Abstract
In human and experimental myocardial infarction (MI), cessation of blood supply leads to rapid necrosis of cardiac myocytes in the ischaemic heart. Immediately after injury, various intra- and intercellular pathways contribute to healing the myocardial wound in order to achieve tissue integrity and function. MI and the consequent loss of myocardium are the major aetiology for heart failure. Despite aggressive primary therapy, prognosis remains poor in patients with large infarction and severe left ventricular dysfunction. Thus, it would be highly desirable to improve healing of the cardiac wound to maintain structure and function of the heart. Healing in the heart occurs in overlapping phases. Herein, we review the inflammatory phase as a trigger of tissue formation.
Keywords: Myocardial infarction, Healing, Inflammation
1. Introduction
One major determinant of remodelling post-myocardial infarction (MI) is infarct size.1 Infarct size depends on the amount of myocardium supplied by the infarct-related coronary artery, the time to effective reperfusion therapy, and myocardial energy consumption during coronary occlusion. Early reperfusion and beta-blockers are therefore standard therapy today. However, slow-flow or no-reflow phenomena are quite frequent post-MI and may contribute to ongoing ischaemia. After reperfusion, ‘the infarct’ in most instances will consist of highly inhomogeneous tissue which may immediately recover, be stunned, or be apoptotic/necrotic. Sensitivity to ischaemia varies among myocardial cells, vasculature, and connective tissue adding to inhomogeneity of infarct tissue composition.2 In addition, inflammatory cells and macrophages may invade from circulating blood and start as well as maintain processes of inflammation, clearing debris, and wound healing. Toxic or protective mediators, circulating and locally released by autochthonous or recruited cells, add to the complexity. It may be of major therapeutic value to influence myocardial healing as it opens a new time window and addresses new mechanisms for therapy. However, specific measures have not yet been developed mostly due to a lack of precise knowledge of processes contributing to wound healing. The present review (i) puts forward the hypothesis that a reperfused MI may be considered a healing wound, (ii) compiles evidence for cells and factors controlling the inflammatory phase of wound healing, and (iii) proposes potential anti-inflammatory mechanisms as targets for therapeutic research.
2. Myocardial infarction: a healing wound
The capacity for regeneration and reparation certainly was of selection value in evolution and is highly variable among species. Some species may restore organs or limbs in total; mammals are mostly restricted to reparation; and variability is high among individual organs. Healing of external wounds is a conditio sine qua non for survival and therefore secured by multiple redundant mechanisms. Nevertheless, it may vary substantially among individuals. There appear to be differences in wound healing with regard to sex, age, and race, whereas genetics of wound healing have not been clarified. A large body of empirical knowledge has been accumulated on measures to support wound healing. More recently, essential factors for wound healing have been identified, but implications of such ‘healing factors’ for healing of internal wounds remain unclear. Two types of ‘wounds’ are particularly frequent and highly clinically relevant in the cardiovascular system: rupture of an atherosclerotic plaque and MI. For the latter, if survived, healing is essential for further prognosis. Both of them are modern diseases and thus evolution has not yet developed specific strategies by selection. Thus, general strategies of coping with stress and wound healing take place.
The healing process may first be dominated by inflammation (degradation of extracellular matrix, inhibition of tissue proliferation, and release of inflammatory mediators = ‘inflammatory phenotype’) and turn then to reparation (increased matrix synthesis, proliferation of fibroblasts and inflammatory cells, and release of fibrosis- promoting cytokines leading to scar formation = ‘activated phenotype’). The analysis of these processes may be of major therapeutic importance. Herein, we will focus on the early inflammatory phase as the trigger of tissue formation.
3. The inflammatory phase of wound healing
3.1. Triggers of inflammation after cardiac injury
What is the trigger of an inflammatory reaction after cardiac injury? Indeed, activation of the immune system after cardiac injury follows the pattern of immune activation after infection: most microorganisms encountered daily by a healthy individual are detected and destroyed within hours by defence mechanisms that are not antigen-specific, the so-called innate immune system. In contrast to adaptive immunity, whereby specific antigen receptors are generated by somatic hypermutation and selection, the innate immune system uses germline-encoded proteins that recognize specific patterns shared by groups of pathogens, but not the host. These receptors, called ‘pattern recognition receptors’, detect largely invariant patterns, for example, lipopolysaccharides (LPS) of bacteria or double-stranded RNA of viruses.3–5 They are constitutively expressed; thus, defence mechanisms are readily available and need not be upregulated. The heart itself expresses all parts of the innate immune system, including pattern recognition receptors and effector proteins.
