression
Cells were plated at 250 000 cells per well in six-well plate. Forty-eight hours later, the cells were treated with BMP-4 (300 ng/ml) for 72 h, followed by binding and cross-linking of [125I] TGF-β1 as described previously (13).
Reverse transcription–polymerase chain reaction
RNA was isolated from cells using the RNAEasy kit (Qiagen, Valencia, CA). The RNA was reverse transcribed using random primers (Invitrogen, Carlsbad, CA). Polymerase chain reaction was performed using primers specific for BMP-4 (5), BMPR2, ALK3 and ALK6 (14), Smad1 and Smad5 (15), Smad4 (16), inhibitor of differentiation-1 (ID1) (17), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (18), E-cadherin (19), Slug (20) and MMP-2 (21). The products were analyzed on a 2% agarose gel and images were acquired using a Bio-Rad Gel Doc.
Smad1 phosphorylation
Seventy percent confluent cells were serum starved for 4 h, followed by BMP-4 treatment with indicated concentrations and times. Cells were lysed and subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis. Western blotting was performed using a phospho-specific Smad1 antibody (Cell Signaling, Danvers, MA), and a total Smad1 antibody generated by Biosource was used to control for equal protein loading.
Induction of EMT
Panc-1 cells were plated at 150 000 cells per well in a six-well dish. Twenty-four hours later, the cells were treated with the indicated concentrations of TGF-β, BMP-2, BMP-4 or BMP-7 for 72 h. The cells were then assayed for EMT by phase-contrast microscopy, RNA was isolated, as described above, and immunofluorescence was performed on EMT markers.
Immunofluorescence
Cells were fixed with a 1:1 solution of methanol and acetone for 10 min at −20°C. Normal rabbit serum (1% in phosphate-buffered saline) was used to block. E-cadherin antibody (BD Biosciences, San Jose, CA) was used at 1:200 with a rabbit anti-mouse antibody conjugated to Alexa 488 (Molecular Probes, Carlsbad, CA). F-actin was detected after fixation in 3.7% formaldehyde solution and permeabilization with 0.1% Triton X. The cells were blocked in bovine serum albumin (1% in phosphate-buffered saline) and incubated with Phalloidin conjugated to Texas Red (1:40 in 1% bovine serum albumin in phosphate-buffered saline) (Molecular Probes). Slides were mounted using 4′,6-diamidino-2-phenylindole-containing media (Vector Laboratories, Burlingame, CA). Immunofluorescence images were acquired using a NIKON inverted microscope.
Matrigel invasion assays
In vitro invasion assays were performed using 24-well Matrigel-coated transwells (BD Biosciences). Cells (50 000) were plated in the upper chamber in serum-free media. Media containing 10% fetal bovine serum was placed in the lower chamber as a chemoattractant. After 48 h, cells on the upper surface of the filter were removed and migrating cells on the underside were fixed and stained with the Three Step Stain Set (VWR, West Chester, PA). The filters were removed and mounted onto glass slides. Each filter was examined using a NIKON inverted microscope at ×10 magnification. For invasion assays with ALK3(QD), cells were infected for 3 days with 50 multiplicity of infection (MOI) of ALK3(QD) adenovirus or green fluorescent protein (GFP)-expressing adenovirus. A total of 100 000 cells were plated in Matrigel transwells and incubated for 48 h. For invasion assays with Smad1 small interfering RNA (siRNA), cells were infected for 48 h with 100 MOI of two Smad1 siRNAs, which have been published previously (22), and a scrambled control siRNA at 200 MOI. Cells were then treated for 72 h with ligand, and 100 000 cells were plated into the Matrigel transwells and incubated for 48 h.
Gelatin zymography
After treatment (48 h), cells were serum starved for 24 h in 500 μl of media with or without ligand. Thirty microliter of media was removed and added to an equivalent amount of Laemmli sample buffer lacking β-mercaptoethanol. Samples were separated using sodium dodecyl sulphate–polyacrylamide gel electrophoresis (10%) containing 0.1% gelatin at 4°C. The gel was incubated in 2.5% Triton X for 1 h and then placed in activation buffer (50 mM Tris–HCL, 150 mM NaCl, 10 mM CaCl2 and 0.02% Brij) for 16 h at 37°C. Gel was stained with Coomassie Blue for 45 min and destained for 15 min.
Smad1 knockdown
Panc-1 cells were plated at 150 000 cells per well and infected with 100 MOI of two Smad1 short hairpin RNA (shRNA) adenovirus constructs or scrambled shRNA control generously provided by Dr Maria Trojanowska. Forty-eight hours later, the cells were treated with BMP-4 to induce EMT. MMP-2 activity and invasiveness were assayed as described above.
Results
The BMP-signaling pathway is functional in pancreatic cancer cell lines
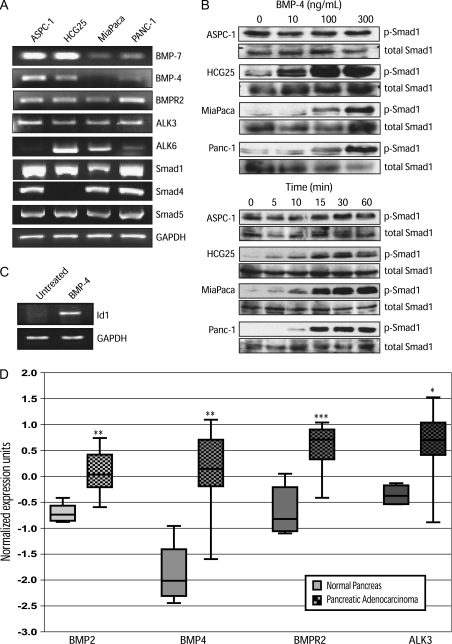
Loss of TGF-β responsiveness in pancreatic cancer as a result of altered expression and mutation of cascade components has been well characterized (1). However, responsiveness to its closely related family member, BMP, has not been extensively defined. To determine whether pancreatic cancer cell lines are BMP responsive, we assessed whether BMP signaling components were expressed by several human pancreatic cancer cell lines. All cell lines examined expressed the BMP ligands, BMP-4 and BMP-7, with the exception of BMP-4 in MiaPaca cells (Figure 1A). BMP-2 was also detected at low levels in ASPC-1 cells (data not shown). All the cell lines examined also expressed mRNA for BMP type I receptors (ALK3 and ALK6) and BMPR2. ALK6 mRNA expression was the most variable in the cell lines tested (Figure 1A). In addition, all cell lines examined expressed the BMP-responsive Smads (Smad1 and Smad5). The common Smad, Smad4, a frequent target of deletion in pancreatic cancer, was expressed by all cell lines except for HCG25, a highly metastatic pancreatic cancer cell line (23).
