Abstract
The study of experimental colon carcinogenesis in rodents has a long history, dating back almost 80 years. There are many advantages to studying the pathogenesis of carcinogen-induced colon cancer in mouse models, including rapid and reproducible tumor induction and the recapitulation of the adenoma–carcinoma sequence that occurs in humans. The availability of recombinant inbred mouse panels and the existence of transgenic, knock-out and knock-in genetic models further increase the value of these studies. In this review, we discuss the general mechanisms of tumor initiation elicited by commonly used chemical carcinogens and how genetic background influences the extent of disease. We will also describe the general features of lesions formed in response to carcinogen treatment, including the underlying molecular aberrations and how these changes may relate to the pathogenesis of human colorectal cancer.
Rodent colon tumor models (history and overview)
The study of experimental colon carcinogenesis in rodents has had a remarkably long history, dating back almost 80 years (1). Perhaps the earliest published study of Lorenz et al. (2) demonstrated tumorigenesis in the forestomach and intestine of mice following feeding with the polycyclic aromatic hydrocarbon, methylcholanthrene. Lisco et al. (3) reported that feeding radioactive yttrium to rats induced a high proportion of colon tumors. Walpole et al. (4) reported that white rats given injections of 4-aminodiphenyl and 3,2-dimethyl-4-aminodiphenyl developed colon tumors. However, the most commonly used model for sporadic colorectal cancer (CRC) takes advantage of the organotropism of the colon carcinogens, 1,2-dimethylhydrazine (DMH) and azoxymethane (AOM). Identification of these carcinogens arose initially from an early population study of Guamanians in which hydrazines were reported to be possible colon carcinogens with the hydrazine source being consumed cycad flour (5). When large quantities of cycad flour were fed to rats, adenocarcinomas in the colon arose in some of the animals. The carcinogen in cycad flour was subsequently found to be cycasin, a form of methylazoxymethanol (MAM) (6).
DMH, a metabolic precursor of MAM, was used in several early studies to induce tumors in rats (7–9). Repetitive treatment with this methylating agent was reported to produce colon tumors in rodents that exhibit many of the pathological features associated with the human disease (10–13). Thus, DMH has provided cancer researchers with a reproducible experimental system for studying ‘sporadic’ (non-familial) forms of CRC (14). However, AOM offers advantages over DMH, including enhanced potency and greater stability in dosing solution (15,16). Since then, several thousand studies using AOM have been published (17–19). We have used this mouse model extensively for 15 years and have compiled considerable data on sensitivity and lesion characteristics across a number of mouse lines (20–27).
The occurrence of spontaneously occurring cancers within the gastrointestinal tract of rodents is not as rare as might be expected. For example, Rowlatt et al. (28) described the occurrence of a wide range of tumors of the intestinal tract in aged C57BL/6 mice, although the majority of the lesions were located in the small intestine. Miwa et al. (29) reported 28 cases of spontaneous colon tumors in male Wistar rats. Vandenberghe et al. (30) in a study of Campylobacter-like bacteria reported 17 naturally occurring primary endophytic adenonocarcinomas in the ascending colon of Wistar rats. An examination of animal husbandry also demonstrated the presence of adenocarcinomas in the ascending colon of ACI rats foster bred by an Osaka female rat, perhaps depending on milk factor and humidity (31). Newmark et al. (32) found that maintaining mice on a semipurified diet, referred to as the new Western diet, designed to recapitulate a human Western diet, was capable of inducing colon tumors in 42% of 18-month-old C57BL/6 mice. Richie et al. (33) recently showed an induction of colon tumors in p53 knockout mice by causing glutathione depletion with buthionine sulfoximine treatment.
In summary, there are a number of advantages to studying the pathogenesis of carcinogen-induced colon cancer in rodent models. The models are highly reproducible, they can be readily tested on animals with different genetic backgrounds and the pathogenesis recapitulates human CRC, at least at the early stages. Historically, the majority of colon carcinogenesis studies have been carried out in rats (34,35). However, the high frequency of tumors generated within the distal colon of mice, as well as the histogenesis of multiple adenomas with subsequent development of adenocarcinomas, also validate the importance of this species for studying the pathogenesis of colon cancer (36,37). An advantage of murine models is the availability of extensive genetic information on individual mouse lines, the existence of recombinant inbred mouse panels and the ever-increasing number of transgenic, knockout and knockin genetic models that are available for study. Thus, the focus of this review will be mainly on murine models. One notable disadvantage of these models is the general lack of invasive and metastatic phenotype. However, in general, rodent models recapitulate the adenoma–carcinoma sequence that is found in human CRC. They have been used extensively to study cancer chemoprevention agents and dietary factors, although we will not discuss these studies in detail. In this review, we will discuss the general mechanisms of tumor initiation by these agents, describe the general features of the lesions formed by them and describe how they relate to human CRC.
Genetic background modulates carcinogen-induced tumors in rodents
Strain sensitivity in mice
As in human populations, genetic background of laboratory animals is a significant component of organ-specific carcinogenesis. Genetically defined inbred mouse strains that differ in their sensitivity to colon carcinogens offer distinct advantages for studying organ-specific carcinogenesis (Table I). For example, AKR/J and DBA/2J mice have been shown to be relatively resistant to colon tumor development, whereas Balb/cHea and SWR/J are moderately sensitive and A/J, P/J, STS/A and ICR/Ha are highly sensitive to DMH (21,38–44). These reported differences, however, are often variable and probably the result of environmental conditions under which the animals are maintained. For example, in one study, the relatively resistant AKR strain was found to develop a high proportion of invasive tumors in 16/21 female mice exposed to DMH for 25 weeks (44). Interestingly, route of administration is also important and directly influences colon tumor incidence (45). Izumi et al. (45) compared the carcinogenicity of DMH when given by oral, intragrastric and subcutaneous (s.c.) administration. The s.c. treatment was most effective at yielding organospecificy to the colon. Addressing the wide variation in dosing protocols, Bissahoyo et al. (46) reported that in A/J mice, s.c. injection of AOM yielded smaller tumors than following intraperitoneal (i.p.) delivery, a difference that was not observed in SWR/J mice.
Table I.
Differential sensitivity to carcinogens across mouse lines
| Straina | Carcinogen | No. of tumors per mouse | References |
| A/J | DMH, AOM | 9.2–41.6 | (26,42,46,254,255) |
| STS/A | DMH | 8.2–18.4 | (56) |
| SWR/J | DMH, AOM | 9.5–16 | (26,41,42,255) |
| FVB/N | AOM | 3.6 | (37) |
| C57Bl/6b | DMH, AOM, MAM acetate | 0–5.5 | (37,41,49,255–257) |
| BALB/cJ | AOM | 1–2 | (24,44,45) |
| ICR/Hac | DMH | n.d. | (41,257) |
| DBA/2J | 3-MC, DMH | 0–1 | (44,256,257) |
| AKR/J | DMH, AOM, MAM | <1.0 | (26,37,38,42,44,46,255) |
| 129/SV | AOM | 0 | (37) |
| BALB/cHeA | DMH | 0 | (56) |
3-MC, methylcholanthrene.
Tumor incidence in the most sensitive strain that typically approaches 100%.
Tumor incidences in the C57Bl/6 line are dependent upon the particular subline (N, J and Ha), ranging from 10 to 85% (251). n.d., not determined.
Incidence was 100%, but total number of tumors per colon was not reported.
Our laboratory has identified a panel of inbred mouse lines that differ markedly in their susceptibility to AOM at both early and late stages of tumorigenesis (21,27,47). SWR/J and A/J mice are remarkably sensitive to AOM exposure. For example, A/J mice develop up to 20 tumors in the distal colon, whereas tumors in AKR/J mice are very rare (25,27,37). These differences were also reported by Bissahoyo et al. (46) who confirmed the importance of AOM dosing regimens and environmental conditions on tumorigenesis in C57Bl/6J, DBA/2J, AKR/J, SWR/J and A/J mice. Such differential strain sensitivity to chemical carcinogens provides an excellent experimental tool for modeling interindividual sporadic cancer sensitivity that is evident within human populations.
A number of potential molecular mechanisms that may contribute to differential cancer sensitivity have been considered. These include rates of carcinogen activation/detoxification, the extent of acute DNA alkylation and the efficiency of repair (16). In addition, the balance between proliferation and apoptosis of initiated colon crypt cells has also been examined (39,47). Based on proliferation kinetics, it was suggested that AKR expresses a protective or resistance factor (repressor gene) that impedes progression of carcinogen-induced foci into colon tumors (39). Deschner et al. (48) further suggested that the differential sensitivity of inbred mice to MAM may be predicted by the acute colonic proliferative response to carcinogen exposure, in which the sensitive SWR/J colons demonstrated the largest expansion of the epithelial proliferative compartment and the resistant AKR/J colons the least.
A limited set of mouse progenitor strains are commonly used for constructing gene knockout and transgenic animals. As part of our long-standing interest in differential sensitivity to colon carcinogens, our laboratory established the AOM sensitivity of a panel of inbred mouse lines (FVB/N, 129/SvJ, C57Bl/6J and BALB/CJ) that are commonly used for constructing knockout and transgenic mouse strains (37). We reported that FVB/N developed on average 3.6 tumors per mouse and Balb/C mice developed one tumor per mouse (Table I). The 129SvJ and C57Bl/6J mice failed to develop tumors after four weekly injections of AOM (37), although Diwan et al. (49) did report differences in sensitivity to DMH of a panel of C57Bl/6 sublines (N, J and Ha). Despite these differences in tumor multiplicity, however, tumors shared a similar morphology regardless of strain. Of note, there was a lack of invasiveness and metastasis in even the most sensitive strains, an observation that underlies the vast majority of murine colon tumor models (50).
Jacoby et al. (51) conducted a genetic analysis of colon cancer susceptibility to DMH in mice. Mice were selected for study based on their stark differences in sensitivity to DMH. The prediction was that DMH-induced carcinogenesis would be a phenotype controlled by several unlinked loci. Matings were performed between C57BL/6Ha and ICR/Ha strains with DMH sensitivity of 0 versus 100%, respectively. Tumors were found in 40 of 122 progeny. Segregation analysis revealed that multiple loci contribute to the phenotype, but with significant linkage to a novel locus, Ccs1 on chromosome 12. Subsequent analysis identified a DNA mismatch repair gene, Mlh3, that physically maps to Ccs1, although there were no mutations identified in the protein-coding region of the gene in either susceptible or resistant mouse strains (52).
Since a large number of laboratories are using carcinogens to induce colon tumors in mice, the question of intralaboratory variability and standardization has emerged. Bissahoyo et al. (46) assessed AOM-induced tumorigenesis in A/J, SWR/J and AKR/J mouse strains using a variety of dosing regimens, as well as dietary factors. Using A/J mice, these investigators confirmed the utility of using 10 mg/kg body wt of AOM to produce a maximal effect in terms of tumor multiplicity, size and penetrance (26,37,42) although the maximum effect was achieved with only four doses (46). Importantly, no sex-dependent differences were reported and in utero exposure to AOM was not found to be carcinogenic (46). Histologically, these investigators reported that in SWR/J tumors from the 8 week treatment group, there was increased tumor progression, and in 10% of the colons, there was evidence for local invasion through the basement membrane, suggesting that AOM may have tumor-promoting properties in addition to its known ability to initiate tumors.
Recombinant inbred lines
Inbred mouse lines have also been used to map susceptibility quantitative trait loci that influence colon cancer. The Demant laboratory developed a series of recombinant congenic mouse strains referred to as the CcS/Dem that were made from BALB/cHeA and STS/A strains (53–55). Each substrain has a different random subset of ∼12.5% of genes from STS/A and 87.5% of genes from BALB/cHeA (53). Using DMH, five susceptibility loci were identified (Scc1–Scc5) using 192 F2 mice between the susceptible recombinant congenic strain CsS-19 and BALB/c (56). A candidate for Scc1 was subsequently identified as protein tyrosine phosphatase receptor type-J (Ptprj) (57). A follow-up study using AOM confirmed the original five Scc loci and also mapped five additional new Scc loci (Scc11–Scc15) (58). Interestingly, a different subset of susceptibility genes was involved in the formation of adenomas and premalignant aberrant crypt foci (ACF) (59). Recently, Sevignani et al. (60) reported that microRNA (miRNA) genes are frequently located near mouse cancer susceptibility loci. Using a genome-wide approach, these investigators compared the genomic position of mouse cancer susceptibility loci with the positions of a panel of mouse miRNAs. Using a random effect Poisson regression model, they reported that a statistically significant number of miRNAs are located close to tumor susceptibility loci. Overall, 41.9% of miRNAs were located <5 megabase from the peak of the closest locus. These data thus provide a catalog of miRNAs in inbred mice that could represent a new family of genes involved in the development of human solid tumors (60).
ApcMin/+ mice
One mouse line that has been used to a limited extent for carcinogenesis studies is the ApcMin/+ mouse strain, developed from the results of a germ line mutagenesis study with N-ethyl-N-nitrosourea combined with phenotypic screening (61). Although ApcMin/+ mice spontaneously form a large number of benign adenomas in the small intestine, colon tumors develop in fewer than half of the animals. AOM treatment, however, can increase tumor incidence in the colons of these mice by up to 6-fold (62,63), significantly decreasing the number of animals required for a single experiment. It should also be noted that the number of colon tumors formed in ApcMin/+ mice are significantly greater than that formed in wild-type C57BL/6 mice, which is a relatively resistant background (63). Although the number of studies in which AOM has been given to ApcMin/+ is limited, this model has proven to be useful for studying the effects of chemopreventive compounds (64), where differences in agent metabolism between the large and small intestine may prove to be important. The tumors that form in AOM-treated ApcMin/+ mice also have interesting genetic features as well. On most mouse strain backgrounds, AOM induces tumors with activating mutations in the β-catenin gene (see below). However, colon tumors formed in AOM-treated ApcMin/+ mice frequently undergo loss of heterozygosity at the normal Apc locus (62). Although it is not clear how AOM might promote loss of heterozygosity in this model, it is through this mechanism by which colon tumors frequently form in human familial adenomatous polyposis patients, which is an advantage of this model.
Metabolism of DMH and AOM
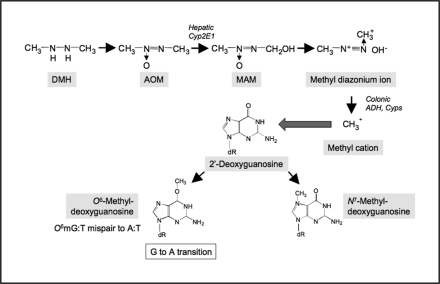
DMH and its metabolite, AOM, are procarcinogens that require metabolic activation to form DNA-reactive products (65–67). Metabolism of these compounds involves multiple xenobiotic-metabolizing enzymes (Figure 1), which proceeds through several N-oxidation and hydroxylation steps, including the formation of MAM following hydroxylation of AOM. The reactive metabolite, MAM, readily yields a methyldiazonium ion, which can alkylate macromolecules in the liver and colon (65,68,69), including the addition of methyl groups at the O6 or N7 position of guanine (O6-methyl-deoxyguanosine and N7-methyl-deoxyguanosine). MAM is a substrate of the nicotinamide adenine dinucleotide+-dependent dehydrogenase present in the colon and liver, suggesting that the active metabolite of MAM might be the corresponding aldehyde (70). A direct role for the alcohol-inducible cytochrome P-450 isoform, CYP2E1, in activation of AOM and MAM has been established (71). These metabolites of CYP2E1 are transported to the colon via the bloodstream. The ability of AOM and DMH to target the colonic mucosa is probably a consequence of the relative stability of the hydroxylated metabolite, MAM (72); with a half-life of ∼12 h, there is sufficient time for MAM to distribute to the colon (73). Further activation of blood-borne metabolites may then proceed via non-P450-dependent mechanisms, including possible oxidation of MAM, directly within the colon (71,74).