Although it is commonly accepted that the innate immune system is activated by microbial patterns, Matzinger and co-workers6 assume in the ‘Danger’ model that the presence of potentially infectious patterns does not necessarily trigger an immune response, unless there is evidence of host tissue injury by the so-called ‘alarm’ signals. In support of this hypothesis, Matzinger and co-workers have demonstrated that, in the absence of any foreign pathogens, resting dendritic cells can be activated by virally infected or necrotic cells, but not by healthy cells or cells undergoing programmed cell death (apoptosis). Potential mediators include reactive oxygen species (ROS), heat shock proteins (HSP), and fibronectin.7 Thus, this work suggests that certain products of tissue injury, such as ROS and intracellular proteins released from necrotic cells, initiate an inflammatory response, leading to the activation of pattern recognition receptors such as Toll-like receptors (TLRs), the transcription nuclear factor kappa B (NF-κB), and complement.
3.1.1. Toll-like receptors
TLRs have emerged as the primary, non-antigen-specific defence mechanisms that enable innate immune detection of foreign pathogens. Thus, TLRs could be important for the initiation of the inflammatory phase after tissue injury.
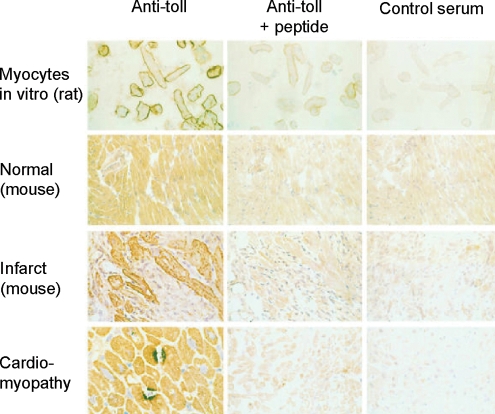
To date, 11 human and 13 mouse TLRs have been cloned.5 The ligands for TLRs are molecular motifs produced by pathogens, but also certain host-derived proteins such as HSP or fibronectin. As recently reviewed by us,7,8 TLR2, -3, -4, and -6 are expressed in cardiac myocytes, whereas TLR1 and -5 are not.9,10 TLRs and their signalling components are activated in experimental or clinical heart failure. TLR4 expression is increased in the myocardium of patients with advanced heart failure.10,11 In addition, there is a change in the TLR expression pattern: whereas in normal murine and human myocardium the TLR4 expression is diffuse and predominantly confined to cardiac myocytes, myocardium from patients with advanced heart failure displays focal areas of intense TLR4 staining10 (Figure 1).
Figure 1.
TLR4 in rat, murine, and human myocardium. Primary isolates of adult rat ventricular myocytes 24 h after isolation, stained with a polyclonal antibody targeted to a TLR4-specific epitope adjacent to the cytoplasmic TIR domain of hTLR4 (upper panel). Normal murine cardiac muscle (magnification 200×; second panel) exhibited diffuse, homogeneous myocyte staining. However, cardiac myocytes adjacent to an area of ischaemic injury induced by coronary artery ligation exhibited intense sarcolemmal TLR4 staining. Finally, cardiomyocytes from humans with dilated cardiomyopathy (lower panel) displayed intensely stained focal expression of TLR4 (figure reprinted with permission of Frantz et al.10).
The data on myocardial wound healing and TLRs are very limited. After coronary artery ligation, mortality and left ventricular dilatation were significantly reduced, and left ventricular function was preserved in TLR2−/− and TLR4−/− mice.12,13 In ischaemia/reperfusion experiments, the invasion of inflammatory cells as well as infarct size were significantly reduced in TLR4 KO animals.14 Conclusively, the data suggest a role of TLRs for the activation of inflammatory cells after cardiac injury.