Fig. 1.
The BMP-signaling cascade is functional in pancreatic cancer cell lines. (A) Reverse transcription–polymerase chain reaction of BMP ligands (BMP-7 and BMP-4), BMPR2, type I receptors (ALK3 and ALK6), BMP-activated Smads (Smad1 and Smad5) and the common Smad, Smad4, from indicated pancreatic cancer cell lines. (B) Pancreatic cancer cells were immunoblotted for phosphorylated and total Smad1 following stimulation with indicated concentrations of BMP-4 for 10 min (top set of panels) or with 300 ng/ml of BMP-4 for the indicated times (bottom set of panels). (C) Reverse transcription–polymerase chain reaction for the BMP-responsive gene, ID1, after treatment with BMP-4 (300 ng/ml) for 72 h. GAPDH serves as a total complementary DNA control. All images are representative of three independent experiments. (D) Summary of publicly available microarray data from Oncomine for message levels of BMP-signaling cascade components in normal pancreas (left in each pair) compared with pancreatic adenocarcinoma (checkered boxes, right in each pair). ***P < 0.0002, **P < 0.002, *P < 0.02.
To establish whether the BMP pathway was functional, we stimulated the cell lines with BMP-4 and assessed Smad1 phosphorylation. HCG25, MiaPaca and Panc-1 cells all exhibited robust Smad1 phosphorylation following BMP-4 treatment in a dose- (Figure 1B) and time-dependent manner (Figure 1B). ASPC-1 cells exhibited higher basal levels of phosphorylated Smad1 making it difficult to assess induction of phosphorylated Smad1, but still appeared to have some time-dependent increases in phosphorylated Smad1 (Figure 1B). Induction of Smad1 phosphorylation was also observed upon BMP-2 treatment of these cell lines (data not shown). To verify active BMP responsiveness in pancreatic cancer cells, transcription of the BMP-responsive target gene, inhibitor of differentiation-1 (ID1), was examined. BMP-4 treatment induced ID1 expression in Panc-1 cells (Figure 1C). Taken together, these data demonstrate that pancreatic cancer cell lines express active BMP receptor complexes that are capable of activating the downstream BMP effector Smad1 and increasing transcription of a BMP-responsive target gene.
Multiple BMP signaling components are upregulated in pancreatic cancer
To determine whether the BMP-signaling cascade was altered in human pancreatic cancer specimens, we used publicly available microarray data sets on Oncomine (http://www.oncomine.org) to determine the relative level of several BMP ligands and receptors in the normal human pancreas and in human pancreatic adenocarcinomas (24). Analysis of the available BMP cascade components demonstrated that expression of BMP-2 and BMP-4 along with their receptors, BMPR2 and ALK3, were significantly increased in pancreatic cancer compared with normal pancreatic tissue (Figure 1D). In contrast, ALK6 and BMP-7 expression were not altered in pancreatic cancer relative to normal pancreatic tissue, whereas alterations in Smad1 expression were inconsistent between data sets. Unfortunately, information was not available on tumor grade, stage or prognosis, preventing insight into how altered BMP signaling may affect pancreatic tumor biology or patient outcome. However, there was significant upregulation of known BMP transcriptional target genes including osteocalcin, bambi, osteoprotegerin and osteopontin in pancreatic adenocarcinoma as compared with normal tissue (P value < 0.03, data not shown). These data suggest that BMP signaling is increased during human pancreatic carcinogenesis and that elevated BMP signaling may contribute to pancreatic cancer progression.
BMP family members induce EMT in a pancreatic cancer cell line
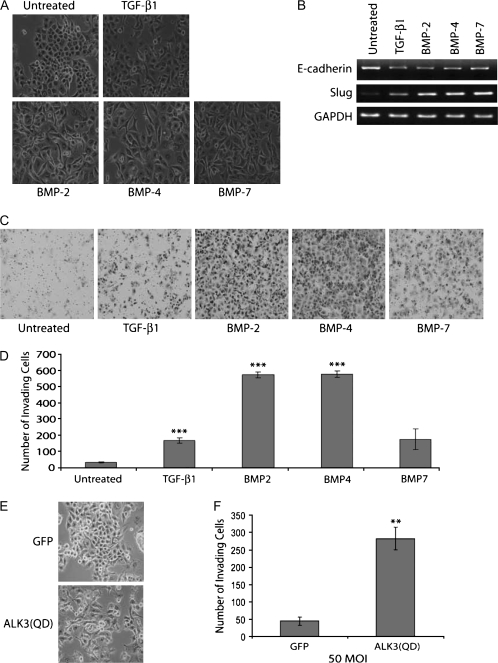
EMT is an important contributor to pancreatic cancer progression (2), as demonstrated from immunohistochemical analysis of EMT markers in human pancreatic cancer specimens including increased Slug expression (25), and the specific induction of EMT by BMP-4 in Panc-1 cells (8), a well-established pancreatic cancer cell line model of TGF-β1-induced EMT (8,13,26,27). To assess the consequences of an increase in multiple BMP family members and activation of downstream signaling in pancreatic cancer, we investigated the ability of additional BMP family members to induce EMT in Panc-1 cells. As previously reported, treatment with TGF-β1 or BMP-4 resulted in robust changes in the morphology of Panc-1 cells, from a cobblestone to a spindle-like phenotype, consistent with EMT [(Figure 2A) (8,26)]. In addition, here we demonstrate that BMP-2 and BMP-7 are also capable of inducing this morphological change in pancreatic cancer cells (Figure 2A).
Fig. 2.
BMP family members induce EMT and invasiveness in Panc-1. (A) Phase-contrast images of Panc-1 cells incubated with TGF-β1 (10 ng/ml), BMP-2 (300 ng/ml), BMP-4 (300 ng/ml) or BMP-7 (300 ng/ml) for 72 h. (B) Reverse transcription–polymerase chain reaction of E-cadherin and the mesenchymal marker, Slug. GAPDH serves as a total complementary DNA control. (C) Panc-1 cells were treated with TGF-β1 (10 ng/ml) or BMP-2, BMP-4 or BMP-7 (300 ng/ml) to induce EMT and plated into Matrigel chambers. Images are cells on the underside of the Matrigel chamber taken at ×20. The images are representative of three independent experiments. (D) Graphical representation of the average number of invading cells from three independent fields. Error bars are the standard error of the mean. (E) Phase-contrast images of Panc-1 cells adenovirally infected with a GFP control or constitutively active ALK3 [ALK3(QD)] (50 MOI), as indicated. (F) Matrigel filter images and graphical representation of the average number of invading ALK3(QD)-infected Panc-1 cells. ***P < 0.0002, **P < 0.002, Student’s t-test. Images are representative of three independent experiments.