Fig. 1.
Metabolism of AOM involves multiple transcriptionally regulated xenobiotic-metabolizing enzymes.
It was originally hypothesized that the differential sensitivity of mouse strains to DMH may be attributed to differential activation of the procarcinogen (26). Sohn et al. (71) suggested that MAM is activated by alcohol dehydrogenase directly in rat colon to its proximate mutagen, methyldiazonium ion. To determine whether this ultimate metabolic step is critical to the activation of MAM and strain sensitivity, our laboratory evaluated the capacity of colon cytosols to activate MAM in an in vitro system (75). Due to its reactivity, MAM was generated from MAM acetate by acetylcholinesterase immediately prior to use. However, colon cytosols isolated from 7-week-old sensitive or resistant mice (SWR/J and AKR/J mice, respectively) had only minimal capacity to metabolize MAM, even at the highest concentration of MAM tested (2 mM). This result suggests that metabolism of MAM occurs by processes in the mouse that are independent of colonic alcohol dehydrogenase (75). The primary promutagenic lesion produced by AOM is methylation at the O6 position of guanine (76). Maximum alkylation of target tissue DNA occurs 6–12 h after injection with DMH (77,78). To examine the possibility that differential sensitivity to DMH/AOM correlates with DNA methylation, Papanikolaou et al. (16) measured DNA adduct levels 6 h after exposure of mice to AOM (i.p. 20 mg/kg). Using both immunoslot blot and high-performance liquid chromatography-fluorescence analyses, adduct levels were found to be significantly higher (2- to 3-fold) in the colons of the resistant AKR/J mice 6 h after exposure. In a second experiment, adduct levels were slightly higher (1.4-fold) in the sensitive SWR/J colons at 12 h, but had been markedly reduced by 48 h, whereas in the AKR/J, levels were unchanged (16). Furthermore, incubation of colon microsomes with AOM and calf thymus DNA showed comparable levels of O6-methyl-deoxyguanosine, demonstrating equivalent metabolic capacity in both strains. From these experiments, it can be concluded that the differential strain sensitivity is not the result of differences in carcinogen metabolism, but instead probably involves subsequent carcinogenic steps such as tumor promotion.
Other colon carcinogens
In addition to MAM acetate, AOM and DMH, there are other carcinogens that induce colon tumors to varying extents, including: (i) heterocyclic amines (HCAs), e.g. 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and 2-amino-33-methylimidazo[4,5-f]quinoline (IQ); (ii) aromatic amines, e.g. 3,2′-dimethyl-4-aminobiphenyl (DMAB) and (iii) alkylnitrosamide compounds, e.g. methylnitrosourea (MNU) and N-methyl-N′-nitro-N-nitrosoguanidine (MNNG). These carcinogens are summarized in Table II. Earlier studies tested the carcinogenicity of these agents, and subsequent studies were performed to evaluate their effects on histogenesis, cell proliferation kinetics, interaction with genetic susceptibility traits, dietary components, fecal stream components (e.g. bile acids) and bacterial flora, among other factors (79,80). The spectrum of epithelial lesions including malignancies induced in the colon by these carcinogens is similar to neoplastic lesions observed within the human colon.
Table II.
Summary of carcinogens used to induce colon cancer in rodents
| Carcinogens | Route of administration | Species | References |
| MNNG | Rectal infusion, 0.5 ml of 0.25% MNNG solution/day × 32 | Rats (Donryu), females | (108) |
| MAM acetate | Rectal infusion, 1 mg MAM acetate in 0.5 ml deionized water/day × 7, 14 or 26 | Rats (Donryu), males | (258) |
| MAM acetate | i.p. 20 or 35 mg/kg body wt ×1; intravenous, 35 mg/kg body wt × 1 | Rats (Sprague-Dawley), males | (259) |
| N-nitroso-N-butylurea | i.p. 75 or 150 mg/kg body wt × 1 | Mice (C57BL/6), males | (260) |
| Methylnitrosourea | Intrarectal, 2.5 mg/rat/week × 2 | Rats (F344), males | (261) |
| DMN-OAc | i.p. 0.1 mmol/kg body wt × 1 | Rats (Sprague-Dawley), males | (262) |
| PhIP | Diet, 400 p.p.m., 52 weeks | Rats (F344) | (96) |
| IQ | Diet, 300 p.p.m., 55 weeks (male), 72 weeks (female) | Rats (F344) | (263) |
| DMAB | s.c. 100 mg/kg, 20 weeks | Rats (F344) | (264) |
HCAs
The formation of mutagens upon broiling of fish and meat was first discovered by the Sugimura laboratory (81–83). Since then, considerable progress has been made in this field. For example, IQ, produced from food pyrolysis, was first isolated from broiled fish and subsequently from a variety of broiled or cooked fish and meat (84,85). IQ is a strong mutagen in Salmonella typhimurium and also induces mutations in Chinese hamster lung cells and hepatocellular carcinomas in rodents and non-human primates (86,87). IQ has genotoxic activity in different organs (88,89). Other cooked food mutagens include 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline, 2-amino-3,4-dimethylimidazo[4,5-f]quinoline and PhIP. Among a number of HCAs that have been demonstrated to be highly mutagenic and tumorigenic in rodent models, IQ and PhIP have received considerable attention because they demonstrate a multitarget organospecificity, with cancer induction in colon, mammary gland and prostate of rodents (90–92). The precursors of IQ-type HCAs are creatinine, amino acids and sugars in meat and fish (93). It has been shown that IQ requires metabolic activation by liver microsomes for conversion to its ultimate carcinogen, while forming high levels of DNA adducts in a number of organs (94,95).
Although it is not clear whether HCAs directly contribute to human carcinogenesis, it is certain that these compounds are present in cooked foods and thus pose a credible risk to human populations. For example, colon tumors were induced in male F344 rats by administering PhIP in the diet at 100 and 400 p.p.m. for 52 and 104 weeks (90,96). Although the incidence of colon tumors was ≈43 and 55% in animals given PhIP at 100 and 400 p.p.m., respectively, severe toxicity resulted from the PhIP feeding regimen. Other investigators have also utilized IQ and PhIP to induce colon tumors. The results of these studies indicate that tumor incidence is very low, ranging from 5 to 28% when these agents were administered in the diet for up to 52 weeks (97). Most of the tumors were localized to the small intestine (20%) and very few tumors were found in the colon (8%). Based on these findings, Nakagama et al. (98) developed a novel animal model to enhance the formation of colon lesions by using a combination of PhIP and a high-fat diet. Short-term intermittent feeding of 400 p.p.m. PhIP in combination with a high-fat diet resulted in accelerated tumor formation. Since HCAs may contribute to human colon cancer development, this latter model may be useful for inducing colon carcinogenesis in animal model systems and to investigate the chemopreventive activity of potential agents against colon carcinogenesis. Recently, Doi et al. (99) identified a set of PhIP-altered genes (e.g. Stat1 and VEGF) in rat colon tumors by giving an initiating dose of AOM followed by varying doses of PhIP (up to 200 p.p.m.), a tumor-enhancing effect that was clearly dose dependent (100).
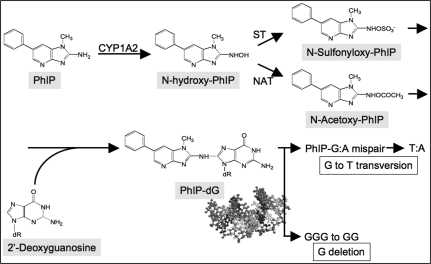
Following its administration, PhIP is rapidly absorbed from the alimentary tract and widely distributed throughout the body. PhIP is then metabolically activated by Phase I and Phase II enzymes mainly in the liver, similar to other HCAs, and probably in terminal target organs as well (82,101). As shown in Figure 2, cytochrome P450-mediated N-hydroxylation of PhIP occurs predominantly by CYP1A2 in liver microsomes (82,101). N-hydroxylated metabolites of PhIP bind covalently to DNA to form adducts, but are further metabolised by Phase II enzymes, namely N- and O-acetyltransferases (NAT1 and NAT2) and sulfotransferases, and are required to generate carcinogenic species. The Phase II enzymes add N-acetoxy or N-sulfonyl moieties to N-hydroxylated PhIP in the liver and partly in the terminal target organs, and arylnitrenium ions (R-NH+) derived from these metabolites react with DNA to generate PhIP-adducted bases (101), which can induce mutations. PhIP is also DNA reactive, wherein the exocyclic amino group of PhIP covalently binds to the C8 of the guanine base (dG-C8-PhIP). This is the major adduct found in calf thymus DNA reacted in vitro with N-acetoxy-PhIP (82,101) and is also found in DNA from rats exposed to PhIP. PhIP is detoxified primarily via glutathione and glutathione transferases. In addition, detoxification of N-hydroxylated PhIP occurs in the liver through the generation of glucuronate conjugates (102). In this case, glucuronate-conjugated PhIP is secreted into the bile, de-conjugated by gut flora and reabsorbed from the gut through enterohepatic circulation. As a result, the colonic mucosa may undergo repeated exposures to activated PhIP in vivo.
Fig. 2.
Metabolic pathway for PhIP activation and induced mutations.
Aromatic amines
In 1941, Lorenz et al. (2) initially observed the chemical induction of intestinal tumors in mice fed with the polyaromatic hydrocarbons, dibenzanthracene or methylcholanthrene. The carcinogenic action of DMAB was first recorded by Walpole et al. (4), who described the induction of colon tumors in rats by s.c. administration of DMAB. The carcinogenic action of DMAB to produce colon tumors was also evaluated (103,104). A total of 20 weekly s.c. injections of DMAB to male F344 rats at a dose level of 50 mg/kg body wt induced multiple colon tumors in ∼26 to 30% of animals fed with a low-fat diet and 74% of animals fed with a high-fat diet. DMAB induced both benign (adenomas) and malignant (adenocarcinomas with invasion into muscularis mucosa) epithelial neoplasms, with a multiplicity of 1.2–2.7 tumors per tumor-bearing rat. The adenocarcinomas observed were both histopathologically well differentiated and poorly differentiated. One of the major weaknesses of this model, however, is that it requires multiple injections of DMAB to induce colon tumors. On a molar equivalent basis, DMAB is less potent in rodent models than the series of compounds derived from DMH or AOM. Another weakness of this model system is the induction of neoplasms in various other tissues, such as adenocarcinomas of mammary glands in female rats, sarcomas of the salivary glands, squamous cell carcinomas of the ear duct and skin, forestomach squamous cell papillomas, sarcomas and lymphomas and urothelial carcinomas of urinary bladder (11,105,106).
Alkylnitrosamide compounds
MNNG and MNU are direct alkylating agents that do not require metabolic activation and thus are potent topical carcinogens (89). Intrarectal instillation of MNU or MNNG has been reported to induce colorectal tumors in rodent models (7,107–110). In rats and mice, most of the induced colonic tumors are sessile or polypoid lesions. Because biochemical activation is not required, these carcinogens are ideal for inducing colon tumors in animals and studying the modifying effects of xenobiotics without involving the metabolism of the initiating carcinogen (110). Intrarectal administration of MNNG at a dose rate of 1–3 mg/rat/week for 20 weeks induced colon tumors in 100% of male F344 rats, of which 43% tumors were adenocarcinomas and 57% were adenomas. The neoplasms were all located in the distal colon and rectum where MNNG and MNU were applied. The adenocarcinomas induced were mostly well-differentiated types with infiltration into the submucosa, whereas some were poorly differentiated showing mucinous cancer cells infiltrating into the submucosa. Metastases were not usually observed (111,112). Because MNNG and MNU given intrarectally selectively induce tumors in the distal colon and rectum, these models have been widely used. The major weakness of this model is that intrarectal injection poses a significant technical challenge, and quantification of carcinogens instilled intrarectally is difficult.
Chemically induced gene mutations
There are a number of pathways leading to colon cancer in humans, including the sporadic and FAP-associated adenoma–carcinoma sequence, hereditary non-polyposis colorectal cancer and colitis-related cancer, and each is driven by distinct molecular events (113,114). In human sporadic CRC, a large number of mutational events occur, and the ‘genomic landscape’ of human CRC has recently been described (115). Genomic sequencing of human CRCs has revealed frequent somatic mutations and deletions in a spectrum of tumor-related genes, including K-Ras, adenomatous polyposis coli (Apc) and p53 (116). Chemical agents used to induce tumors in rodents target many of the same genes or related genetic pathways. In this section, we discuss the genetic alterations associated with colon carcinogenesis induced by chemical carcinogens in rodent models. In the section that follows, we will focus on the effects of these mutations on a number of tumor-associated pathways.
A number of tumor-related genes are often targeted by colon carcinogens in rodent colon cancer models. One common genetic target in human and carcinogen-induced rodent CRC is the K-Ras gene. Jacoby et al. (117) showed that DMH caused K-Ras activating mutations (G to A) in 66% of colon carcinomas. Similar K-Ras mutations occurred in premalignant colonic mucosa in 2 of 11 rats by 15 weeks, suggesting that this was an early mutational event (117). Interestingly, this group further showed that only 2.5% of MNU-generated adenomas had this mutation, whereas 33% of carcinomas had a G to A transition in K-Ras (118). In AOM-induced lesions, K-Ras mutations are frequently observed, whereas Apc and p53 mutations are less frequently found (20,119,120). These differences cannot be explained simply by species differences since p53 mutations have been detected in MNU-induced rat colon tumors and Apc mutations have been found in PhIP-induced tumors (121,122). Furthermore, K-Ras mutations do not appear to be a feature of PhIP-induced colon tumors (123). It has also been reported that in the rat, PhIP treatment causes a specific guanine deletion at the 5′-GGGA-3′ site in the Apc gene, resulting in a protein truncation (121). These different mutations probably result from the types of DNA damage induced by the different carcinogens. AOM causes point mutations (G:C to A:T transversions) in the K-Ras gene (121,124,125), which may result from base misincorporation resulting from the O6-methyl-deoxyguanine adducts (65,126). Recently, Calcagno et al. (127) established an unequivocal role for K-Ras activation in neoplastic initiation and progression in mice. Transgenic mice in which an oncogenic K-Ras (G12D) allele was activated in the colon developed spontaneous ACF. Interestingly, the biological fate of these lesions was dependent upon the anatomical region of the colon in which they formed since oncogenic K-Ras-induced ACF were capable of progression only in the proximal colon (127). Thus, the differences in genetic mutations that arise in chemically induced colon tumor models are largely carcinogen specific. K-Ras activation appears to play an important role in the AOM and DMH models, whereas this pathway is not frequently targeted by PhIP. Conversely, MNU and PhIP have been shown to target p53 and Apc, respectively.