3.1.2. Nuclear factor kappa B (NF-κB)
TLR signalling converges on the activation of the transcription factor NF-κB, a key signalling component for early inflammatory activation. As for TLRs, the role of NF-κB in healing can only indirectly be deduced from heart failure as well as ischaemia/reperfusion models. NF-κB-dependent signalling mechanisms in ischaemia/reperfusion injury are well defined by now.15 After ischaemia/reperfusion injury, NF-κB activation is biphasic, with peaks after 15 min and 3 h, i.e. early in the healing process.16,17 As for TLR, ischaemia/reperfusion injury is reduced by the inhibition of NF-κB using molecular [inhibition of p65 by double-stranded oligonucleotides18 and use of an IκB triple mutant (S32A, S36A, and Y42A) completely abrogating NF-κB activation19], as well as pharmacological methods (IKK inhibition20). We recently demonstrated that mice with targeted deletion of the NF-κB subunit p50 are protected against ischaemia/reperfusion injury.21 KO and WT animals underwent 30 min of coronary artery ligation and 24 h of reperfusion in vivo. Ischaemia–reperfusion damage was significantly attenuated in the p50 KO, compared with WT mice. Although adhesion molecules such as intercellular adhesion molecule-1 (ICAM) were upregulated in left ventricles of p50 KO animals, fewer neutrophils infiltrated the infarct area, suggesting leukocytes as a potential mediator of protection observed in p50 KO. This was confirmed in adoptive transfer experiments: transplantation of KO bone marrow in KO animals sustained the protective effect on ischaemia–reperfusion injury, whereas transplantation of WT bone marrow in KO animals abolished it. Thus, impaired NF-κB activation in p50 KO leukocytes attenuated cardiac damage.
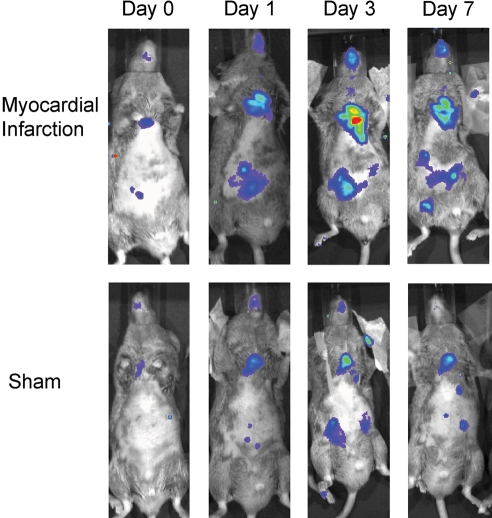
After permanent coronary artery ligation, activation of NF-κB peaks after 3 days (Figure 2).22,23 Mice with targeted deletion of the NF-κB subunit p50 are protected from left ventricular dilatation after MI and have preserved left ventricular function. Collagen content and matrix metalloproteinase (MMP)-9 expression are significantly lower in KO mice after MI and may account for improved left ventricular remodelling.24
Figure 2.
NF-κB activation in the heart after ischaemic injury. In transgenic mice that express a luciferase reporter whose transcription is dependent on NF-κB, light generated at the site of NF-κB activation within the transgenic mouse is sufficiently intense to be detected externally by a light-sensitive camera upon injection of luciferin. Myocardial infarction induced NF-κB-dependent in vivo luminescence in the heart of transgenic mice when compared with sham-operated mice. Maximal NF-κB activity was observed 3 days after myocardial infarction by serial molecular imaging (figure reprinted with permission of Tillmanns et al.22).
Thus, NF-κB is important for activation of inflammation and healing after MI. The effects on healing seem to be cell type-specific.
3.1.3. Complement
The complement system is central to the innate immune system. It can be activated via the classical, alternative, and lectin pathway. Complement has long been implicated in mediating tissue injury in ischaemic organs, with or without reperfusion.25–28 Activation of complement is an early event in ischaemia/reperfusion injury and healing, although the mechanism(s) leading to complement activation has only recently been identified. Carroll and co-workers28,29 have demonstrated that selective depletion of natural IgM is sufficient to abrogate most ischaemic–reperfusion injury in both murine hindlimb28 or murine intestinal29 reperfusion injury models. The self-target for this monoclonal natural IgM is non-muscle myosin heavy chain type II A and C.30 These data could also be reproduced for the heart.31 Mice baring an altered natural IgM repertoire (Cr2−/−) were protected from ischaemia/reperfusion injury and had a reduction in inflammatory infiltrates. This effect could be blunted by IgM reconstitution, suggesting that neoepitopes recognized by natural IgM appear on the surface of endothelium damaged by reperfusion injury. In conclusion, complement is activated and an important mediator of neutrophil and monocyte recruitment early after injury.32
3.1.4. Oxidative stress
ROS are atoms or molecules with unpaired electrons in their outer orbit. It can directly react with lipids, proteins, and DNA causing cell injury and death. It can trigger cytokine and chemokine release partially mediated by NF-κB. Oxidative stress produces myocardial contractile dysfunction and structural damage and has been implicated in the development of heart failure and left ventricular remodelling following MI.33–35 Indeed, cardiac tissue itself is a rich source of ROS, and NADPH oxidases, xanthine oxidases, and mitochondria are critical determinants of myocardial ROS generation.36,37
The normal heart possesses substantial ability to neutralize ROS; however, in the injured heart, the antioxidant defence is overwhelmed, resulting in the generation of oxygen-related free radicals. Markers of oxidative stress are elevated after MI.38 Various antioxidant approaches reduce adverse cardiac remodelling,39 for example, overexpression of glutathione peroxidase.40 Despite the damaging effects of oxidation itself, ROS can directly influence signalling, for example, increased ROS production promotes the development of interstitial fibrosis and extracellular matrix turnover, in part through activation of matrix metalloproteinases. Thus, ROS are able to generate an inflammatory response of the healing phase by necrosis and redox signalling.