EMT is usually associated with the loss of the epithelial adherens junction protein, E-cadherin, mediated through increases in expression of transcriptional repressors including Snail and Slug (2). TGF-β1, BMP-2, BMP-4 and BMP-7 all induced loss of E-cadherin at both the protein (supplementary Figure 1 is available at Carcinogenesis Online, data not shown) and mRNA level (Figure 2B), altered the localization of F-actin (supplementary Figure 1 is available at Carcinogenesis Online, data not shown) and induced expression of Slug at the mRNA level (Figure 2B). In contrast, none of the BMP ligands induced EMT in either the ASPC-1 or HCG25 pancreatic cancer cell lines as assessed by an absence of morphological alterations and unchanged levels of E-cadherin and Slug mRNA (data not shown). Taken together, these data demonstrate that BMP-2, BMP-4 and BMP-7 induce EMT in Panc-1, suggesting that increased BMP signaling could contribute to pancreatic cancer progression through inducing EMT.
BMP increases the invasiveness of Panc-1 cells
EMT is intimately associated with the acquisition of motility and invasiveness (2). BMP-4 has recently been demonstrated to increase the motility of pancreatic cancer cells (8); however, the contribution of BMP to pancreatic cancer cell invasiveness has not been investigated. Accordingly, we assessed the effects of BMP on the invasiveness of Panc-1 and ASPC-1 cells. BMP-2 and BMP-4 treatment of Panc-1 cells dramatically increased their invasion through Matrigel (Figure 2C and D). BMP-7 also increased invasiveness of Panc-1 cells, but not as dramatically as its family members (Figure 2C and D). In contrast, BMP-4-treated ASPC-1 cells did not exhibit significant changes in invasiveness as compared with untreated cells, consistent with the lack of BMP-induced EMT (data not shown). These data indicate that multiple members of the BMP family are capable of increasing the invasiveness of Panc-1 cells in the context of EMT.
Gene expression data suggested a preferential upregulation of the BMP type I receptor, ALK3, in human pancreatic cancers (Figure 1D). To gain insight into the mechanism by which BMP increases invasiveness, we expressed a constitutively active form of ALK3, ALK3(QD), in Panc-1 cells. Expression of this activated BMP receptor in Panc-1 cells resulted in a dramatic morphological change indicative of EMT (Figure 2E) and was accompanied by an increase in invasiveness (Figure 2F). These data suggest that the pathways responsible for EMT and its associated increase in invasiveness are downstream of BMP activation of ALK3.
BMP-induced EMT is associated with loss of transforming growth factor-β type III receptor expression
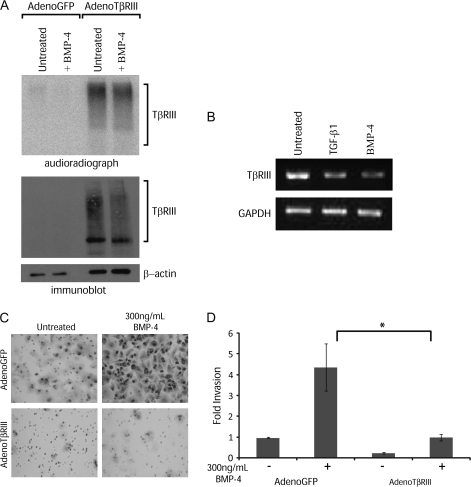
We have previously demonstrated that loss of expression of the transforming growth factor-β type III receptor (TβRIII) occurs during TGF-β-induced EMT in pancreatic cancer cells, preceding loss of E-cadherin expression, and that this loss is required for the increased invasiveness of pancreatic cancer cells (13). We have also demonstrated that TβRIII expression is downregulated in response to TGF-β, its well-characterized ligand (28). Additionally, we have recently shown that TβRIII can bind to several members of the BMP family and serve as a BMP receptor (29). To determine the effect of BMP on TβRIII expression and whether TβRIII expression is lost during BMP-mediated EMT, we induced EMT with BMP-4 and assayed TβRIII protein (Figure 3A) and mRNA levels (Figure 3B). Reduction of TβRIII protein and mRNA expression occurred with BMP-4 treatment (Figure 3), suggesting that loss of TβRIII expression is robustly associated with EMT induction, irrespective of the EMT-inducing ligand.
Fig. 3.
TβRIII expression is reduced during BMP-induced EMT and increasing TβRIII expression inhibits BMP-induced invasion. (A) Audioradiograph (top panel) of 125I-TGF-β cross-linked to endogenous TβRIII (left lanes) and exogenous HA-tagged TβRIII (right lanes) and immunoblot controls for TβRIII (middle panel) and β-actin (bottom panel). (B) TβRIII mRNA was determined by reverse transcription–polymerase chain reaction with primers specific to TβRIII (top panel, anti-HA antibody) and GAPDH served as a total RNA control (bottom panel). The images are representative of three independent experiments. (C) Matrigel filter images of Panc-1 cells adenovirally infected (50 MOI) with GFP control virus or wild-type human TβRIII-IRES-GFP, followed by treatment with BMP-4 (300 ng/ml). The images are representative of three independent experiments. (D) Graphical representation of the number of invading cells as determined from three independent experiments. *P < 0.02, Student’s t-test.
Increasing TβRIII expression suppresses BMP-mediated increases in invasiveness of Panc-1 cells
The specific loss of TβRIII expression at both the mRNA and protein levels during BMP-induced EMT suggested that loss of TβRIII expression might have functional significance, as it had during TGF-β-induced EMT, where its loss was a prerequisite for the acquisition of motility and invasiveness (13). Based on TβRIII’s potent suppressive effects on invasiveness, we investigated whether increasing TβRIII protein levels affected BMP-induced invasion. As observed previously (Figure 2C), BMP-4 increased the invasiveness of GFP-infected Panc-1 cells (Figure 3C, top panels). When complete loss of TβRIII expression was prevented during BMP-induced EMT in Panc-1 using a hemagglutinin (HA)-tagged TβRIII-expressing adenovirus (Figure 3A, right lanes), the BMP-4-induced invasiveness was significantly suppressed (Figure 3C and D), as was the basal invasiveness of Panc-1 cells (Figure 3C and D). In contrast, we did not observe any effects of increasing TβRIII levels on morphological alterations associated with BMP-induced EMT (data not shown). These studies suggest that loss of TβRIII expression is required for the increased invasiveness associated with BMP-induced EMT.