A role for the β-catenin/T-cell factor (Tcf) pathway in carcinogen-induced rodent colon cancer models has been clearly established. Using immunohistochemistry, Maltzman et al. (128) were the first to show that loss of wild-type Apc protein occurs in AOM-induced mouse colon tumors. Takahashi et al. (129) reported pronounced cytoplasmic and nuclear β-catenin staining in AOM-induced rat colon tumors. single strand conformation polymorphism analysis of the glycogen synthase kinase-3β phosphorylation consensus motif revealed eight mutations in 6/8 carcinomas (129). A similar high frequency of β-catenin mutations were found in AOM-induced colon tumors in ICR mice (130). Using single strand conformation polymorphism analysis, Takahashi et al. (130) reported that 9/10 β-catenin mutations in colon tumors were G:C → A:T transitions, with a concomitant increase in cytoplasmic and nuclear staining. Koesters et al. (131) tested the possibility that long-term treatment with a carcinogen may alter the mutational spectrum of β-catenin mutations in rat colon. Rats were treated for 20 weeks with DMH and β-catenin was sequenced. While mutations were found in 12/33 tumors (36%), only one mutation affected the codon 33 mutation cluster region, whereas 11/12 (>90%) of the mutations were G–C mutations at codon 41. The authors further speculate that codon 41 mutations may have greater oncogenic potential, but require a sustained carcinogenic exposure to enable selection in tumors (131). β-Catenin mutations have also been found in PhIP-induced rat colon tumors without Apc mutations (132). PhIP was also shown to induce β-catenin mutations in rat adenomas (133) and even in dysplastic ACF (134). Similarly, a high frequency of β-catenin mutations was found in rat colon tumors induced by IQ treatment (135). In mice, there is considerable evidence for both β-catenin mutation and alterations in cellular localization (130). Guda et al. (23) showed that in A/J mice, AOM treatment caused nuclear accumulation of β-catenin. The secondary bile acid, deoxycholic acid, has also recently been shown to increase nuclear β-catenin levels in the normally resistant AKR/J mice treated with AOM, an effect that was not associated with a loss of plasma membrane E-cadherin (22). However, in AOM-exposed ApcMin mice, Suzui et al. (63) reported that mutations in the β-catenin gene are less contributory to tumorigenesis than in C57BL/6 wild-type mice, suggesting different genetic pathways for colon cancer development in this model. Shitashige et al. (136) have focused on the potential role of splicing factor-1 (SF1), a component of the β-catenin–Tcf-4 complex. They report that AOM treatment resulted in a significant increase in the volume and number of colon tumors in the Sf1 heterozygous mice (137). These results indicate that consistent with human colon cancers, the Wnt/Apc/β-catenin-signaling pathway indeed plays an important role in chemical-induced rodent colon carcinogenesis.
A common genetic alteration found in human CRC that is not typically observed in rodent carcinogen models is a missense mutation of the p53 gene (120,138–141). The p53 gene remains unmutated, even though p53 knockout animals have an increased sensitivity to AOM (142,143). Although the p53 gene is sequence-normal in AOM-induced tumors, it is expressed at elevated levels, much like what is observed in p53-mutant lesions where the mutant p53 cannot activate expression of its negative regulator, Mdm2 (139), the analysis of p53 target gene expression and DNA binding activity revealed that the overexpressed p53 protein has extremely low activity. Although it is not entirely clear how the phenotypic repression of p53 activity is accomplished in these tumors, evidence for a central role for Mdm2 has been obtained (144). Therefore, with regard to the p53 pathway, AOM-induced tumors may be a good model human CRC in which p53 is normal (∼50%), or lesions with a wild-type p53 and overexpressed Mdm2, which have been estimated to account for 15% of human CRC (145).
Phenotypic changes associated with chemically induced colon carcinogenesis
As noted above, many of the cellular and biochemical defects found in human carcinomas are recapitulated in chemical-induced rodent models, supporting the use of experimental colon carcinogenesis in animal systems as a model for human disease. These defects include dysregulation of arachidonic acid (AA) metabolism, including elevated cyclooxygenase (COX-2) activity with concomitant prostaglandin E2 production (22,146), alterations in ornithine decarboxylase (ODC) (147) and polyamine levels (148,149) and upregulation of a number of proinflammatory cytokines, including tumor necrosis factor (TNF)-α and interleukin-1a/β (150). In this regard, the rodent carcinogen models have proven to be particularly useful for testing and studying anticancer agents that target overexpressed proteins in human colon cancers.
AA/COX-2 pathway
Cellular fatty acid metabolism and downstream signaling events play a critical role in colon carcinogenesis. Evidence suggests that the modulation of AA metabolism with Cox inhibitors [e.g. non-steroidal anti-inflammatory drugs (NSAIDs)] or dietary factors can be an effective approach for lowering colon cancer risk or preventing polyp recurrence (151–157). However, a number of important issues regarding how lipid-signaling pathways impact early and late stages of colon carcinogenesis remain unresolved. A better understanding of the mechanisms by which AA, AA metabolites and related lipid-signaling pathways affect colon carcinogenesis could greatly improve our ability to target appropriate patient populations and design more effective treatment protocols that reduce colon cancer risk. Rodent carcinogen models are well suited for studying the role of lipid signaling in colon cancer.
A number of studies have reported that colon carcinogens, particularly AOM, can increase COX-2 expression and consequently prostaglandin E2 (PGE2) levels within adenocarcinomas (130,158–163). Elevated COX-2 expression has been shown to occur throughout the carcinogenic process, beginning in rats within normal-appearing colonic mucosa as early as 1 week following AOM exposure, an effect that was enhanced by administration of a high-fat corn oil diet (163). Elevated levels of COX-2 in AOM-induced rat tumors were also shown by Rao et al. (164) using a high-fat diet containing mixed lipids. Dong et al. (159) reported elevated COX-2 expression in A/J mice 24 weeks after AOM treatment accompanied by increased PGE2 levels. Importantly, the formation of carcinogen-induced colon lesions can be suppressed by NSAIDs or COX-2 inhibitors. For example, the COX-2 inhibitor NS-398 significantly reduced the number of ACF in rats given AOM (165). In addition, sulindac was shown to be effective against certain types of ACF, including β-catenin-accumulated crypt (BCAC) and hexosaminidase-negative foci in rats (166), whereas the selective COX-2 inhibitor, JTE-522, was shown to suppress total ACF counts in DMH-treated rats (167). This same group, however, reported that COX-2 inhibition was effective when applied during the initiation phase, but not the progression phase, of DMH-induced carcinogenesis (167). These findings are, however, in contrast to those reported by Reddy et al. (168) showing that Celecoxib treatment was effective throughout the carcinogenic process in rats. Finally, Riehl et al. (169) have shown that COX-1-derived PGE2 may be important during early phases of colon carcinogenesis following AOM, possibly by affecting stem cell survival in response to acute damage.
Other components of the AA cascade have also been shown to affect colon cancer pathogenesis. For example, coordinate with the COXs, the secretory phospholipase and cytosolic phospholipase A2 (cPLA2) control the production of lipid mediators, including PGE2, by regulating the release of AA from phospholipids membranes (170). Overexpression of cPLA2 has been reported in cholangiocarcinomas, hepatomas, non-small cell lung cancers and small intestinal tumors (171–173). Using AOM, our laboratory reported a strict inverse relationship between cPLA2 and COX-2 expression in human and murine colon tumors (159,174), with cPLA2 levels reduced in neoplastic lesions at both the messenger RNA and protein level. We have also reported that genetic deletion of cPLA2 dramatically enhances the tumorigenic effects of AOM in the colon of Balb/c mice (24). The mechanism for this enhanced tumor response may be related to impaired apoptosis within the colonic mucosa of cPLA2-deficient mice (24), an effect that is related to reduced ceramide production in this tissue, as initially proposed by Chan et al. (175).
15-Hydroxyprostaglandin dehydrogenase (15-PGDH), a prostaglandin-degrading enzyme that is regulated by epidermal growth factor receptor (EGFR) signaling through the transcription factor Snail, is downregulated in colon cancer (176,177). Mann et al. (176) showed that pharmacologic disruption of EGFR signaling with erlotinib eliminated tumor growth in a xenograft model while restoring 15-PGDH expression and reducing intratumoral PGE2 levels. Myung et al. (177) directly tested the role of 15-PGDH in carcinogenesis by generating 15-PGDH-null mice on the otherwise colon carcinogen resistant C57Bl/6J background. As predicted, the absence of 15-PGDH resulted in a marked increase in susceptibility to AOM, both in the incidence of tumors as well as precancerous ACF.
As an alternative approach to reduce the levels of PGE2, without affecting the production of other prostaglandins, our laboratory recently tested the role of the terminal microsomal PGE2 synthase 1, mPGES-1, on intestinal tumorigenesis (178). While mPGES-1 deletion caused a marked reduction in intestinal tumors in Apc-mutant mice, it also suppressed the formation and growth of ACF induced by AOM, suggesting that promotion associated with PGE2 may also occur early in carcinogenesis (178). Reduced ACF size was associated with an attenuated nuclear translocation of β-catenin, demonstrating for the first time that PGE2 may exert direct control over Wnt signaling in vivo.
ODC and polyamines
A number of biochemical pathways are altered in colon cancer cells to sustain the high cell proliferation rates observed within tumors (179,180). An important example of one pathway is cellular polyamine synthesis. Polyamines (putrescine, spermidine and spermine) are necessary for cell growth and proliferation. They exist primarily in complexes with RNA and their effect on cell proliferation is likely to arise in part from a stimulation of protein synthesis (181). ODC is the rate-limiting enzyme in polyamine synthesis that generates putrescine from the decarboxylation of ornithine. ODC is intimately involved in normal cellular proliferation and is likely to play a role in colon carcinogenesis (182,183). Increased ODC activity and polyamine synthesis is a phenomenon common to cells undergoing rapid proliferation, including colon cancer (reviewed in 184). As shown by Luk et al. (185), AOM treatment resulted in elevated ODC activity corresponding to early (initiation) and late (promotion) phases of cancer development. As shown by Pizzi et al. (186), AOM treatment caused an extension of the crypt proliferative compartment to the upper third of crypts with a corresponding upward shift of DNA-synthesizing cells. These proliferation abnormalities were accompanied by increased ODC activity. Since human colon cancers also display elevated levels of polyamine synthesis and ODC activity, it was reasoned that ODC inhibition by agents like difluoromethylornithine (DFMO) may be able to suppress colon cancer development. The rodent carcinogen models played a central role in demonstrating the effectiveness of DFMO for colon cancer prevention (187–189). DFMO was subsequently tested in combination with sulindac in clinical trials by Meyskens et al. (190) and was found to reduce the risk of recurrent colorectal adenomas by up to 95%, with relatively low levels of toxicity. The interaction with sulindac may stem in part from sulindac’s ability to stimulate polyamine export from the cell (191). The common ODC phenotype of human and carcinogen-induced rodent colon cancers was therefore instrumental in the preclinical development of DFMO.
Transforming growth factor-β and other cytokines
The transforming growth factor (TGF)-β pathway has been demonstrated to play an important role in modulating colon carcinogenesis in humans (192). In the normal colon, TGF-β has an antiproliferative effect on epithelial cells, inducing growth arrest in G1 and apoptosis (193–195). These antiproliferative effects are mediated through the type I and type II TGF-β receptors, which dimerize upon TGF-β binding leading to the activation of the transcriptional regulatory SMAD proteins, SMAD2, 3 and 4. TGF-β may also influence colon cancer development through its potent immunosuppressive activities (196). Disruption of the TGF-β pathway is often observed in human colon cancers with microsatellite instability. In these cancers, carcinogenesis proceeds through the silencing of genes involved in DNA mismatch repair, which leads to the generation of frame-shift mutations in TGF-βRII. TGF-β signaling may also become compromised in repair-competent colon cancers due to point mutations in TGF-βRII or mutations in post-receptor signaling molecules such as SMAD2 and SMAD4 (197–202).
Evidence has been obtained that this pathway becomes disrupted in carcinogen-induced rodent models and may be an important factor for determining the sensitivity of different mouse species to AOM-induced carcinogenesis (203). Although the TGF-βRII is not mutated in the AOM model, the expression of this receptor is significantly downregulated in sensitive A/J mice, at both the messenger RNA and protein level (203). Repression of TGF-βRII may render epithelial cells insensitive to TGF-β-mediated growth arrest. AOM tumors are also deficient for processing latent TGF-β1, with approximately half of the TGF-β1 in the tumors present in an unprocessed, inactive form (204). Finally, inhibition of the TGF-β pathway in AOM tumors is supported by microarray analysis showing that many TGF-β-activated genes are not expressed at elevated levels in tumors (204). The AOM model therefore provides investigators interested in understanding the role of TGF-β signaling in colon cancer a tractable model in which to test different hypotheses.
TGF-β and other cytokines can have a profound, yet complex effect on colon cancer. TGF-β for example may suppress early stages of colon cancer, whereas its ability to stimulate stromal cells may ultimately promote cancer invasion and metastasis. Chemical-induced colon cancers in rodents support the expression of numerous cytokines. Furthermore, these tumors can be promoted by the application of inflammatory agents such as dextran sodium sulfate (DSS) or 2,4,6-trinitrobenzene sulfonic acid. These rodent models are particularly well suited for analyzing the role of different cytokines and cytokine profiles in colon carcinogenesis. Using knockout mouse strains, Osawa et al. (205) obtained evidence that the cytokine profile of a Th2 inflammatory responses (as observed in ulcerative colitis patients) is generally tumor promoting. Specifically, interferon-gamma knockout mice had a heightened tumor response relative to interleukin-4-deficient animals. The role of other cytokines has also been explored, with TNF-α being a particularly intriguing case. Although TNF-α knockout mice are more susceptible to colonic inflammation than wild-type animals (206), TNF-R1 knockout animals are resistant to cancer development in the AOM/DSS model (207), suggesting that signaling through different receptors may strongly influence colon cancer development. The rodent carcinogen models promise to play a central role in refining our understanding of the highly complex role of the immune response and inflammatory cytokines in colon cancer.
Tumor progression in rodent models
Tumor histology
In a study from our laboratory (37), we described the histology of tumors in A/J, FVB/N and Balb/CJ mice treated with four to six injections of AOM (i.p.) and killed at 24 weeks after the last injection. In each of these carcinogen-sensitive strains, tumors were localized to the distal colon and could not be detected, either grossly or histologically within the proximal colon. Tumors were intramucosal, ranging in size from 2 to 8 mm diameter and were exophytic, polypoid and tan to red with irregular surfaces in all strains. In the A/J mouse, colons were covered by multiple, coalescing tumors with minimal intervening areas of macroscopically normal epithelium (37). This ‘carpeting’ of the distal colonic mucosa with tumors can present a challenge in that it is often difficult to identify normal adjacent mucosa for comparison. Histologically, the cellular morphology within tumors in the A/J, FVB/N and Balb/CJ mice show similarities. At higher magnification, the intramucosal, expansile tumors are hypercellular, comprised of large numbers of closely packed epithelial cells, arranged in crypt-like structures with occasional cystic lumina, loss of goblet cell differentiation and supported by fibrovascular stroma. A common histological feature of these murine tumors is the paucity of goblet cells as demonstrated by markedly reduced periodic acid Schiff staining (37). These tumor crypts are arranged in a disorganized manner with general loss of polarity and minimal infiltration of the lamina propria by isolated islands of tumor cells derived from the epithelial compartment. The nuclei are large, pseudostratified, hyperchromatic, finely stippled and elongate to oval with mild to moderate anisokaryosis. Moderate to high numbers of lymphocytes and neutrophils are found within the lesions. Few macrophages infiltrate the stroma of the tumors but are present within the crypt lumina. It is interesting to note that the greatest degree of inflammatory cell infiltrate is present in tumors of the highly sensitive A/J mouse strain, suggesting a strong inflammatory component in the progression of these tumors (37).
In general, rodent colon tumor models tend to be non-metastatic and rarely show evidence of tumor cell invasion (50,111,112,208). However, in the study of Nambiar et al. (37), infiltrative cords of epithelial cells were occasionally found to breach the muscularis mucosa and another focal nest of epithelial cells were present in the submucosal lymphatics of one tumor, indicating that progression to infiltrating carcinoma is possible, although infrequent, in this model. Another tumor that was present in an A/J mouse at the recto-anal junction also showed infiltration into the submucosa. Finally, there was evidence of a squamous cell carcinoma at the recto-anal junction that infiltrates into the rectal wall, and large numbers of neutrophils were present in this tumor. Thus, while metastasis is rare in these rodent carcinogen models, the potential certainly exists that these behaviorally benign tumors can be promoted under certain conditions (e.g. genetically or through the use of tumor-promoting agents), although this potential application has yet to be fully exploited. Recently, Maggio-Price et al. (209) demonstrated environmental conditions that favor neoplastic progression in otherwise unaffected mice. SMAD3−/− mice maintained free of Gram-negative enterohepatic Helicobacter spp. for up to 9 months do not develop colon cancer, whereas infection of these mice with Helicobacter was found to trigger colon cancer within the cecum of 50–66% of the animals as early as 5 weeks post-infection. Histologically, the majority of these tumors were found to be infiltrative mucinous adenocarcinomas with evidence of tumor cell penetration through the colon wall into the serosa and mesentery (209).
Preneoplastic lesions
The observation that chemical carcinogens may induce macroscopic alterations within the colonic epithelium has a long and interesting history. Initial descriptions of preneoplastic alterations within the colonic mucosa were made by Delapierre et al. (210) who monitored the descending colon of rats by transmission electron microscopy and observed preneoplastic lesions at 3 weeks after treatment with DMH. Wargovich et al. (211) reported a number of histological alterations, including mucin changes and alterations in crypt proliferation rates after four injections of DMH to C57BL/6J and CF1 mice. While these and other studies (18,212–214) have identified colon crypts with altered histology, it was not until Bird (215) developed a methodology that could readily identify and quantify macroscopic alterations on the surface of the colon that they referred to as ACF. Using methylene blue dye staining, it was found that carcinogen-induced cryptal lesions could be distinguished by their increased size, thicker epithelial lining and increased pericryptal zone (216). ACF are morphological lesions that represent an early stage in the stepwise progression of colon cancer (216,217). These surface abnormalities often appear in the distal colon within 2 weeks of carcinogen treatment. Sequential analyses suggest that these early lesions increase in size and multiplicity and often exhibit nuclear atypia and dysplasia (42,217–219). ACF also show increased proliferative activity, growth factor signaling and K-Ras mutations, suggesting that at least a subset of ACF are putative precursors of colon cancer (20,127). Human ACF share a similar morphology and are present in grossly normal-appearing colon tissue from patients with CRC (220,221). It has been suggested that the nature and order of acquired genetic changes can profoundly impact ACF morphology and likelihood of tumor progression (222,223).
Since these early observations, a number of other investigators have described subsets of ACF with various histological criteria, including hexosaminidase-altered foci, BCAC, flat dysplasia and mucin-depleted foci (reviewed in 219). The Pretlow laboratory (224,225) demonstrated a marked reduction in hexosaminidase activity in methacrylate-embedded aberrant crypts taken from the distal colons of F344 rats treated with AOM. Tsukamoto et al. (226) then examined the transcription of the hexosaminidase α and β subunits in altered foci and reported that both markers were downregulated in aberrant crypts of DMH-treated rats. The same group then examined by scanning electron microscopy the three-dimensional structure of DMH-exposed rat colons and found that hexosaminidase-altered foci had abnormal budding within the middle of the crypt body, distinct from the symmetrical budding that occurred in hexosaminidase-normal crypts (227). BCAC, described initially by (228), are reportedly a premalignant lesion in the colon that lack some of the characteristic histological features of traditional ACF, but may have a greater likelihood for malignant transformation than ACF. When the frequency of BCAC was studied in AOM-exposed resistant and sensitive mouse strains, BCAC was found at a greater frequency in the distal colon of the sensitive strain at a site associated with subsequent tumor formation (229). On the other hand, ACF were more common in the proximal colon and occurred at a similar frequency in the sensitive and resistant strains, suggesting that BCAC may be a more reliable prognostic lesion (229). Mucin-depleted foci, originally characterized by their defective mucin production by the Caderni et al. (230,231), are dysplastic lesions that are found in the colons of carcinogen-exposed rats. These lesions are induced by DMH, have a higher degree of dysplasia than ACF, increase in response to tumor promoters such as cholic acid or heme-rich diets and carry alterations in the Wnt-signaling pathway, including mutations in β-catenin (232). In addition, it was shown that the cancer-preventing agent polyethylene glycol suppressed their growth, whereas a high-fat diet resulted in a modest increase in mucin-depleted foci/colon with no change in ACF density (233). Finally, Paulsen et al. (233) identified flat dysplastic ACF in the colons of ApcMin mice that have different macroscopic characteristics than carcinogen-induced ACF, as well as histological features consistent with microadenomas. More recently, these investigators have identified flat dysplastic ACF in the colons of AOM-treated A/J mice, as well as F344 rats (234). In both species, these flat ACF grew more rapidly than classical elevated ACF, possibly as a result of Wnt activation and accelerated crypt multiplication. Although limited in numbers, flat dysplastic ACF appeared to be more likely to become tumors than the elevated ACF (234). Clearly, the expanding diversity of ACF identified in rodent carcinogen models provides researchers with exciting new tools for studying the earliest stages of CRC. Lesions characterized in these models may ultimately become useful for predicting cancer risk in humans.
AOM/DSS model
Colon cancer is the most serious complication associated with long-standing inflammatory bowel disease (IBD), including ulcerative colitis and Crohn’s disease (235,236). The risk of CRC increases with the extent and duration of the disease. Several animal models have been reported that recapitulate many of the features associated with IBD. Perhaps the most commonly used mouse model employs DSS (237). CRC development in the DSS colitis model typically requires a relatively long exposure period or cycles of DSS administration, and the incidence and/or multiplicity of induced tumors are relatively low (238). Many studies have suggested that chronic or repeated mucosal inflammation may result in tumorigenesis through several proposed mechanisms. These mechanisms include induction of genetic mutations, increased cryptal cell proliferation, changes in crypt cell metabolism, changes in bile acid enterohepatic circulation and alterations in bacterial flora (239,240). CRC development following DSS-induced inflammation supports the hypothesis that chronic inflammation in IBD plays a critical role in epithelial malignant neoplasia in the large bowel (239). In inflammatory and/or angiogenic environments, superoxide anion may be produced by infiltrating mast cells (241) or macrophages (242) to facilitate cancer development.
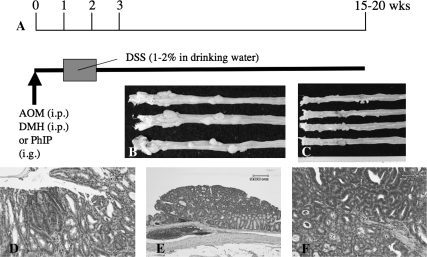
For understanding the pathogenesis of IBD-related CRC, we developed a novel colitis-related mouse CRC model (a two-stage mouse colon carcinogenesis model) initiated with AOM and promoted by DSS (243). In this model, mice develop tumors after a relatively short-term DSS exposure, compared with DSS alone. Male Crj:CD-1 (ICR) mice were given a single i.p. administration (10 mg/kg body wt) of AOM, followed by a 1 week oral exposure (2% in drinking water) to DSS (molecular weight 40 000). Within 20 weeks, numerous colonic neoplasms (adenocarcinomas, 100% incidence with a multiplicity of 5.60 ± 2.42 and adenomas, 38% incidence with a multiplicity of 0.20 ± 0.40) and dysplastic lesions were observed (Figure 3). Immunohistochemically, dysplasia and neoplasms showed positive staining for β-catenin, COX-2 and inducible nitric oxide synthase, but did not show evidence of p53 immunoreactivity. Thus, this novel mouse model combining AOM with DSS can be used for investigating colitis-related colon. Interestingly, numerous mast cells are found surrounding or within these carcinomas (243). Although the role of mast cells in IBD development is still a matter of debate (244,245), inducible nitric oxide synthase expression by these cells may be involved IBD and IBD-related colon carcinogenesis (246).
Fig. 3.
(A) Protocol for AOM/DSS-induced colon carcinogenesis. Animals received a single i.p. injection (10 mg/kg body wt for AOM and 10 or 20 mg/kg body wt for DMH), or intragastric intubation (PhIP, 200 mg/kg body wt), followed by a 1 week exposure of DSS (1–2%) in drinking water to induce colonic neoplasms. Between 15 and 20 weeks, colon tumors in mice (B) and rats (C) develop. (D) Dysplastic crypts induced by the combination of AOM/DSS in mice. (E) Tubular adenoma induced by AOM/DSS in mice. (F) A representative tubular adenocarcinoma induced by AOM/DSS in mice. Scale bar = 60 μm.
Our recent studies on inflammation-related carcinogenesis, where mice received a single dose of various colon carcinogens (i.e. AOM, PhIP and DMH) followed by 1 week of exposure to 2% DSS, have shown a high incidence of tumor formation within 20 weeks (247,248). Importantly, we also observed differential sensitivities to AOM/DSS-induced colon carcinogenesis among four different mouse lines (BALB/c, C3H/HeN, C57BL/6N and DBA/2N) (249). When these mice were given a single i.p. injection of AOM (10 mg/kg body wt), followed by 1% DSS (wt/vol, molecular weight 36 000–50 000) in drinking water for 4 days, the incidence of colonic adenocarcinoma was 100% with a multiplicity of 7.7 ± 4.3 in Balb/c mice, whereas only 50% of C57BL/6N mice developed tumors with a multiplicity of 1.0 ± 1.2. Moreover, only a few colonic adenomas, but no adenocarcinomas, developed in C3H/HeN or DBA/2N mice. The inflammation and immunohistochemical nitrotyrosine positivity score of mice treated with AOM and DSS were (in decreasing order) as follows: C3H/HeN > BALB/c = ICR > DBA/2N > C57BL/6N and BALB/c = ICR > C57BL/6N > C3H/HeN > DBA/2N, respectively. These findings suggest that susceptibility to AOM/DSS-induced colonic tumorigenesis may be directly influenced by the degree of nitrosative stress in colonic tissue.
In a dose–response study of DSS as a tumor promoter (249), male ICR mice were given a single i.p. injection of AOM (10 mg/kg body wt), followed by DSS (molecular weight 40 000) at dose levels ranging from 0.1 to 2% (wt/vol) in the drinking water for 1 week. At week 14, in the mice that received AOM and 2% DSS, the incidence and multiplicity of colonic tubular adenoma (75% and 1.25 ± 1.26 per mouse) and adenocarcinoma (100%, 2.75 ± 2.22 per mouse) were the highest. Colon tumors did not develop in mice that received AOM + 0.25% DSS and AOM + 0.1% DSS, although dysplastic crypts were observed in these mice. Scoring of inflammation and nitrotyrosine immunoreactivity suggested that severe inflammation and nitrosative stress was caused by high doses (2 and 1%) of DSS. Thus, the tumor-promoting effect of DSS is dose dependent, occurring at 1% or higher, and the effect corresponds to the degree of inflammation and nitrosative stress, assessed in this study by increased (2-fold) nitrotyrosine immunoreactivity that was observed within a variety of cell types (neoplastic, cryptal and endothelial cells, as well as infiltrative mononuclear cells) within the colonic mucosa (249).
In a time–course study, sequential analysis of pathological alterations during carcinogenesis was conducted to determine the influence of inflammation on colon carcinogenesis. Male ICR mice were given a single i.p. injection of AOM (10 mg/kg body wt) followed by 2% (wt/vol) DSS for 7 days, starting 1 week after AOM exposure. Colon adenomas were identified in 2/5 mice at week 3 and colon adenocarcinomas developed in 2/5 mice at week 4. Tumor incidence gradually increased with time and reached 100% with a multiplicity of 6.20 ± 2.48 at week 6. At week 14, the multiplicity of adenocarcinomas was 9.75 ± 2.49. In addition, colonic dysplasia was observed at all time points. The scores of colonic inflammation and nitrotyrosine immunohistochemistry were extremely high at early time points and were well correlated. These results indicate that the AOM/DSS model generates neoplasms that develop through dysplastic lesions in the colonic mucosa.
Although the incidence of colon tumors in ApcMin/+ mice is generally low, numerous colon tumors developed within 5 weeks in male and female ApcMin/+ mice when they received 2% DSS in drinking water for 7 days (248). Further molecular analysis revealed a high incidence (80–100%) of β-catenin gene mutations in AOM-induced colonic adenocarcinomas (247,248). In DMH/DSS-induced adenocarcinomas, 10 of 11 (90%) adenocarcinomas had β-catenin gene mutations. About half of the mutations were detected at codons 37 or 41 (247). In the AOM/DSS-induced colonic adenocarcinomas, mutations of the β-catenin gene were present in codons 32 to 34 (248). These mutations differ from those induced by AOM alone that were found in codons 33, 37 and 41 (130). From these findings and other reports (130,131), we postulate that mutations within codons 33 and 34 might be caused by AOM exposure, whereas codon 32 mutations result from DSS exposure. Furthermore, we suggest that DSS promotes loss of Apc in the ApcMin model.
Within the inflamed colon of mice that received a combination of AOM and DSS, gene expression was altered when compared with that of untreated mice or mice treated with either AOM or DSS alone (250). Global gene expression analysis was performed on normal-appearing colonic mucosa at weeks 5 and 10 following treatment. Significantly upregulated genes in the AOM/DSS group at week 5 include the Wnt inhibitory factor 1 (Wif1), plasminogen activator (Plat) and myelocytomatosis oncogene (Myc). At week 10, the level of phospholipase A2 group IIA (platelets and synovial fluid) (Plscr2) was increased. The combination of AOM/DSS also resulted in the downregulation of the peroxisome proliferator activated receptor-binding protein (Pparbp) and the Tgfb3 at 10 weeks. In addition, the inflammation-related gene, peroxisome proliferator activated receptor-γ (Pparg), was also downregulated at 5 weeks. The impact of these gene expression changes on cancer development has not yet been evaluated.
In summary, the AOM/DSS animal model has proven to be a powerful tool for investigating the pathogenesis and chemoprevention of colitis-related colon carcinogenesis. Modifications of this model might facilitate the detection of environmental carcinogens (251) and other tumor risk modifiers (252), as well as novel chemopreventive agents (253) against IBD-related CRC.
Funding
National Institutes of Health (5R01CA081428).