BMP increases the expression and activity of MMP-2
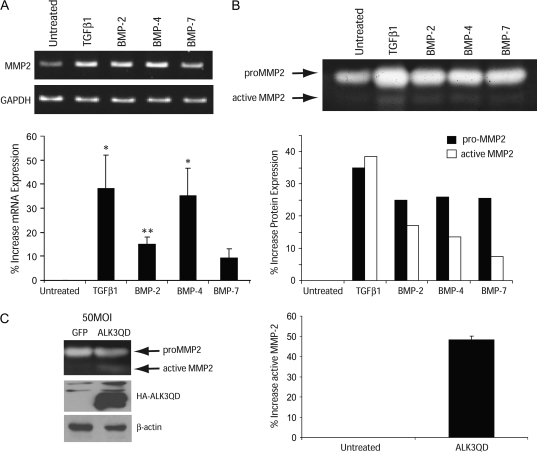
As these studies demonstrated, for the first time, BMP-induced invasiveness of Panc-1 cells, we further explored the mechanism for this effect. TGF-β1, a related family member, induces EMT and increases the invasiveness of Panc-1 cells through an increase in MMP-2 expression (30). However, to our knowledge, the ability of BMPs to alter MMP-2 expression or activity has not been explored in any cancer model. Accordingly, we investigated whether BMP alters MMP-2 expression or activity in the Panc-1 model. BMP-2 and BMP-4 both significantly increased MMP-2 mRNA levels in Panc-1, whereas BMP-7 treatment only slightly increased MMP-2 expression (Figure 4A). This increase in mRNA expression correlated with an increase in pro-MMP-2 levels, along with a significant increase in the active form of MMP-2 as measured by gelatin zymography (Figure 4B). Since we observed an increase in invasiveness with expression of constitutively active ALK3, we determined whether ALK3 mediated the increase in invasiveness through MMP-2 by assessing the effects of ALK3(QD) on MMP-2 protein expression and activity. Expression of ALK3(QD) in Panc-1 increased expression of active MMP-2 protein by ∼50% (Figure 4C). Taken together, these data demonstrate that BMP treatment activates ALK3, inducing EMT, which is accompanied by an increase in MMP-2 activity and an increase in the invasiveness of Panc-1 cells.
Fig. 4.
BMP increases MMP-2 expression and activity. (A) Reverse transcription–polymerase chain reaction of MMP-2 (top panel) in Panc-1 cells following treatment with either TGF-β1 (10 ng/ml), BMP-2, BMP-4 or BMP-7 (300 ng/ml), as indicated. GAPDH served as a total complementary DNA control (bottom panel). MMP-2 was normalized to GAPDH levels using ImageJ densitometry and the values were normalized to untreated samples (bottom graph). Graphically represented is the mean ± SD of three independent experiments. (B) Gelatin zymography images of MMP-2 protein expression from the media of EMT-induced treated cells. The images are representative of four independent experiments. The intensity of the pro-MMP-2 and active MMP-2 bands were determined by densitometry (ImageJ) and normalized to untreated samples and graphically represented as percent increase (bottom graph). (C) Gelatin zymography (top panel) of Panc-1 cells adenovirally infected with GFP or ALK3(QD). HA-ALK3(QD) expression and total protein was verified by western blot with HA (top) and β-actin (bottom), respectively. The intensity of active MMP-2 was determined by densiometry (ImageJ) and normalized to GFP-infected cells and graphically represented (right). Images represent four independent experiments. Statistics are determined by Student’s t-test. **P < 0.002, *P < 0.02.
TβRIII suppresses BMP-mediated increases in Smad1 phosphorylation
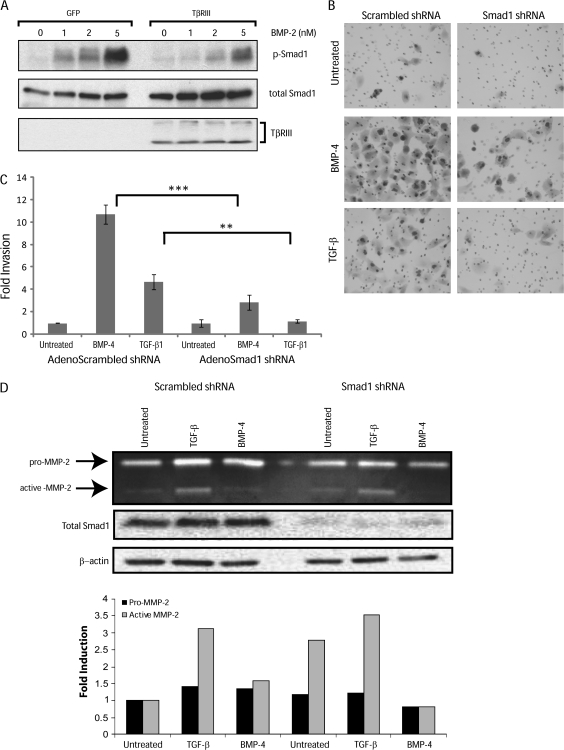
TβRIII abrogates both BMP- (Figure 3C) and TGF-β-mediated increases in invasion (13), and we have previously demonstrated that TβRIII can mediate suppression of MMP expression and activation in ovarian cancer cells (31). TβRIII is traditionally thought to bind ligand and facilitate ligand binding to the TGF-β type II receptor to increase downstream signaling (32). However, we have shown previously that TβRIII suppresses activation of Smad2 after TGF-β-induced EMT in pancreatic cancer cells (13). Therefore, we determined the effect of altering TβRIII expression on activation of the BMP-responsive Smad, Smad1, in Panc-1 cells. Using adenoviral constructs, we increased TβRIII expression with an HA-tagged full-length TβRIII and assessed BMP induction of Smad1 phosphorylation. An increase in TβRIII expression resulted in a significant decrease in Smad1 phosphorylation compared with the response in Panc-1 expressing GFP control virus (Figure 5A). These data suggest that TβRIII-mediated regulation of Smad1 signaling may affect the invasiveness of pancreatic cancer cells.
Fig. 5.