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AA
arachidonic acid
- ACF
aberrant crypt foci
- AOM
azoxymethane
- Apc
adenomatous polyposis coli
- BCAC
β-catenin-accumulated crypt
- COX-2
cyclooxygenease-2
- cPLA2
cytosolic phospholipase A2
- CRC
colorectal cancer
- DFMO
difluoromethylornithine
- DMAB
3,2′-dimethyl-4-aminobiphenyl
- DMH
1,2-dimethylhydrazine
- DSS
dextran sodium sulfate
- HCA
heterocyclic amine
- IBD
inflammatory bowel disease
- i.p.
intraperitoneal
- IQ
2-amino-33-methylimidazo[4,5-f]quinoline
- MAM
methylazoxymethanol
- miRNA
microRNA
- MNNG
N-methyl-N′-nitro-N-nitrosoguanidine
- MNU
methylnitrosourea
- ODC
ornithine decarboxylase
- PGDH
15-hydroxyprostaglandin dehydrogenase
- PGE2
prostaglandin E2
- PhIP
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine
- s.c.
subcutaneous
- TGF
transforming growth factor
- TNF
tumor necrosis factor
References
- 1.Krebs C. Experimenteller Alkoholkrebs bei weissen Mäusen. Z. Immun. Exp. Therap. 1928;50:203–218. [Google Scholar]
- 2.Lorenz E, et al. Intestinal carcinoma and other lesions in mice following oral administration of 1,2,5,6-dibenzanthracene and 20-methyleholanthrene. J. Natl Cancer Inst. 1941;1:17–40. [Google Scholar]
- 3.Lisco H, et al. Carcinoma of the colon in rats following the feeding of radioactive yttrium. Cancer Res. 1947;7:721–725. [Google Scholar]
- 4.Walpole AL, et al. The carcinogenic action of 4-aminodiphenyl and 3:2′-dimethyl-4-amino-diphenyl. Br. J. Ind. Med. 1952;9:255–263. doi: 10.1136/oem.9.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laqueur GL, et al. Carcinogenic Properties of nuts from Cycas circinalis L. indigenous to Guam. J. Natl Cancer Inst. 1963;31:919–951. [PubMed] [Google Scholar]
- 6.Laqueur GL. Carcinogenic effects of cycad meal and cycasin, methylazoxymethanol glycoside, in rats and effects of cycasin in germfree rats. Fed. Proc. 1964;23:1386–1388. [PubMed] [Google Scholar]
- 7.Druckrey H, et al. Selective production of intestinal cancer in rats by 1,2-dimethylhydrazine. Naturwissenschaften. 1967;54:285–286. doi: 10.1007/BF00620890. [DOI] [PubMed] [Google Scholar]
- 8.Schauer A, et al. Cancerization of the rat intestine by 1,2-dimethylhydrazine. Z. Gesamte Exp. Med. 1969;150:87–93. [PubMed] [Google Scholar]
- 9.Thurnherr N, et al. Induction of colonic carcinoma in mice using 1,2-dimethylhydrazine hydrochloride. Schweiz. Med. Wochenschr, 1975;105:585–586. [PubMed] [Google Scholar]
- 10.Haase P, et al. Histogenesis of colonic tumours in mice induced by dimethyl hydrazine. J. Pathol. 1973;109:Px. [PubMed] [Google Scholar]
- 11.Martin MS, et al. An experimental model for cancer of the colon and rectum. Intestinal carcinoma induced in the rat 1,2-dimethylhydrazine. Digestion. 1973;8:22–34. doi: 10.1159/000197298. [DOI] [PubMed] [Google Scholar]
- 12.Shamsuddin AK, et al. Preneoplastic and neoplastic changes in colonic mucosa in Crohn′s disease. Arch. Pathol. Lab. Med. 1981;105:283–286. [PubMed] [Google Scholar]
- 13.Ward JM. Morphogenesis of chemically induced neoplasms of the colon and small intestine in rats. Lab. Invest. 1974;30:505–513. [PubMed] [Google Scholar]
- 14.LaMont JT, et al. Experimental colon cancer. Gastroenterology. 1978;75:1157–1169. [PubMed] [Google Scholar]
- 15.Neufert C, et al. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat. Protoc. 2007;2:1998–2004. doi: 10.1038/nprot.2007.279. [DOI] [PubMed] [Google Scholar]
- 16.Papanikolaou A, et al. Initial levels of azoxymethane-induced DNA methyl adducts are not predictive of tumor susceptibility in inbred mice. Toxicol. Appl. Pharmacol. 1998;150:196–203. doi: 10.1006/taap.1998.8393. [DOI] [PubMed] [Google Scholar]
- 17.Deschner EE, et al. Colonic neoplasms in mice produced with six injections of 1,2-dimethylhydrazine. Oncology. 1977;34:255–257. doi: 10.1159/000225236. [DOI] [PubMed] [Google Scholar]
- 18.Thurnherr N, et al. Induction of adenocarcinomas of the colon in mice by weekly injections of 1,2-dimethylhydrazine. Cancer Res. 1973;33:940–945. [PubMed] [Google Scholar]
- 19.Toth B, et al. Production of intestinal and other tumours by 1,2-dimethylhydrazine dihydrochloride in mice. II. Scanning electron microscopic and cytochemical study of colonic neoplasms. Br. J. Exp. Pathol. 1976;57:696–705. [PMC free article] [PubMed] [Google Scholar]
- 20.Bolt AB, et al. Azoxymethane induces KI-ras activation in the tumor resistant AKR/J mouse colon. Mol. Carcinog. 2000;27:210–218. [PubMed] [Google Scholar]
- 21.Delker DA, et al. Quantitative assessment of azoxymethane-induced aberrant crypt foci in inbred mice. Exp. Mol. Pathol. 1999;65:141–149. doi: 10.1016/s0014-4800(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 22.Flynn C, et al. Deoxycholic acid promotes the growth of colonic aberrant crypt foci. Mol. Carcinog. 2007;46:60–70. doi: 10.1002/mc.20253. [DOI] [PubMed] [Google Scholar]
- 23.Guda K, et al. Carcinogen-induced colon tumors in mice are chromosomally stable and are characterized by low-level microsatellite instability. Oncogene. 2004;23:3813–3821. doi: 10.1038/sj.onc.1207489. [DOI] [PubMed] [Google Scholar]
- 24.Ilsley JN, et al. Cytoplasmic phospholipase A2 deletion enhances colon tumorigenesis. Cancer Res. 2005;65:2636–2643. doi: 10.1158/0008-5472.CAN-04-3446. [DOI] [PubMed] [Google Scholar]
- 25.Nambiar PR, et al. Genetic signatures of high- and low-risk aberrant crypt foci in a mouse model of sporadic colon cancer. Cancer Res. 2004;64:6394–6401. doi: 10.1158/0008-5472.CAN-04-0933. [DOI] [PubMed] [Google Scholar]
- 26.Papanikolaou A, et al. Azoxymethane-induced colon tumors and aberrant crypt foci in mice of different genetic susceptibility. Cancer Lett. 1998;130:29–34. doi: 10.1016/s0304-3835(98)00101-3. [DOI] [PubMed] [Google Scholar]
- 27.Papanikolaou A, et al. Expression analysis of the group IIA secretory phospholipase A(2) in mice with differential susceptibility to azoxymethane-induced colon tumorigenesis. Carcinogenesis. 2000;21:133–138. doi: 10.1093/carcin/21.2.133. [DOI] [PubMed] [Google Scholar]
- 28.Rowlatt C, et al. Naturally occurring tumors and other lesions of the digestive tract in untreated C57BL mice. J. Natl Cancer Inst. 1969;43:1353–1364. [PubMed] [Google Scholar]
- 29.Miwa M, et al. Spontaneous colon tumors in rats. J. Natl Cancer Inst. 1976;56:615–621. doi: 10.1093/jnci/56.3.615. [DOI] [PubMed] [Google Scholar]
- 30.Vandenberghe J, et al. Spontaneous adenocarcinoma of the ascending colon in Wistar rats: the intracytoplasmic presence of a Campylobacter-like bacterium. J. Comp. Pathol. 1985;95:45–55. doi: 10.1016/0021-9975(85)90076-3. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto M, et al. Adenocarcinoma in the ascending colon of ACI strain rat foster bred by WF-Osaka female rat. Med. J. Osaka Univ. 1991;40:1–3. [PubMed] [Google Scholar]
- 32.Newmark HL, et al. A Western-style diet induces benign and malignant neoplasms in the colon of normal C57Bl/6 mice. Carcinogenesis. 2001;22:1871–1875. doi: 10.1093/carcin/22.11.1871. [DOI] [PubMed] [Google Scholar]
- 33.Richie JP, Jr, et al. Induction of colon tumorigenesis by glutathione depletion in p53-knock-out mice. Int. J. Oncol. 2007;30:1539–1543. [PubMed] [Google Scholar]
- 34.Madara JL, et al. Evidence for an adenoma-carcinoma sequence in dimethylhydrazine-induced neoplasms of rat intestinal epithelium. Am. J. Pathol. 1983;110:230–235. [PMC free article] [PubMed] [Google Scholar]
- 35.Weisburger JH, et al. Colon cancer: its epidemiology and experimental production. Cancer. 1977;40:2414–2420. doi: 10.1002/1097-0142(197711)40:5+<2414::aid-cncr2820400904>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 36.Chang WW. Histogenesis of symmetrical 1,2-dimethylhydrazine-induced neoplasms of the colon in the mouse. J. Natl Cancer Inst. 1978;60:1405–1418. doi: 10.1093/jnci/60.6.1405. [DOI] [PubMed] [Google Scholar]
- 37.Nambiar PR, et al. Preliminary analysis of azoxymethane induced colon tumors in inbred mice commonly used as transgenic/knockout progenitors. Int. J. Oncol. 2003;22:145–150. [PubMed] [Google Scholar]
- 38.Boffa LC, et al. Differences in colonic nuclear proteins of two mouse strains with different susceptibilities to 1, 2-dimethylhydrazine-induced carcinogenesis. Cancer Res. 1980;40:1774–1780. [PubMed] [Google Scholar]
- 39.Deschner EE, et al. Susceptibility to 1,2-dimethylhydrazine-induced colonic tumors and epithelial cell proliferation characteristics of F1, F2, and reciprocal backcrosses derived from SWR/J and AKR/J parental mouse strains. Cancer. 1988;61:478–482. doi: 10.1002/1097-0142(19880201)61:3<478::aid-cncr2820610312>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 40.Diwan BA, et al. Effects of methylazoxymethanol acetate on inbred mice: influence of genetic factors on tumor induction. Proc. Soc. Exp. Biol. Med. 1979;161:347–349. doi: 10.3181/00379727-161-40550. [DOI] [PubMed] [Google Scholar]
- 41.Evans JT, et al. Genetics of colon carcinogenesis in mice treated with 1,2-dimethylhydrazine. Cancer Res. 1977;37:134–136. [PubMed] [Google Scholar]
- 42.Papanikolaou A, et al. Sequential and morphological analyses of aberrant crypt foci formation in mice of differing susceptibility to azoxymethane-induced colon carcinogenesis. Carcinogenesis. 2000;21:1567–1572. [PubMed] [Google Scholar]
- 43.Rosenberg DW, et al. Induction of aberrant crypts in murine colon with varying sensitivity to colon carcinogenesis. Cancer Lett. 1995;92:209–214. doi: 10.1016/0304-3835(95)03797-z. [DOI] [PubMed] [Google Scholar]
- 44.Turusov VS, et al. Strain differences in susceptibility of female mice to 1,2-dimethylhydrazine. Carcinogenesis. 1982;3:603–608. doi: 10.1093/carcin/3.6.603. [DOI] [PubMed] [Google Scholar]
- 45.Izumi K, et al. Carcinogenicity of 1,2-dimethylhydrazine dihydrochloride in BALB/c mice. Influence of the route of administration and dosage. Virchows Arch. A Pathol. Anat. Histol. 1979;384:263–267. doi: 10.1007/BF00428228. [DOI] [PubMed] [Google Scholar]
- 46.Bissahoyo A, et al. Azoxymethane is a genetic background-dependent colorectal tumor initiator and promoter in mice: effects of dose, route, and diet. Toxicol. Sci. 2005;88:340–345. doi: 10.1093/toxsci/kfi313. [DOI] [PubMed] [Google Scholar]
- 47.Guda K, et al. Strain-specific homeostatic responses during early stages of azoxymethane-induced colon tumorigenesis in mice. Int. J. Oncol. 2007;31:837–842. [PubMed] [Google Scholar]
- 48.Deschner EE, et al. Differential susceptibility of inbred mouse strains forecast by acute colonic proliferative response to methylazoxymethanol. J. Natl Cancer Inst. 1984;72:195–198. doi: 10.1093/jnci/72.1.195. [DOI] [PubMed] [Google Scholar]
- 49.Diwan BA, et al. Differential susceptibility of 3 sublines of C57BL/6 mice to the induction of colorectal tumors by 1,2-dimethylhydrazine. Cancer Lett. 1980;9:111–115. doi: 10.1016/0304-3835(80)90114-7. [DOI] [PubMed] [Google Scholar]
- 50.Cespedes MV, et al. Orthotopic microinjection of human colon cancer cells in nude mice induces tumor foci in all clinically relevant metastatic sites. Am. J. Pathol. 2007;170:1077–1085. doi: 10.2353/ajpath.2007.060773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacoby RF, et al. Genetic analysis of colon cancer susceptibility in mice. Genomics. 1994;22:381–387. doi: 10.1006/geno.1994.1399. [DOI] [PubMed] [Google Scholar]
- 52.Lipkin SM, et al. MLH3: a DNA mismatch repair gene associated with mammalian microsatellite instability. Nat. Genet. 2000;24:27–35. doi: 10.1038/71643. [DOI] [PubMed] [Google Scholar]
- 53.Groot PC, et al. The recombinant congenic strains for analysis of multigenic traits: genetic composition. FASEB J. 1992;6:2826–2835. doi: 10.1096/fasebj.6.10.1634045. [DOI] [PubMed] [Google Scholar]
- 54.Moen CJ, et al. Scc-1, a novel colon cancer susceptibility gene in the mouse: linkage to CD44 (Ly-24, Pgp-1) on chromosome 2. Oncogene. 1992;7:563–566. [PubMed] [Google Scholar]
- 55.van Wezel T, et al. Gene interaction and single gene effects in colon tumour susceptibility in mice. Nat. Genet. 1996;14:468–470. doi: 10.1038/ng1296-468. [DOI] [PubMed] [Google Scholar]
- 56.Moen CJ, et al. Different genetic susceptibility to aberrant crypts and colon adenomas in mice. Cancer Res. 1996;56:2382–2386. [PubMed] [Google Scholar]
- 57.Ruivenkamp CA, et al. Ptprj is a candidate for the mouse colon-cancer susceptibility locus Scc1 and is frequently deleted in human cancers. Nat. Genet. 2002;31:295–300. doi: 10.1038/ng903. [DOI] [PubMed] [Google Scholar]
- 58.Ruivenkamp C, et al. LOH of PTPRJ occurs early in colorectal cancer and is associated with chromosomal loss of 18q12-21. Oncogene. 2003;22:3472–3474. doi: 10.1038/sj.onc.1206246. [DOI] [PubMed] [Google Scholar]
- 59.Moen CJ, et al. Fine mapping of colon tumor susceptibility (Scc) genes in the mouse, different from the genes known to be somatically mutated in colon cancer. Proc. Natl Acad. Sci. USA. 1996;93:1082–1086. doi: 10.1073/pnas.93.3.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sevignani C, et al. MicroRNA genes are frequently located near mouse cancer susceptibility loci. Proc. Natl Acad. Sci. USA. 2007;104:8017–8022. doi: 10.1073/pnas.0702177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moser AR, et al. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 62.Mollersen L, et al. Loss of heterozygosity and nonsense mutation in Apc in azoxymethane-induced colonic tumours in min mice. Anticancer Res. 2004;24:2595–2599. [PubMed] [Google Scholar]
- 63.Suzui M, et al. Enhanced colon carcinogenesis induced by azoxymethane in min mice occurs via a mechanism independent of beta-catenin mutation. Cancer Lett. 2002;183:31–41. doi: 10.1016/s0304-3835(02)00114-3. [DOI] [PubMed] [Google Scholar]
- 64.Issa AY, et al. Green tea selectively targets initial stages of intestinal carcinogenesis in the AOM-ApcMin mouse model. Carcinogenesis. 2007;28:1978–1984. doi: 10.1093/carcin/bgm161. [DOI] [PubMed] [Google Scholar]
- 65.Fiala ES. Investigations into the metabolism and mode of action of the colon carcinogens 1,2-dimethylhydrazine and azoxymethane. Cancer. 1977;40:2436–2445. doi: 10.1002/1097-0142(197711)40:5+<2436::aid-cncr2820400908>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 66.Fiala ES, et al. Mechanism of benzylselenocyanate inhibition of azoxymethane-induced colon carcinogenesis in F344 rats. Cancer Res. 1991;51:2826–2830. [PubMed] [Google Scholar]
- 67.Weisburger JH. Colon carcinogens: their metabolism and mode of action. Cancer. 1971;28:60–70. doi: 10.1002/1097-0142(197107)28:1<60::aid-cncr2820280113>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 68.Fiala ES, et al. Non-alcohol dehydrogenase-mediated metabolism of methylazoxymethanol in the deer mouse, Peromyscus maniculatus. Cancer Res. 1984;44:2885–2891. [PubMed] [Google Scholar]
- 69.Fiala ES, et al. Inhibition of the metabolism of the colon carcinogen, azoxymethane, by pyrazole. Cancer Res. 1978;38:4515–4521. [PubMed] [Google Scholar]
- 70.Zedeck MS, et al. Metabolism of the colon carcinogen methylazoxymethanol acetate. Front. Gastrointest. Res. 1979;4:32–37. doi: 10.1159/000402283. [DOI] [PubMed] [Google Scholar]
- 71.Sohn OS, et al. Differential effects of CYP2E1 status on the metabolic activation of the colon carcinogens azoxymethane and methylazoxymethanol. Cancer Res. 2001;61:8435–8440. [PubMed] [Google Scholar]
- 72.Nagasawa HT, et al. Decomposition of methylazoxymethanol, the aglycone of cycasin, in D 2 O. Nature. 1972;236:234–235. doi: 10.1038/236234a0. [DOI] [PubMed] [Google Scholar]
- 73.Feinberg A, et al. Production of a highly reactive alkylating agent from the organospecific carcinogen methylazoxymethanol by alcohol dehydrogenase. Cancer Res. 1980;40:4446–4450. [PubMed] [Google Scholar]
- 74.Schoental R. The mechanisms of action of the carcinogenic nitroso and related compounds. Br. J. Cancer. 1973;28:436–439. doi: 10.1038/bjc.1973.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Delker DA, et al. The role of alcohol dehydrogenase in the metabolism of the colon carcinogen methylazoxymethanol. Toxicol. Sci. 1998;45:66–71. doi: 10.1006/toxs.1998.2499. [DOI] [PubMed] [Google Scholar]
- 76.Pegg AE. Methylation of the O6 position of guanine in DNA is the most likely initiating event in carcinogenesis by methylating agents. Cancer Invest. 1984;2:223–231. doi: 10.3109/07357908409104376. [DOI] [PubMed] [Google Scholar]
- 77.Cooper HK, et al. DNA alkylation in mice with genetically different susceptibility to 1,2-dimethylhydrazine-induced colon carcinogenesis. Cancer Res. 1978;38:3063–3065. [PubMed] [Google Scholar]
- 78.Herron DC, et al. In vivo kinetics of O6-methylguanine and 7-methylguanine formation and persistence in DNA of rats treated with symmetrical dimethylhydrazine. Cancer Res. 1981;41:3967–3972. [PubMed] [Google Scholar]
- 79.Malt RA, et al. Colonic Carcinogenesis. Falk Symposium 31. Titisee, West Germany: MTP Press Ltd; 1981. pp. 1–406. [Google Scholar]
- 80.Scheppach W, et al. Exogenous Factors in Colonic Carcinogenesis. Falk Symposium 128. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2003. pp. 1–324. [Google Scholar]
- 81.Nagao M, et al. Mutagenicities of smoke condensates and the charred surface of fish and meat. Cancer Lett. 1977;2:221–226. doi: 10.1016/s0304-3835(77)80025-6. [DOI] [PubMed] [Google Scholar]
- 82.Sugimura T, et al. Heterocyclic amines: mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004;95:290–299. doi: 10.1111/j.1349-7006.2004.tb03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wakabayashi K, et al. Food-derived mutagens and carcinogens. Cancer Res. 1992;52:2092s–2098s. [PubMed] [Google Scholar]
- 84.Barnes WS, et al. High pressure liquid chromatographic method for the analysis of 2-amino-3methyl[4,5-f]imidazo quinoline, a mutagen formed from the cooking of food. J. Agric. Food Chem. 1983;31:883–886. [Google Scholar]
- 85.Felton JS, et al. Isolation and characterization of new mutagens from fried ground beef. Carcinogenesis. 1984;5:95–102. doi: 10.1093/carcin/5.1.95. [DOI] [PubMed] [Google Scholar]
- 86.Adamson RH, et al. Carcinogenicity of 2-amino-3-methylimidazo[4,5-f]quinoline in nonhuman primates: induction of tumors in three macaques. Jpn. J. Cancer Res. 1990;81:10–14. doi: 10.1111/j.1349-7006.1990.tb02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sugimura T, et al. Heterocyclic amines produced in cooked food: unavoidable xenobiotics. Princess Takamatsu Symp. 1990;21:279–288. [PubMed] [Google Scholar]
- 88.Tanaka T, et al. Multipotential carcinogenicity of the fried food mutagen 2-amino-3-methylimidazo[4,5-f]quinoline in rats. Jpn. J. Cancer Res. 1985;76:570–576. [PubMed] [Google Scholar]
- 89.Weisburger JH, et al. Experimental Colon Carcinogenesis. Boca Raton, FL: CRC Press; 1983. [Google Scholar]
- 90.Hasegawa R, et al. Dose-dependence of 2-amino-1-methyl-6-phenylimidazo[4,5-b]-pyridine (PhIP) carcinogenicity in rats. Carcinogenesis. 1993;14:2553–2557. doi: 10.1093/carcin/14.12.2553. [DOI] [PubMed] [Google Scholar]
- 91.Ito N, et al. Carcinogenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in the rat. Mutat. Res. 1997;376:107–114. doi: 10.1016/s0027-5107(97)00032-8. [DOI] [PubMed] [Google Scholar]
- 92.Shirai T, et al. The prostate: a target for carcinogenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) derived from cooked foods. Cancer Res. 1997;57:195–198. [PubMed] [Google Scholar]
- 93.Grivas S, et al. Isolation and identification of the food mutagens IQ and MeIQx from a heated model system of creatinine, glycine and fructose. Food Chem. 1986;20:127–136. [Google Scholar]
- 94.Kato R. Metabolic activation of mutagenic heterocyclic aromatic amines from protein pyrolysates. Crit. Rev. Toxicol. 1986;16:307–348. doi: 10.3109/10408448609037466. [DOI] [PubMed] [Google Scholar]
- 95.Schut HA, et al. DNA adduct formation of the carcinogen 2-amino-3-methylimidazo[4,5-f]-quinoline in target tissues of the F-344 rat. Cancer Lett. 1988;41:345–352. doi: 10.1016/0304-3835(88)90296-0. [DOI] [PubMed] [Google Scholar]
- 96.Ito N, et al. A new colon and mammary carcinogen in cooked food, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) Carcinogenesis. 1991;12:1503–1506. doi: 10.1093/carcin/12.8.1503. [DOI] [PubMed] [Google Scholar]
- 97.Reddy BS, et al. Inhibitory effect of Bifidobacterium longum on colon, mammary, and liver carcinogenesis induced by 2-amino-3-methylimidazo[4,5-f]quinoline, a food mutagen. Cancer Res. 1993;53:3914–3918. [PubMed] [Google Scholar]
- 98.Nakagama H, et al. Modeling human colon cancer in rodents using a food-borne carcinogen, PhIP. Cancer Sci. 2005;96:627–636. doi: 10.1111/j.1349-7006.2005.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Doi K, et al. Altered gene expression in rat colonic adenocarcinomas induced in an azoxymethane plus 2-amino-1-methyl-6-phenylimidazo[4,5-b]- pyridine initiation-promotion model. Oncology. 2007;73:252–260. doi: 10.1159/000127423. [DOI] [PubMed] [Google Scholar]
- 100.Doi K, et al. Lack of large intestinal carcinogenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine at low doses in rats initiated with azoxymethane. Int. J. Cancer. 2005;115:870–878. doi: 10.1002/ijc.20960. [DOI] [PubMed] [Google Scholar]
- 101.Schut HA, et al. DNA adducts of heterocyclic amine food mutagens: implications for mutagenesis and carcinogenesis. Carcinogenesis. 1999;20:353–368. doi: 10.1093/carcin/20.3.353. [DOI] [PubMed] [Google Scholar]
- 102.Felton JS, et al. Human exposure to heterocyclic amine food mutagens/carcinogens: relevance to breast cancer. Environ. Mol. Mutagen. 2002;39:112–118. doi: 10.1002/em.10070. [DOI] [PubMed] [Google Scholar]
- 103.Reddy BS, et al. Effect of intestinal microflora and dietary fat on 3,2′-dimethyl-4-aminobiphenyl-induced colon carcinogenesis in F344 rats. Cancer Res. 1981;41:1363–1367. [PubMed] [Google Scholar]
- 104.Spjut HJ, et al. Experimental induction of tumors of the large bowel of rats. A review of the experience with 3-2′ dimethyl-4-aminobiphenyl. Cancer. 1971;28:29–37. doi: 10.1002/1097-0142(197107)28:1<29::aid-cncr2820280107>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 105.Decaens C, et al. Induction of rat intestinal carcinogenesis with single doses, low and high repeated doses of 1,2-dimethylhydrazine. Carcinogenesis. 1989;10:69–72. doi: 10.1093/carcin/10.1.69. [DOI] [PubMed] [Google Scholar]
- 106.Ward JM. Dose response to a single injection of azoxymethane in rats. Induction of tumors in the gastrointestinal tract, auditory sebaceous glands, kidney, liver and preputial gland. Vet. Pathol. 1975;12:165–177. doi: 10.1177/030098587501200302. [DOI] [PubMed] [Google Scholar]
- 107.Narisawa T, et al. Promoting effect of bile acids on colon carcinogenesis after intrarectal instillation of N-methyl-N′-nitro-N-nitrosoguanidine in rats. J. Natl Cancer Inst. 1974;53:1093–1097. doi: 10.1093/jnci/53.4.1093. [DOI] [PubMed] [Google Scholar]
- 108.Narisawa T, et al. Carcinoma of the colon and rectum of rats by rectal infusion of N-methyl-N′-nitro-N-nitrosoguanidine. Gann. 1971;62:231–234. [PubMed] [Google Scholar]
- 109.Narisawa T, et al. Colon cancer induction in mice by intrarectal instillation of N-methylnitosorurea (38498) Proc. Soc. Exp. Biol. Med. 1975;148:166–169. doi: 10.3181/00379727-148-38498. [DOI] [PubMed] [Google Scholar]
- 110.Reddy BS, et al. Animal models for the study of dietary factors and cancer of the large bowel. Cancer Res. 1975;35:3421–3426. [PubMed] [Google Scholar]
- 111.Heijstek MW, et al. Mouse models of colorectal cancer and liver metastases. Dig. Surg. 2005;22:16–25. doi: 10.1159/000085342. [DOI] [PubMed] [Google Scholar]
- 112.Kobaek-Larsen M, et al. Review of colorectal cancer and its metastases in rodent models: comparative aspects with those in humans. Comp. Med. 2000;50:16–26. [PubMed] [Google Scholar]
- 113.Miyamoto A, et al. Lower risk of death from gastric cancer among participants of gastric cancer screening in Japan: a population-based cohort study. Prev. Med. 2007;44:12–19. doi: 10.1016/j.ypmed.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 114.Yasui Y, et al. Molecular mechanism of colorectal carcinogenesis. In: Tanaka T, editor. Cancer: Disease Progression and Chemoprevention. Kerala, India: Reseach Signpost; 2007. pp. 45–56. [Google Scholar]
- 115.Wood LD, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 116.Takahashi M, et al. Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci. 2004;95:475–480. doi: 10.1111/j.1349-7006.2004.tb03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jacoby RF, et al. Mutations in the K-ras oncogene induced by 1,2-dimethylhydrazine in preneoplastic and neoplastic rat colonic mucosa. J. Clin. Invest. 1991;87:624–630. doi: 10.1172/JCI115039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jacoby RF, et al. K-ras oncogene mutations in rat colon tumors induced by N-methyl-N-nitrosourea. Carcinogenesis. 1992;13:45–49. doi: 10.1093/carcin/13.1.45. [DOI] [PubMed] [Google Scholar]
- 119.De Filippo C, et al. Mutations of the Apc gene in experimental colorectal carcinogenesis induced by azoxymethane in F344 rats. Br. J. Cancer. 1998;77:2148–2151. doi: 10.1038/bjc.1998.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Erdman SH, et al. Assessment of mutations in Ki-ras and p53 in colon cancers from azoxymethane- and dimethylhydrazine-treated rats. Mol. Carcinog. 1997;19:137–144. doi: 10.1002/(sici)1098-2744(199707)19:2<137::aid-mc8>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 121.Kakiuchi H, et al. Specific 5′-GGGA-3′–>5′-GGA-3′ mutation of the Apc gene in rat colon tumors induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Proc. Natl Acad. Sci. USA. 1995;92:910–914. doi: 10.1073/pnas.92.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Matsumoto K, et al. Demonstration of ras and p53 gene mutations in carcinomas in the forestomach and intestine and soft tissue sarcomas induced by N-methyl-N-nitrosourea in the rat. Jpn. J. Cancer Res. 1997;88:129–136. doi: 10.1111/j.1349-7006.1997.tb00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kakiuchi H, et al. Rare frequency of activation of the Ki-ras gene in rat colon tumors induced by heterocyclic amines: possible alternative mechanisms of human colon carcinogenesis. Mol. Carcinog. 1993;8:44–48. doi: 10.1002/mc.2940080110. [DOI] [PubMed] [Google Scholar]
- 124.Stopera SA, et al. Evidence for a ras gene mutation in azoxymethane-induced colonic aberrant crypts in Sprague-Dawley rats: earliest recognizable precursor lesions of experimental colon cancer. Carcinogenesis. 1992;13:2081–2085. doi: 10.1093/carcin/13.11.2081. [DOI] [PubMed] [Google Scholar]
- 125.Vivona AA, et al. K-ras mutations in aberrant crypt foci, adenomas and adenocarcinomas during azoxymethane-induced colon carcinogenesis. Carcinogenesis. 1993;14:1777–1781. doi: 10.1093/carcin/14.9.1777. [DOI] [PubMed] [Google Scholar]
- 126.Rogers KJ, et al. Formation of O6-methylguanine by alkylation of rat liver, colon, and kidney DNA following administration of 1,2-dimethylhydrazine. Cancer Res. 1977;37:4082–4087. [PubMed] [Google Scholar]
- 127.Calcagno SR, et al. Oncogenic K-ras promotes early carcinogenesis in the mouse proximal colon. Int. J. Cancer. 2008;122:2462–2470. doi: 10.1002/ijc.23383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maltzman T, et al. AOM-induced mouse colon tumors do not express full-length APC protein. Carcinogenesis. 1997;18:2435–2439. doi: 10.1093/carcin/18.12.2435. [DOI] [PubMed] [Google Scholar]
- 129.Takahashi M, et al. Beta-catenin is frequently mutated and demonstrates altered cellular location in azoxymethane-induced rat colon tumors. Cancer Res. 1998;58:42–46. [PubMed] [Google Scholar]
- 130.Takahashi M, et al. Frequent mutations of the beta-catenin gene in mouse colon tumors induced by azoxymethane. Carcinogenesis. 2000;21:1117–1120. [PubMed] [Google Scholar]
- 131.Koesters R, et al. Predominant mutation of codon 41 of the beta-catenin proto-oncogene in rat colon tumors induced by 1,2-dimethylhydrazine using a complete carcinogenic protocol. Carcinogenesis. 2001;22:1885–1890. doi: 10.1093/carcin/22.11.1885. [DOI] [PubMed] [Google Scholar]
- 132.Dashwood RH, et al. High frequency of beta-catenin (ctnnb1) mutations in the colon tumors induced by two heterocyclic amines in the F344 rat. Cancer Res. 1998;58:1127–1129. [PubMed] [Google Scholar]