TβRIII suppresses Smad1 phosphorylation and loss of Smad1 suppresses BMP induction of MMP activity and pancreatic cancer cell invasiveness. (A) Panc-1 cells adenovirally infected with either GFP- or HA-tagged TβRIII-IRES-GFP were treated with the indicated concentrations of BMP-4 for 10 min. Total cell lysates were immunoblotted for phosphorylated Smad1 (top panel), total Smad1 (middle panel) and HA-tagged TβRIII expression was determined by immunoblot for HA. (B) Representative images of Matrigel filters of Panc-1 cells that were adenovirally infected (100 MOI) with two Smad1 shRNA constructs or a scrambled shRNA control, followed by treatment with BMP-4 (300 ng/ml). (C) Graphical representations of the fold invasion from three independent experiments. ***P < 0.0002, **P < 0.002, Student’s t-test. (D) Gelatin zymography (top panel) of media from Panc-1 cells infected with shRNA constructs against Smad1 and treated with indicated ligands to induce EMT. Smad1 levels (middle panel) and β-actin for total protein levels (bottom panel) were determined by immunoblotting. Images are representative of three independent experiments.
BMP-4 requires Smad1 to increase invasiveness during EMT
The BMP-signaling cascade is active and potently activates Smad1 in pancreatic cancer cells (Figure 1B), while dominant-negative Smad1 has been demonstrated to inhibit endothelial cell migration (33). Taken together with the effects of increasing TβRIII expression on inhibition of Smad1 phosphorylation and BMP-induced invasion, we investigated the contribution of Smad1 signaling to BMP induction of MMP-2 expression and invasiveness. Smad1 was reduced by combining two Smad1 shRNA adenoviruses (a scrambled shRNA was used as a control) resulting in significant reduction in Smad1 levels (Figure 5D, middle panel). There was no significant effect of loss of Smad1 on the morphological alterations associated with BMP- or TGF-β-induced EMT (data not shown), suggesting that Smad1-mediated signaling may not be required for these aspects of EMT. However, reducing Smad1 levels in Panc-1 significantly suppressed the induction of invasiveness by BMP-4 and TGF-β (Figure 5B and C). To determine whether this suppression of invasion resulted from modulation of MMP-2 in Panc-1 cells, we assessed the effects of reducing Smad1 levels on MMP-2 protein expression and activity using gelatin zymography. Knocking down Smad1 attenuated BMP-mediated increases in the expression of the pro and active forms of MMP-2 (Figure 5D); however, loss of Smad1 did not alter TGF-β-mediated induction of MMP-2 (Figure 5D). Taken together, these data suggest that BMP increases Panc-1 invasiveness via a Smad1-dependent signaling pathway.
Discussion
While the TGF-β pathway is often disrupted in pancreatic cancer, alterations in the BMP pathway aside from co-Smad, Smad4, have not been frequently reported. Our data demonstrate that human pancreatic cancer cells and human pancreatic cancers retain a functional BMP-signaling pathway. In addition, we demonstrate that the BMP pathway is important for the induction of EMT in Panc-1, one of the most extensively studied pancreatic cancer cell lines and the most established and robust model for pancreatic cancer cell EMT (8,13,26,27,34,35). While the effect of BMP on EMT in Panc-1 confirms a recent report using BMP-4 (8), the current studies extend these findings by demonstrating that multiple BMP family members, including BMP-2 and BMP-7, induce EMT in Panc-1 cells as demonstrated by morphological alterations, increased Slug expression, loss of TβRIII expression and increases in EMT-associated invasion. We also provide mechanistic insight into how BMP mediates these processes by demonstrating that: (i) ALK3 has a role in mediating the effects of BMP on invasiveness, potentially by increasing MMP-2 expression; (ii) TβRIII suppresses Smad1 activation and BMP-induced invasion and (iii) loss of Smad1 abrogates BMP-mediated increases in invasiveness, with Smad1 signaling partially contributing to BMP-induced MMP-2 activity. Along with the demonstration of an intact BMP-signaling pathway in pancreatic cancers, this study suggests that BMP family members contribute to pancreatic cancer progression, particularly invasion and metastasis. Although we observed upregulation of BMP-2 and BMP-4, whereas BMP-7 expression was unaltered, the relative contribution of individual BMP isoforms to human pancreatic cancer progression remains to be determined.
We demonstrate that several BMP ligands are able to increase the invasiveness of a pancreatic cancer cell model of EMT Panc-1 cells. Studies in Panc-1 were paralleled by studies in ASPC-1 pancreatic cancer cells, where BMP ligands did not significantly alter Smad1 phosphorylation (Figure 1B) nor induce EMT or invasion (data not shown), further suggesting a role for Smad1 activation in pancreatic cancer cell invasion. Additionally, ASPC-1 do not possess a functional Smad4 protein, therefore Smad1-mediated signals are not propagated in this cell line (36). The Panc-1 data presented here are consistent with the increased BMP-4 expression observed in the PanIN and PDAC mouse models of pancreatic tumor progression (11). In addition, although EMT in response to BMP ligands could only be demonstrated in the Panc-1 cell model, the importance of understanding the molecular contributions of EMT to pancreatic cancer is supported by the demonstration of EMT in human pancreatic cancer specimens (25), and the significant upregulation of the mediators of EMT, Snail (P = 0.044) and Slug (P = 9.1 × 10−5), in human pancreatic tumors (Oncomine).
Here we demonstrate for the first time that the acquisition of invasiveness is associated with BMP-mediated increases in the expression and activity of MMP-2, an extracellular matrix-remodeling enzyme that has frequently been implicated to contribute to invasion (3,30). We demonstrate that BMP-7 induced less MMP-2 expression and activity than BMP-2 and BMP-4 (Figure 4A). Accordingly, BMP-7 was less potent than BMP-2 and BMP-4 in increasing Panc-1 invasiveness (Figure 2C and D). As the degree of BMP-mediated increase in MMP-2 expression correlates with the increase in invasiveness, these data support a role for MMP-2 in BMP-induced invasion. Analysis of pancreatic cancer data sets in Oncomine indicates that MMP-2 expression is also dramatically upregulated in pancreatic adenocarcinomas relative to normal pancreatic tissue (P = 8.8 × 10−4), suggesting that elevated MMP-2 expression also contributes to human pancreatic cancer progression. However, loss of Smad1 in Panc-1 cells had a minimal effect on MMP-2 activity, suggesting that Smad1 is likely not a main component of the pathway contributing to the induction of MMP-2 expression and activity in response to TGF-β superfamily ligands. BMPs signal via non-canonical pathways, including activation of mitogen-activated protein kinase-signaling cascades (9). Future studies will be necessary to determine whether these non-canonical signaling pathways contribute to the induction of MMP-2.
Further studies will also be necessary to determine whether changes in BMP signaling components correlate with pancreatic tumor biology, including metastasis, since it is very difficult to get sufficient information on pancreatic tumor grade and prognosis with currently available clinical specimens. BMP ligands have previously been demonstrated to increase the invasiveness of ovarian and colon cancer cells (5,10). Whether BMP functions through induction of MMP-2 in a broad spectrum of human cancers remains to be established. In addition, the contribution of additional BMP transcriptional targets, including ID1, which has previously been shown to be critical for breast cancer invasion and metastasis (37), in pancreatic cancer metastasis remains to be elucidated.