- 133.Tsukamoto T, et al. More frequent beta-catenin gene mutations in adenomas than in aberrant crypt foci or adenocarcinomas in the large intestines of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-treated rats. Jpn. J. Cancer Res. 2000;91:792–796. doi: 10.1111/j.1349-7006.2000.tb01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ochiai M, et al. Characterization of dysplastic aberrant crypt foci in the rat colon induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Am. J. Pathol. 2003;163:1607–1614. doi: 10.1016/S0002-9440(10)63517-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Blum CA, et al. Mutational analysis of Ctnnb1 and Apc in tumors from rats given 1,2-dimethylhydrazine or 2-amino-3-methylimidazo[4,5-f]quinoline: mutational ‘hotspots’ and the relative expression of beta-catenin and c-jun. Mol. Carcinog. 2003;36:195–203. doi: 10.1002/mc.10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shitashige M, et al. Involvement of splicing factor-1 in beta-catenin/T-cell factor-4-mediated gene transactivation and pre-mRNA splicing. Gastroenterology. 2007;132:1039–1054. doi: 10.1053/j.gastro.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 137.Shitashige M, et al. Increased susceptibility of Sf1(+/−) mice to azoxymethane-induced colon tumorigenesis. Cancer Sci. 2007;98:1862–1867. doi: 10.1111/j.1349-7006.2007.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Makino H, et al. Absence of p53 mutations in rat colon tumors induced by 2-amino-6-methyldipyrido[1,2-a:3′,2′-d]imidazole, 2-amino-3-methylimidazo[4,5-f]quinoline, or 2-amino-1-methyl-6-phenylimidazo]4,5-b]pyridine. Jpn. J. Cancer Res. 1994;85:510–514. doi: 10.1111/j.1349-7006.1994.tb02388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nambiar PR, et al. Role of the alternating reading frame (P19)-p53 pathway in an in vivo murine colon tumor model. Cancer Res. 2002;62:3667–3674. [PubMed] [Google Scholar]
- 140.Shivapurkar N, et al. Absence of p53 gene mutations in rat colon carcinomas induced by azoxymethane. Cancer Lett. 1995;96:63–70. doi: 10.1016/0304-3835(95)03947-u. [DOI] [PubMed] [Google Scholar]
- 141.Toyota M, et al. Genetic alterations in rat colon tumors induced by heterocyclic amines. Cancer. 1996;77:1593–1597. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1593::AID-CNCR26>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 142.Hu Y, et al. Absence of acute apoptotic response to genotoxic carcinogens in p53-deficient mice is associated with increased susceptibility to azoxymethane-induced colon tumours. Int. J. Cancer. 2005;115:561–567. doi: 10.1002/ijc.20876. [DOI] [PubMed] [Google Scholar]
- 143.Tyner SD, et al. Increased tumor cell proliferation in murine tumors with decreasing dosage of wild-type p53. Mol. Carcinog. 1999;24:197–208. doi: 10.1002/(sici)1098-2744(199903)24:3<197::aid-mc6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 144.Aizu W, et al. Circumvention and reactivation of the p53 oncogene checkpoint in mouse colon tumors. Biochem. Pharmacol. 2006;72:981–991. doi: 10.1016/j.bcp.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 145.Tachibana M, et al. Dysfunction of p53 pathway in human colorectal cancer: analysis of p53 gene mutation and the expression of the p53-associated factors p14ARF, p33ING1, p21WAF1 and MDM2. Int. J. Oncol. 2004;25:913–920. [PubMed] [Google Scholar]
- 146.Kawamori T, et al. Enhancement of colon carcinogenesis by prostaglandin E2 administration. Carcinogenesis. 2003;24:985–990. doi: 10.1093/carcin/bgg033. [DOI] [PubMed] [Google Scholar]
- 147.Tempero M. Bile acids, ornithine decarboxylase, and cell proliferation in colon cancer: a review. Dig. Dis. 1986;4:49–56. doi: 10.1159/000171137. [DOI] [PubMed] [Google Scholar]
- 148.Milovic V, et al. Polyamines and colon cancer. Biochem. Soc. Trans. 2003;31:381–383. doi: 10.1042/bst0310381. [DOI] [PubMed] [Google Scholar]
- 149.Tanaka T, et al. Chemoprevention of azoxymethane-induced rat colon carcinogenesis by the naturally occurring flavonoids, diosmin and hesperidin. Carcinogenesis. 1997;18:957–965. doi: 10.1093/carcin/18.5.957. [DOI] [PubMed] [Google Scholar]
- 150.Yoshimi N, et al. Expression of cytokines, TNF-alpha and IL-1 alpha, in MAM acetate and 1-hydroxyanthraquinone-induced colon carcinogenesis of rats. Carcinogenesis. 1994;15:783–785. doi: 10.1093/carcin/15.4.783. [DOI] [PubMed] [Google Scholar]
- 151.Arber N, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N. Engl. J. Med. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 152.Gupta RA, et al. Combinations for cancer prevention. Nat. Med. 2000;6:974–975. doi: 10.1038/79664. [DOI] [PubMed] [Google Scholar]
- 153.Hallak A, et al. Rofecoxib reduces polyp recurrence in familial polyposis. Dig. Dis. Sci. 2003;48:1998–2002. doi: 10.1023/a:1026130623186. [DOI] [PubMed] [Google Scholar]
- 154.Hawk E, et al. The adenoma prevention with celecoxib and prevention of colorectal sporadic adenomatous polyps trials: stepping stones to progress. Cancer Epidemiol. Biomarkers Prev. 2007;16:185–187. doi: 10.1158/1055-9965.EPI-06-1086. [DOI] [PubMed] [Google Scholar]
- 155.Janne PA, et al. Chemoprevention of colorectal cancer. N. Engl. J. Med. 2000;342:1960–1968. doi: 10.1056/NEJM200006293422606. [DOI] [PubMed] [Google Scholar]
- 156.Subbaramaiah K, et al. Inhibition of cyclooxygenase: a novel approach to cancer prevention. Proc. Soc. Exp. Biol. Med. 1997;216:201–210. doi: 10.3181/00379727-216-44170. [DOI] [PubMed] [Google Scholar]
- 157.Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis (part I) J. Natl Cancer Inst. 1998;90:1529–1536. doi: 10.1093/jnci/90.20.1529. [DOI] [PubMed] [Google Scholar]
- 158.Bissonnette M, et al. Mutational and nonmutational activation of p21ras in rat colonic azoxymethane-induced tumors: effects on mitogen-activated protein kinase, cyclooxygenase-2, and cyclin D1. Cancer Res. 2000;60:4602–4609. [PubMed] [Google Scholar]
- 159.Dong M, et al. Inverse association between phospholipase A2 and COX-2 expression during mouse colon tumorigenesis. Carcinogenesis. 2003;24:307–315. doi: 10.1093/carcin/24.2.307. [DOI] [PubMed] [Google Scholar]
- 160.DuBois RN, et al. Increased cyclooxygenase-2 levels in carcinogen-induced rat colonic tumors. Gastroenterology. 1996;110:1259–1262. doi: 10.1053/gast.1996.v110.pm8613017. [DOI] [PubMed] [Google Scholar]
- 161.Kishimoto Y, et al. Molecular changes in the early stage of colon carcinogenesis in rats treated with azoxymethane. J. Exp. Clin. Cancer Res. 2002;21:203–211. [PubMed] [Google Scholar]
- 162.Shao J, et al. Coordinate regulation of cyclooxygenase-2 and TGF-beta1 in replication error-positive colon cancer and azoxymethane-induced rat colonic tumors. Carcinogenesis. 1999;20:185–191. doi: 10.1093/carcin/20.2.185. [DOI] [PubMed] [Google Scholar]
- 163.Singh J, et al. Dietary fat and colon cancer: modulation of cyclooxygenase-2 by types and amount of dietary fat during the postinitiation stage of colon carcinogenesis. Cancer Res. 1997;57:3465–3470. [PubMed] [Google Scholar]
- 164.Rao CV, et al. Modulation of experimental colon tumorigenesis by types and amounts of dietary fatty acids. Cancer Res. 2001;61:1927–1933. [PubMed] [Google Scholar]
- 165.Kishimoto Y, et al. Effects of cyclooxygenase-2 inhibitor NS-398 on APC and c-myc expression in rat colon carcinogenesis induced by azoxymethane. J. Gastroenterol. 2002;37:186–193. doi: 10.1007/s005350200019. [DOI] [PubMed] [Google Scholar]
- 166.Kuno T, et al. Induction of apoptosis by sulindac in azoxymethane-induced possible colonic premalignant lesions in rats. Jpn. J. Cancer Res. 2002;93:242–246. doi: 10.1111/j.1349-7006.2002.tb02164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wei M, et al. JTE-522, a selective cyclooxygenase-2 inhibitor, inhibits induction but not growth and invasion of 1,2-dimethylhydrazine-induced tubular adenocarcinomas of colon in rats. Int. J. Cancer. 2005;113:354–358. doi: 10.1002/ijc.20583. [DOI] [PubMed] [Google Scholar]
- 168.Reddy BS, et al. Chemoprevention of colon cancer by specific cyclooxygenase-2 inhibitor, celecoxib, administered during different stages of carcinogenesis. Cancer Res. 2000;60:293–297. [PubMed] [Google Scholar]
- 169.Riehl TE, et al. Azoxymethane protects intestinal stem cells and reduces crypt epithelial mitosis through a COX-1-dependent mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G1062–G1070. doi: 10.1152/ajpgi.00129.2006. [DOI] [PubMed] [Google Scholar]
- 170.Takano T, et al. Cyclooxygenases-1 and 2 couple to cytosolic but not group IIA phospholipase A2 in COS-1 cells. Prostaglandins Other Lipid Mediat. 2000;60:15–26. doi: 10.1016/s0090-6980(99)00033-7. [DOI] [PubMed] [Google Scholar]
- 171.Han C, et al. 85-kDa cPLA(2) plays a critical role in PPAR-mediated gene transcription in human hepatoma cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282:G586–G597. doi: 10.1152/ajpgi.00305.2001. [DOI] [PubMed] [Google Scholar]
- 172.Heasley LE, et al. Induction of cytosolic phospholipase A2 by oncogenic Ras in human non-small cell lung cancer. J. Biol. Chem. 1997;272:14501–14504. doi: 10.1074/jbc.272.23.14501. [DOI] [PubMed] [Google Scholar]
- 173.Wu T, et al. Involvement of 85-kd cytosolic phospholipase A(2) and cyclooxygenase-2 in the proliferation of human cholangiocarcinoma cells. Hepatology. 2002;36:363–373. doi: 10.1053/jhep.2002.34743. [DOI] [PubMed] [Google Scholar]
- 174.Dong M, et al. Cytoplasmic phospholipase A2 levels correlate with apoptosis in human colon tumorigenesis. Clin. Cancer Res. 2005;11:2265–2271. doi: 10.1158/1078-0432.CCR-04-1079. [DOI] [PubMed] [Google Scholar]
- 175.Chan TA, et al. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc. Natl Acad. Sci. USA. 1998;95:681–686. doi: 10.1073/pnas.95.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mann JR, et al. Repression of prostaglandin dehydrogenase by epidermal growth factor and snail increases prostaglandin E2 and promotes cancer progression. Cancer Res. 2006;66:6649–6656. doi: 10.1158/0008-5472.CAN-06-1787. [DOI] [PubMed] [Google Scholar]
- 177.Myung SJ, et al. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc. Natl Acad. Sci. USA. 2006;103:12098–12102. doi: 10.1073/pnas.0603235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nakanishi M, et al. Genetic deletion of mPGES-1 suppresses intestinal tumorigenesis. Cancer Res. 2008;68:3251–3259. doi: 10.1158/0008-5472.CAN-07-6100. [DOI] [PubMed] [Google Scholar]
- 179.Cohen SM, et al. Cell proliferation in carcinogenesis. Science. 1990;249:1007–1011. doi: 10.1126/science.2204108. [DOI] [PubMed] [Google Scholar]
- 180.Pegg AE. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988;48:759–774. [PubMed] [Google Scholar]
- 181.Igarashi K, et al. Polyamines: mysterious modulators of cellular functions. Biochem. Biophys. Res. Commun. 2000;271:559–564. doi: 10.1006/bbrc.2000.2601. [DOI] [PubMed] [Google Scholar]
- 182.LaMuraglia GM, et al. High ornithine decarboxylase activity and polyamine levels in human colorectal neoplasia. Ann. Surg. 1986;204:89–93. doi: 10.1097/00000658-198607000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Luk GD. Essential role of polyamine metabolism in hepatic regeneration. Inhibition of deoxyribonucleic acid and protein synthesis and tissue regeneration by difluoromethylornithine in the rat. Gastroenterology. 1986;90:1261–1267. doi: 10.1016/0016-5085(86)90394-x. [DOI] [PubMed] [Google Scholar]
- 184.Seiler N, et al. Polyamines and the intestinal tract. Crit. Rev. Clin. Lab. Sci. 2007;44:365–411. doi: 10.1080/10408360701250016. [DOI] [PubMed] [Google Scholar]
- 185.Luk GD, et al. Kinetic changes in mucosal ornithine decarboxylase activity during azoxymethane-induced colonic carcinogenesis in the rat. Cancer Res. 1986;46:4449–4452. [PubMed] [Google Scholar]
- 186.Pizzi C, et al. Cell kinetics and polyamine enzymes in the intestinal mucosa of rats with azoxymethane induced tumours. Int. J. Exp. Pathol. 1994;75:305–311. [PMC free article] [PubMed] [Google Scholar]
- 187.Nigro ND, et al. Inhibition of intestinal carcinogenesis in rats: effect of difluoromethylornithine with piroxicam or fish oil. J. Natl Cancer Inst. 1986;77:1309–1313. [PubMed] [Google Scholar]
- 188.Rao CV, et al. Chemoprevention of colon carcinogenesis by dietary administration of piroxicam, alpha-difluoromethylornithine, 16 alpha-fluoro-5-androsten-17-one, and ellagic acid individually and in combination. Cancer Res. 1991;51:4528–4534. [PubMed] [Google Scholar]
- 189.Reddy BS, et al. Chemoprevention of colon carcinogenesis by concurrent administration of piroxicam, a nonsteroidal antiinflammatory drug with D,L-alpha-difluoromethylornithine, an ornithine decarboxylase inhibitor, in diet. Cancer Res. 1990;50:2562–2568. [PubMed] [Google Scholar]
- 190.Meyskens FLJ, et al. Difluoromethylornithine plus Sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev. Res. 2008;1:32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gerner EW, et al. Polyamines and cancer: old molecules, new understanding. Nat. Rev. Cancer. 2004;4:781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 192.Markowitz S. TGF-beta receptors and DNA repair genes, coupled targets in a pathway of human colon carcinogenesis. Biochim. Biophys. Acta. 2000;1470:M13–M20. doi: 10.1016/s0304-419x(99)00031-1. [DOI] [PubMed] [Google Scholar]
- 193.Oberhammer FA, et al. Induction of apoptosis in cultured hepatocytes and in regressing liver by transforming growth factor beta 1. Proc. Natl Acad. Sci. USA. 1992;89:5408–5412. doi: 10.1073/pnas.89.12.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rorke EA, et al. Transforming growth factor-beta 1 (TGF beta 1) enhances apoptosis in human papillomavirus type 16-immortalized human ectocervical epithelial cells. Exp. Cell Res. 1995;216:65–72. doi: 10.1006/excr.1995.1008. [DOI] [PubMed] [Google Scholar]
- 195.Wang CY, et al. Both transforming growth factor-beta and substrate release are inducers of apoptosis in a human colon adenoma cell line. Cancer Res. 1995;55:5101–5105. [PubMed] [Google Scholar]