TGF-β and BMP both induce EMT and invasiveness. Our data indicate that the pathway responsible for BMP-induced EMT and invasion is downstream of the type I BMP receptor ALK3. For the first time, we identify the importance of Smad1 in mediating the invasiveness of pancreatic cancer cells both induced by TGF-β and BMP. To date, mutation or loss of Smad1 during tumor progression has not been reported. The data presented here indicate that both BMP and TGF-β are individually able to drive this process. While BMP appears to induce several characteristics of EMT more significantly than TGF-β, further studies are necessary to determine whether it is a more potent inducer of EMT than TGF-β. In addition, the effect of different combinations of TGF-β superfamily ligands on EMT, Smad1 activation and invasiveness remains to be defined.
Another component common to both TGF-β- and BMP-signaling cascades is Smad4. Smad4 is required for TGF-β1-mediated EMT induction in pancreatic cancer cell lines (30). ASPC-1 cells express mRNA for Smad4 (Figure 1A); however, these cells are known to express substantially lower Smad4 protein levels than Panc-1 cells due to a point mutation that increases the ubiquitin-mediated degradation of Smad4 (36). In the present study, BMP failed to induce morphological changes, induce EMT or increase the invasiveness of ASPC-1 cells (data not shown). We also assessed EMT in HCG25 cells, a cell line that lacks Smad4 mRNA (Figure 1A), and did not observe any loss in E-cadherin or cytoskeletal reorganization with BMP treatment (data not shown). These data suggest that BMP-mediated EMT and invasiveness may also be dependent on Smad4. Loss of Smad4 is a late event in pancreatic cancer progression, with the majority of patients exhibiting loss of Smad4 in PanIN-3 lesions, prior to progression to invasive ductal adenocarcinoma (38). As the role that Smad4 plays in pancreatic carcinogenesis is still not fully understood, current studies are underway to define the role of its binding partners, including Smad1, during pancreatic cancer progression.
The studies presented here also demonstrate for the first time that the recently identified BMP receptor, TβRIII (29), is able to inhibit BMP-induced invasiveness of Panc-1 cells. These data support additional reports from our lab that TβRIII expression inhibits the motility and invasiveness of a number of cancer cells types, including breast, lung, ovarian and prostate cancer (31,39–41). Based on our previous work in Panc-1 cells, we hypothesize that loss of TβRIII expression precedes loss of E-cadherin during BMP-induced EMT and tumor progression; however, the causality of BMP leading to TβRIII and E-cadherin loss during EMT remains to be examined (13). Future studies will be necessary to determine the sequence of events involved in BMP-induced loss of TβRIII, loss of E-cadherin, upregulation of Slug, along with the contribution of TβRIII to BMP-induced invasion of additional cell types, especially lung cancer, for which increased BMP signaling has been documented. Specifically, BMP-2 expression is increased in 98% of lung adenocarcinomas, and human lung tumors have high levels of phosphorylated Smad1 and 5 and Id-1 compared with normal lung tissue (42).
The mechanism by which TβRIII inhibits invasion remains to be determined. Data presented here demonstrate that levels of TβRIII expression modulate activation of Smad1, consistent with its effects on Smad2 phosphorylation (13). Loss of Smad1 abrogated BMP-induced invasion, while not dramatically altering EMT induction or MMP-2 activity. These data suggest that BMP-mediated EMT, MMP-2 upregulation and invasion are each likely to be regulated by distinct signaling pathways activated downstream of ALK3, such as the mitogen-activated protein kinase cascade.
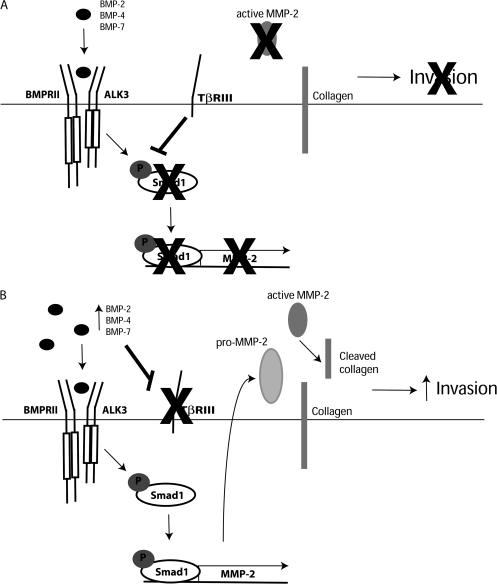
Previous reports by us and others, along with data presented here lead us to establish the following model: (i) BMP ligand expression increases during pancreatic tumorigenesis and (ii) BMP induces loss of TβRIII and induction of EMT, which (a) increases Smad1 signaling and MMP-2-induction and (b) increases invasion. In contrast, maintained TβRIII expression in normal tissue decreases Smad1 phosphorylation and inhibits cell invasion (Figure 6). Future studies will focus on the role of Smad1 phosphorylation and BMP-responsive target genes, in addition to MMP-2, that may be involved in BMP-mediated invasion. Since reduction of Smad1 levels had a dramatic inhibitory effect on pancreatic cancer cell invasiveness but did not completely inhibit the expression or activation of MMP-2, there are probably other pro-invasion pathways regulated by Smad1. These pathways are currently under investigation.
Fig. 6.
Proposed mechanism for BMP-induced invasion of pancreatic cancer cells. (A) In normal pancreatic epithelial cells, BMP family members bind to their receptors (ALK3, BMPR2 and TβRIII) on the cell surface. High expression of TβRIII in normal pancreatic cells modulates the level of Smad1 phosphorylation. Loss of Smad1 activation prevents expression and activity of MMP-2 and associated invasiveness. (B) In pancreatic cancer cells, BMP expression increases, which contributes to loss of TβRIII expression (13) and induction of high levels of Smad1 phosphorylation, leading to increased expression and activity of MMP-2. This increase in MMP-2 activity is associated with an increase in pancreatic cancer cell invasiveness.
The BMP type I receptors, ALK3 and ALK6, are highly homologous. Our data suggest that ALK3 is preferentially upregulated in human pancreatic cancers (Figure 1D), whereas ALK6 expression varies in pancreatic cancer cell lines (Figure 1A). Expression of constitutively active ALK3 is sufficient to induce EMT, increase MMP-2 activity and increase invasiveness in vitro. These studies support a role for ALK3 in BMP-induced invasiveness. Kleeff et al. (12) demonstrated that ALK3 mRNA expression was increased in pancreatic adenocarcinomas and were unable to detect mRNA for ALK6 in human pancreatic cancer cell lines and tumor specimens. We did not observe significant alterations in ALK6 mRNA levels in pancreatic tumors as compared with normal pancreatic tissue using Oncomine data sets. Therefore, alterations in ALK3 may be more relevant to pancreatic cancer progression. The differences in expression of ALK6 may explain the reduced effects of BMP-7, which binds preferentially to ALK6, on EMT and invasion. Future studies will explore whether activated ALK6 can induce EMT and invasion, and delineate the pathways, in addition to Smad1, that may be utilized by each of the BMP ligands and type I BMP receptors to mediate EMT, MMP-2 induction and invasiveness.
BMP has important roles in normal pancreatic cell homeostasis, including the regulation of genes involved in insulin production and secretion (43). The expression of BMP ligands and receptors is tightly regulated during pancreatic morphogenesis (44), with BMP critically regulating pancreatic development by stimulating genes involved in pancreatic epithelial progenitor cell expansion (45). Our data suggest that normal homeostatic functions mediated by BMP may be co-opted by pancreatic cancer cells to transition the cells from epithelial to mesenchymal, leading to increased MMP-2 activity and increased invasion.
Both EMT and invasion are critical mediators of metastasis as they facilitate the process of intravasation. Metastasis is the major cause of death for pancreatic cancer patients as well as for most patients with solid tumors. Thus, targeting signaling pathways with the ability to increase EMT and invasiveness could be of significant benefit in the treatment of human cancers. The present studies suggest that, for pancreatic cancer, the BMP pathway through Smad1 is an important regulator of these processes. Future studies will determine whether targeting components of the BMP pathway, including ALK3, Smad1 or MMP-2, provides an effective therapy for patients with metastatic pancreatic cancer.
Supplementary material
Supplementary Figure 1 can be found at http://carcin.oxfordjournals.org/
Funding
Pre-Doctoral Fellowship from the Department of Defense to K.C.K.; National Institutes of Health (CA106307 to G.C.B.).
Supplementary Material
Acknowledgments
We would like to thank Dr Carlos Arteaga (Vanderbilt) for the generous provision of the ALK3(QD) adenovirus, Dr Maria Trojanowska for the Smad1 siRNA adenoviruses and Dr Mythreye Karthikeyan for her assistance with zymography.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- ALK
activin-receptor like kinase
- BMP
bone morphogenetic protein
- BMPR2
BMP type II receptor gene
- EMT
epithelial to mesenchymal transition
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GFP
green fluorescent protein
- HA
hemagglutinin
- ID1
inhibitor of differentiation-1 gene
- MMP
matrix metalloproteinase
- MOI
multiplicity of infection
- mRNA
messenger RNA
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
- TβRIII
transforming growth factor-β type III receptor
- TGF
transforming growth factor
References
- 1.Li D, et al. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 2.Thiery JP, et al. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 3.Egeblad M, et al. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 4.Ma L, et al. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- 5.Deng H, et al. Bone morphogenetic protein-4 is overexpressed in colonic adenocarcinomas and promotes migration and invasion of HCT116 cells. Exp. Cell Res. 2007;313:1033–1044. doi: 10.1016/j.yexcr.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Rothhammer T, et al. Functional implication of BMP4 expression on angiogenesis in malignant melanoma. Oncogene. 2007;26:4158–4170. doi: 10.1038/sj.onc.1210182. [DOI] [PubMed] [Google Scholar]
- 7.Yang S, et al. Diverse biological effect and Smad signaling of bone morphogenetic protein 7 in prostate tumor cells. Cancer Res. 2005;65:5769–5777. doi: 10.1158/0008-5472.CAN-05-0289. [DOI] [PubMed] [Google Scholar]
- 8.Hamada S, et al. Bone morphogenetic protein 4 induces epithelial-mesenchymal transition through MSX2 induction on pancreatic cancer cell line. J. Cell. Physiol. 2007;213:768–774. doi: 10.1002/jcp.21148. [DOI] [PubMed] [Google Scholar]
- 9.Miyazono K, et al. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16:251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Theriault BL, et al. BMP4 induces EMT and Rho GTPase activation in human ovarian cancer cells. Carcinogenesis. 2007;28:1153–1162. doi: 10.1093/carcin/bgm015. [DOI] [PubMed] [Google Scholar]
- 11.Bardeesy N, et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006;20:3130–3146. doi: 10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleeff J, et al. Bone morphogenetic protein 2 exerts diverse effects on cell growth in vitro and is expressed in human pancreatic cancer in vivo. Gastroenterology. 1999;116:1202–1216. doi: 10.1016/s0016-5085(99)70024-7. [DOI] [PubMed] [Google Scholar]
- 13.Gordon KJ, et al. Loss of type III transforming growth factor beta receptor expression increases motility and invasiveness associated with epithelial to mesenchymal transition during pancreatic cancer progression. Carcinogenesis. 2008;29:252–262. doi: 10.1093/carcin/bgm249. [DOI] [PubMed] [Google Scholar]
- 14.Mazerbourg S, et al. Identification of receptors and signaling pathways for orphan bone morphogenetic protein/growth differentiation factor ligands based on genomic analyses. J. Biol. Chem. 2005;280:32122–32132. doi: 10.1074/jbc.M504629200. [DOI] [PubMed] [Google Scholar]
- 15.Bau B, et al. Bone morphogenetic protein-mediating receptor-associated Smads as well as common Smad are expressed in human articular chondrocytes but not up-regulated or down-regulated in osteoarthritic cartilage. J. Bone Miner. Res. 2002;17:2141–2150. doi: 10.1359/jbmr.2002.17.12.2141. [DOI] [PubMed] [Google Scholar]