- 196.Becker C, et al. TGF-beta as a T cell regulator in colitis and colon cancer. Cytokine Growth Factor Rev. 2006;17:97–106. doi: 10.1016/j.cytogfr.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 197.Chung DC. The genetic basis of colorectal cancer: insights into critical pathways of tumorigenesis. Gastroenterology. 2000;119:854–865. doi: 10.1053/gast.2000.16507. [DOI] [PubMed] [Google Scholar]
- 198.Eppert K, et al. MADR2 maps to 18q21 and encodes a TGFbeta-regulated MAD-related protein that is functionally mutated in colorectal carcinoma. Cell. 1996;86:543–552. doi: 10.1016/s0092-8674(00)80128-2. [DOI] [PubMed] [Google Scholar]
- 199.Grady WM, et al. Mutational inactivation of transforming growth factor beta receptor type II in microsatellite stable colon cancers. Cancer Res. 1999;59:320–324. [PubMed] [Google Scholar]
- 200.Howe JR, et al. Mutations in the SMAD4/DPC4 gene in juvenile polyposis. Science. 1998;280:1086–1088. doi: 10.1126/science.280.5366.1086. [DOI] [PubMed] [Google Scholar]
- 201.Riggins GJ, et al. Mad-related genes in the human. Nat. Genet. 1996;13:347–349. doi: 10.1038/ng0796-347. [DOI] [PubMed] [Google Scholar]
- 202.Tarafa G, et al. DCC and SMAD4 alterations in human colorectal and pancreatic tumor dissemination. Oncogene. 2000;19:546–555. doi: 10.1038/sj.onc.1203353. [DOI] [PubMed] [Google Scholar]
- 203.Guda K, et al. Aberrant transforming growth factor-beta signaling in azoxymethane-induced mouse colon tumors. Mol. Carcinog. 2001;31:204–213. doi: 10.1002/mc.1055. [DOI] [PubMed] [Google Scholar]
- 204.Guda K, et al. Defective processing of the transforming growth factor-beta1 in azoxymethane-induced mouse colon tumors. Mol. Carcinog. 2003;37:51–59. doi: 10.1002/mc.10120. [DOI] [PubMed] [Google Scholar]
- 205.Osawa E, et al. Predominant T helper type 2-inflammatory responses promote murine colon cancers. Int. J. Cancer. 2006;118:2232–2236. doi: 10.1002/ijc.21639. [DOI] [PubMed] [Google Scholar]
- 206.Naito Y, et al. Enhanced intestinal inflammation induced by dextran sulfate sodium in tumor necrosis factor-alpha deficient mice. J. Gastroenterol. Hepatol. 2003;18:560–569. doi: 10.1046/j.1440-1746.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 207.Popivanova BK, et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J. Clin. Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Corpet DE, et al. Point: from animal models to prevention of colon cancer. Systematic review of chemoprevention in min mice and choice of the model system. Cancer Epidemiol. Biomarkers Prev. 2003;12:391–400. [PMC free article] [PubMed] [Google Scholar]
- 209.Maggio-Price L, et al. Helicobacter infection is required for inflammation and colon cancer in SMAD3-deficient mice. Cancer Res. 2006;66:828–838. doi: 10.1158/0008-5472.CAN-05-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Delapierre F, et al. Preneoplastic changes in epithelium and mesenchyme in colon of rats treated with 1,2-dimethylhydrazine. Cancer Detect. Prev. 1981;4:429–437. [PubMed] [Google Scholar]
- 211.Wargovich MJ, et al. Early histopathologic events to evolution of colon cancer in C57BL/6 and CF1 mice treated with 1,2-dimethylhydrazine. J. Natl Cancer Inst. 1983;71:125–131. [PubMed] [Google Scholar]
- 212.Chang WW. Histogenesis of colon cancer in experimental animals. Scand. J. Gastroenterol. Suppl. 1984;104:27–43. [PubMed] [Google Scholar]
- 213.James JT, et al. Comparative study of the morphologic, histochemical, and proliferative changes induced in the large intestine of ICR/Ha and C57BL/Ha mice by 1,2-dimethylhydrazine. J. Natl Cancer Inst. 1983;71:955–964. [PubMed] [Google Scholar]
- 214.Lipkin M, et al. Early proliferative changes in intestinal cells. Cancer Res. 1976;36:2665–2668. [PubMed] [Google Scholar]
- 215.Bird RP. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 1987;37:147–151. doi: 10.1016/0304-3835(87)90157-1. [DOI] [PubMed] [Google Scholar]
- 216.McLellan EA, et al. Specificity study to evaluate induction of aberrant crypts in murine colons. Cancer Res. 1988;48:6183–6186. [PubMed] [Google Scholar]
- 217.McLellan EA, et al. Sequential analyses of the growth and morphological characteristics of aberrant crypt foci: putative preneoplastic lesions. Cancer Res. 1991;51:5270–5274. [PubMed] [Google Scholar]
- 218.McLellan EA, et al. Aberrant crypts: potential preneoplastic lesions in the murine colon. Cancer Res. 1988;48:6187–6192. [PubMed] [Google Scholar]
- 219.Whiteley LO, et al. Aberrant crypt foci in the colonic mucosa of rats treated with a genotoxic and nongenotoxic colon carcinogen. Toxicol. Pathol. 1996;24:681–689. doi: 10.1177/019262339602400602. [DOI] [PubMed] [Google Scholar]
- 220.Pretlow TP, et al. Aberrant crypts: putative preneoplastic foci in human colonic mucosa. Cancer Res. 1991;51:1564–1567. [PubMed] [Google Scholar]
- 221.Roncucci L, et al. Identification and quantification of aberrant crypt foci and microadenomas in the human colon. Hum. Pathol. 1991;22:287–294. doi: 10.1016/0046-8177(91)90163-j. [DOI] [PubMed] [Google Scholar]
- 222.Jen J, et al. Molecular determinants of dysplasia in colorectal lesions. Cancer Res. 1994;54:5523–5526. [PubMed] [Google Scholar]
- 223.Kinzler KW, et al. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 224.Barrow BJ, et al. Enzyme-altered foci in colons of carcinogen-treated rats. Cancer Res. 1990;50:1911–1916. [PubMed] [Google Scholar]
- 225.Pretlow TP, et al. Two types of putative preneoplastic lesions identified by hexosaminidase activity in whole-mounts of colons from F344 rats treated with carcinogen. Am. J. Pathol. 1993;142:1695–1700. [PMC free article] [PubMed] [Google Scholar]
- 226.Tsukamoto T, et al. Hexosaminidase-altered aberrant crypts, carrying decreased hexosaminidase alpha and beta subunit mRNAs, in colon of 1,2-dimethylhydrazine-treated rats. Jpn. J. Cancer Res. 2001;92:109–118. doi: 10.1111/j.1349-7006.2001.tb01072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tsukamoto T, et al. Three-dimensional analysis of isolated hexosaminidase-altered aberrant crypts from colons of 1,2-dimethylhydrazine-treated rats. Exp. Toxicol. Pathol. 2006;57:283–289. doi: 10.1016/j.etp.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 228.Yamada Y, et al. Frequent beta-catenin gene mutations and accumulations of the protein in the putative preneoplastic lesions lacking macroscopic aberrant crypt foci appearance, in rat colon carcinogenesis. Cancer Res. 2000;60:3323–3327. [PubMed] [Google Scholar]
- 229.Hata K, et al. Tumor formation is correlated with expression of beta-catenin-accumulated crypts in azoxymethane-induced colon carcinogenesis in mice. Cancer Sci. 2004;95:316–320. doi: 10.1111/j.1349-7006.2004.tb03209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Caderni G, et al. Identification of mucin-depleted foci in the unsectioned colon of azoxymethane-treated rats: correlation with carcinogenesis. Cancer Res. 2003;63:2388–2392. [PubMed] [Google Scholar]
- 231.Femia AP, et al. Mucin-depleted foci (MDF) in the colon of rats treated with azoxymethane (AOM) are useful biomarkers for colon carcinogenesis. Carcinogenesis. 2004;25:277–281. doi: 10.1093/carcin/bgh005. [DOI] [PubMed] [Google Scholar]
- 232.Femia AP, et al. Mucin-depleted foci have beta-catenin gene mutations, altered expression of its protein, and are dose- and time-dependent in the colon of 1,2-dimethylhydrazine-treated rats. Int. J. Cancer. 2005;116:9–15. doi: 10.1002/ijc.20981. [DOI] [PubMed] [Google Scholar]
- 233.Paulsen JE, et al. Identification and quantification of aberrant crypt foci in the colon of Min mice—a murine model of familial adenomatous polyposis. Scand. J. Gastroenterol. 2000;35:534–539. doi: 10.1080/003655200750023813. [DOI] [PubMed] [Google Scholar]
- 234.Paulsen JE, et al. Identification of flat dysplastic aberrant crypt foci in the colon of azoxymethane-treated A/J mice. Int. J. Cancer. 2006;118:540–546. doi: 10.1002/ijc.21416. [DOI] [PubMed] [Google Scholar]
- 235.Eaden J, et al. Inter-observer variation between general and specialist gastrointestinal pathologists when grading dysplasia in ulcerative colitis. J. Pathol. 2001;194:152–157. doi: 10.1002/path.876. [DOI] [PubMed] [Google Scholar]
- 236.van Hogezand RA, et al. Malignancies in inflammatory bowel disease: fact or fiction? Scand. J. Gastroenterol. Suppl. 2002:48–53. doi: 10.1080/003655202320621454. [DOI] [PubMed] [Google Scholar]
- 237.Okayasu I, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 238.Okayasu I, et al. Dysplasia and carcinoma development in a repeated dextran sulfate sodium-induced colitis model. J. Gastroenterol. Hepatol. 2002;17:1078–1083. doi: 10.1046/j.1440-1746.2002.02853.x. [DOI] [PubMed] [Google Scholar]
- 239.Seril DN, et al. Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis. 2003;24:353–362. doi: 10.1093/carcin/24.3.353. [DOI] [PubMed] [Google Scholar]
- 240.Tanaka T, et al. Colitis-related rat colon carcinogenesis induced by 1-hydroxy-anthraquinone and methylazoxymethanol acetate (review) Oncol. Rep. 2000;7:501–508. doi: 10.3892/or.7.3.501. [DOI] [PubMed] [Google Scholar]
- 241.Malaviya R, et al. Bacteria—mast cell interactions in inflammatory disease. Am. J. Ther. 1995;2:787–792. doi: 10.1097/00045391-199510000-00010. [DOI] [PubMed] [Google Scholar]
- 242.Maeda H, et al. Nitric oxide and oxygen radicals in infection, inflammation, and cancer. Biochemistry (Mosc.) 1998;63:854–865. [PubMed] [Google Scholar]
- 243.Tanaka T, et al. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Alican I, et al. A critical role for nitric oxide in intestinal barrier function and dysfunction. Am. J. Physiol. 1996;270:G225–G237. doi: 10.1152/ajpgi.1996.270.2.G225. [DOI] [PubMed] [Google Scholar]
- 245.Anton PM, et al. Chronic ingestion of a potential food contaminant induces gastrointestinal inflammation in rats: role of nitric oxide and mast cells. Dig. Dis. Sci. 2000;45:1842–1849. doi: 10.1023/a:1005509623060. [DOI] [PubMed] [Google Scholar]
- 246.Seril DN, et al. Inhibition of chronic ulcerative colitis-associated colorectal adenocarcinoma development in a murine model by N-acetylcysteine. Carcinogenesis. 2002;23:993–1001. doi: 10.1093/carcin/23.6.993. [DOI] [PubMed] [Google Scholar]
- 247.Kohno H, et al. Beta-catenin mutations in a mouse model of inflammation-related colon carcinogenesis induced by 1,2-dimethylhydrazine and dextran sodium sulfate. Cancer Sci. 2005;96:69–76. doi: 10.1111/j.1349-7006.2005.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Tanaka T, et al. Colonic adenocarcinomas rapidly induced by the combined treatment with 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and dextran sodium sulfate in male ICR mice possess beta-catenin gene mutations and increases immunoreactivity for beta-catenin, cyclooxygenase-2 and inducible nitric oxide synthase. Carcinogenesis. 2005;26:229–238. doi: 10.1093/carcin/bgh292. [DOI] [PubMed] [Google Scholar]
- 249.Suzuki R, et al. Strain differences in the susceptibility to azoxymethane and dextran sodium sulfate-induced colon carcinogenesis in mice. Carcinogenesis. 2006;27:162–169. doi: 10.1093/carcin/bgi205. [DOI] [PubMed] [Google Scholar]
- 250.Suzuki R, et al. Global gene expression analysis of the mouse colonic mucosa treated with azoxymethane and dextran sodium sulfate. BMC Cancer. 2007;7:84. doi: 10.1186/1471-2407-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Mirvish SS, et al. Total N-nitroso compounds and their precursors in hot dogs and in the gastrointestinal tract and feces of rats and mice: possible etiologic agents for colon cancer. J. Nutr. 2002;132:3526S–3529S. doi: 10.1093/jn/132.11.3526S. [DOI] [PubMed] [Google Scholar]
- 252.Hata K, et al. Beta-catenin-accumulated crypts in the colonic mucosa of juvenile ApcMin/+ mice. Cancer Lett. 2006;239:123–128. doi: 10.1016/j.canlet.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 253.Kohno H, et al. Dietary administration with prenyloxycoumarins, auraptene and collinin, inhibits colitis-related colon carcinogenesis in mice. Int. J. Cancer. 2006;118:2936–2942. doi: 10.1002/ijc.21719. [DOI] [PubMed] [Google Scholar]
- 254.Ilsley JN, et al. Dietary iron promotes azoxymethane-induced colon tumors in mice. Nutr. Cancer. 2004;49:162–169. doi: 10.1207/s15327914nc4902_7. [DOI] [PubMed] [Google Scholar]
- 255.Diwan BA, et al. Genetic differences in the induction of colorectal tumors by 1,2-dimethylhydrazine in inbred mice. J. Natl Cancer Inst. 1977;59:455–458. [Google Scholar]
- 256.Diwan BA, et al. Carcinogenic effects of combined treatment of inbred mice with 3-methylcholanthrene and 1,2-dimethylhydrazine. J. Natl Cancer Inst. 1980;64:1491–1493. doi: 10.1093/jnci/64.6.1491. [DOI] [PubMed] [Google Scholar]
- 257.Evans JT, et al. Differential susceptibility of four mouse strains to induction of multiple large-bowel neoplasms by 1,2-dimethylhydrazine. J. Natl Cancer Inst. 1974;52:999–1000. doi: 10.1093/jnci/52.3.999. [DOI] [PubMed] [Google Scholar]
- 258.Narisawa T, et al. Carcinoma of the large intestine of rats induced by rectal infusion of methylazoxymethanol. Gann. 1973;64:93–95. [PubMed] [Google Scholar]
- 259.Zedeck MS, et al. A model system for studies of colon carcinogenesis: tumor induction by a single injection of methylazoxymethanol acetate. J. Natl Cancer Inst. 1974;53:1419–1421. doi: 10.1093/jnci/53.5.1419. [DOI] [PubMed] [Google Scholar]
- 260.Ward JM, et al. Intestinal tumors in mice treated with a single injection of N-nitroso-N-butylurea. Cancer Res. 1975;35:1938–1943. [PubMed] [Google Scholar]
- 261.Reddy BS, et al. Effect of high-fat diet on colon carcinogenesis in F344 rats treated with 1,2-dimethylhydrazine, methylazoxymethanol acetate, or methylnitrosourea. Cancer Res. 1977;37:4156–4159. [PubMed] [Google Scholar]
- 262.Ward JM, et al. Natural history of intestinal neoplasms induced in rats by a single injection of methyl(acetoxymethyl)nitrosamine. Cancer Res. 1977;37:3046–3052. [PubMed] [Google Scholar]
- 263.Ohgaki H, et al. Carcinogenicities of heterocyclic amines in cooked food. Mutat. Res. 1991;259:399–410. doi: 10.1016/0165-1218(91)90130-e. [DOI] [PubMed] [Google Scholar]
- 264.Reddy BS, et al. Effect of intestinal microflora on 2,2′-dimethyl-4-aminobiphenyl-induced carcinogenesis in F344 rats. J. Natl Cancer Inst. 1978;61:1269–1271. doi: 10.1093/jnci/61.5.1269. [DOI] [PubMed] [Google Scholar]