- 16.Xu G, et al. Expression of TGF-beta signaling genes in the normal, premalignant, and malignant human trophoblast: loss of smad3 in choriocarcinoma cells. Biochem. Biophys. Res. Commun. 2001;287:47–55. doi: 10.1006/bbrc.2001.5533. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhary J, et al. Hormonal regulation and differential actions of the helix-loop-helix transcriptional inhibitors of differentiation (Id1, Id2, Id3, and Id4) in Sertoli cells. Endocrinology. 2001;142:1727–1736. doi: 10.1210/endo.142.5.8134. [DOI] [PubMed] [Google Scholar]
- 18.Zou M, et al. Microarray analysis of metastasis-associated gene expression profiling in a murine model of thyroid carcinoma pulmonary metastasis: identification of S100A4 (Mts1) gene overexpression as a poor prognostic marker for thyroid carcinoma. J. Clin. Endocrinol. Metab. 2004;89:6146–6154. doi: 10.1210/jc.2004-0418. [DOI] [PubMed] [Google Scholar]
- 19.Burke JM, et al. Expression of E-cadherin by human retinal pigment epithelium: delayed expression in vitro. Invest. Ophthalmol. Vis. Sci. 1999;40:2963–2970. [PubMed] [Google Scholar]
- 20.Yokoyama K, et al. Reverse correlation of E-cadherin and snail expression in oral squamous cell carcinoma cells in vitro. Oral Oncol. 2001;37:65–71. doi: 10.1016/s1368-8375(00)00059-2. [DOI] [PubMed] [Google Scholar]
- 21.Givant-Horwitz V, et al. Laminin-induced signaling in tumor cells: the role of the M(r) 67,000 laminin receptor. Cancer Res. 2004;64:3572–3579. doi: 10.1158/0008-5472.CAN-03-3424. [DOI] [PubMed] [Google Scholar]
- 22.Pannu J, et al. Transforming growth factor-beta receptor type I-dependent fibrogenic gene program is mediated via activation of Smad1 and ERK1/2 pathways. J. Biol. Chem. 2007;282:10405–10413. doi: 10.1074/jbc.M611742200. [DOI] [PubMed] [Google Scholar]
- 23.Akagi T, et al. Establishment and characteristics of a human pancreatic cancer cell line (HCG-25) Acta Pathol. Jpn. 1977;27:51–58. doi: 10.1111/j.1440-1827.1977.tb01859.x. [DOI] [PubMed] [Google Scholar]
- 24.Rhodes DR, et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9:166–180. doi: 10.1593/neo.07112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hotz B, et al. Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin. Cancer Res. 2007;13:4769–4776. doi: 10.1158/1078-0432.CCR-06-2926. [DOI] [PubMed] [Google Scholar]
- 26.Ellenrieder V, et al. Transforming growth factor beta1 treatment leads to an epithelial-mesenchymal transdifferentiation of pancreatic cancer cells requiring extracellular signal-regulated kinase 2 activation. Cancer Res. 2001;61:4222–4228. [PubMed] [Google Scholar]
- 27.Nakajima S, et al. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma. Clin. Cancer Res. 2004;10:4125–4133. doi: 10.1158/1078-0432.CCR-0578-03. [DOI] [PubMed] [Google Scholar]
- 28.Hempel N, et al. Expression of the type III TGF-beta receptor is negatively regulated by TGF-beta. Carcinogenesis. 2008;29:905–912. doi: 10.1093/carcin/bgn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirkbride KC, et al. Bone morphogenetic proteins signal through the transforming growth factor-beta type III receptor. J. Biol. Chem. 2008;283:7628–7637. doi: 10.1074/jbc.M704883200. [DOI] [PubMed] [Google Scholar]
- 30.Ellenrieder V, et al. TGF-beta-induced invasiveness of pancreatic cancer cells is mediated by matrix metalloproteinase-2 and the urokinase plasminogen activator system. Int. J. Cancer. 2001;93:204–211. doi: 10.1002/ijc.1330. [DOI] [PubMed] [Google Scholar]
- 31.Hempel N, et al. Loss of betaglycan expression in ovarian cancer: role in motility and invasion. Cancer Res. 2007;67:5231–5238. doi: 10.1158/0008-5472.CAN-07-0035. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Casillas F, et al. Betaglycan presents ligand to the TGF beta signaling receptor. Cell. 1993;73:1435–1444. doi: 10.1016/0092-8674(93)90368-z. [DOI] [PubMed] [Google Scholar]
- 33.Southwood M, et al. Regulation of bone morphogenetic protein signalling in human pulmonary vascular development. J. Pathol. 2008;214:85–95. doi: 10.1002/path.2261. [DOI] [PubMed] [Google Scholar]
- 34.Cannito S, et al. Redox mechanisms switch on hypoxia-dependent epithelial-mesenchymal transition in cancer cells. Carcinogenesis. 2008;29:2267–2278. doi: 10.1093/carcin/bgn216. [DOI] [PubMed] [Google Scholar]
- 35.Jungert K, et al. Sp1 is required for transforming growth factor-beta-induced mesenchymal transition and migration in pancreatic cancer cells. Cancer Res. 2007;67:1563–1570. doi: 10.1158/0008-5472.CAN-06-1670. [DOI] [PubMed] [Google Scholar]
- 36.Wan M, et al. SCF (beta-TrCP1) controls Smad4 protein stability in pancreatic cancer cells. Am. J. Pathol. 2005;166:1379–1392. doi: 10.1016/s0002-9440(10)62356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fong S, et al. Id-1 as a molecular target in therapy for breast cancer cell invasion and metastasis. Proc. Natl Acad. Sci. USA. 2003;100:13543–13548. doi: 10.1073/pnas.2230238100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bardeesy N, et al. Pancreatic cancer biology and genetics. Nat. Rev. Cancer. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 39.Dong M, et al. The type III TGF-beta receptor suppresses breast cancer progression. J. Clin. Invest. 2007;117:206–217. doi: 10.1172/JCI29293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turley RS, et al. The type III transforming growth factor-beta receptor as a novel tumor suppressor gene in prostate cancer. Cancer Res. 2007;67:1090–1098. doi: 10.1158/0008-5472.CAN-06-3117. [DOI] [PubMed] [Google Scholar]
- 41.Finger EC, et al. TβRIII suppresses non-small cell lung cancer invasiveness and tumorigenicity. Carcinogenesis. 2008;29:528–535. doi: 10.1093/carcin/bgm289. [DOI] [PubMed] [Google Scholar]
- 42.Langenfeld EM, et al. Bone morphogenetic protein 2 stimulation of tumor growth involves the activation of Smad-1/5. Oncogene. 2006;25:685–692. doi: 10.1038/sj.onc.1209110. [DOI] [PubMed] [Google Scholar]
- 43.Goulley J, et al. BMP4-BMPR1A signaling in beta cells is required for and augments glucose-stimulated insulin secretion. Cell Metab. 2007;5:207–219. doi: 10.1016/j.cmet.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Kim SK, et al. Signaling and transcriptional control of pancreatic organogenesis. Curr. Opin. Genet. Dev. 2002;12:540–547. doi: 10.1016/s0959-437x(02)00338-6. [DOI] [PubMed] [Google Scholar]
- 45.Hua H, et al. BMP4 regulates pancreatic progenitor cell expansion through Id2. J. Biol. Chem. 2006;281:13574–13580. doi: 10.1074/jbc.M600526200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.