In the clinical arena, the results of antioxidant therapy are discouraging. In the HOPE and Heart Protection study, cardiovascular events were not improved by vitamin E treatment.35 There are a number of reasons why vitamin E supplementation did not have the proposed effect: usage of the wrong antioxidant, necessity of other antioxidative cofactors, binding of vitamin E to the cell membrane while oxidative stress is intracellularly generated, and the problem that a single supplement may not be able to compensate for other coexisting abnormalities. Thus, the final role of antioxidant medication remains to be determined.
3.1.5. Coagulation cascade
In the first stage of wound healing, activation of the coagulation cascade is necessary to prevent ongoing blood and fluid loss. Haemostasis is achieved by the formation of a platelet plug, which becomes a scaffold for infiltrating cells. Several coagulation factors are able to activate an innate immune response, for example, thrombin and factor Xa promote cytokine and chemokines synthesis.41
With respect to healing and inflammation after MI, blood coagulation factor XIII (FXIII) has been most thoroughly investigated. It is activated by thrombin in the final stage of the clotting cascade. FXIII−/− mice invariably die after MI due to left ventricular rupture accompanied by reduced migration of neutrophils into the ischaemic zone.42 FXIII levels are decreased in patients with insufficient healing after MI.43 Gene variants (L34) increasing FXIII activity are associated with improved survival after MI.44 The most likely source of FXIII in healing infarcts are invading macrophages.45
3.2. Mediators of inflammation
Upon activation of the innate immune system by ischaemic injury, several inflammatory mediators are released and inflammatory cells are attracted to the site of injury. All these humoral and cellular factors have distinct function for the healing process.
3.3. Humoral immune response
3.3.1. Cytokines
A variety of cytokines are activated after MI and implicated in healing. We will exemplify their role by focusing on interleukin (IL)-1β and tumour necrosis factor (TNF) in this review.
Blood levels and/or myocardial expression levels of IL-1β are increased in patients with coronary artery disease,46 acute MI,47 dilated cardiomyopathy,48,49 and in patients and animal models of congestive heart failure.50 There are two peaks of IL-1β expression after MI in the rat: there is an initial rise in the healing phase, within 24 h, that appeared on immunohistochemical analysis to be located predominantly in the microvascular endothelium and a second peak at 7 days with predominant staining of infiltrating macrophages in the infarct zone.51
Despite these observations, there have been only a few reports about IL-1β function in ischaemic heart disease. Indeed, in some experimental models, early administration of inflammatory cytokines decreases myocardial injury. Brown et al.52,53 observed protective effects of TNF and IL-1β in a model of ischaemia/reperfusion injury. Pre-treatment with IL-1β in an ischemia/reperfusion model in the isolated rat heart increased the pressure developed in the left ventricle and decreased the area at risk.54 In contrast, long-term activation of cytokines seems to be detrimental: mice lacking the active forms of IL-1β and IL-18 (i.e. a caspase-1 knockout model) exhibited both improved peri-infarct survival and decreased ventricular dilatation after experimental MI, possibly due in part to a decrease in MMP-3 activity and reduction of apoptosis.55,56 With respect to the healing phase, in IL-1 receptor KO mice, myofibroblast infiltration and collagen deposition were decreased as was the development of adverse left ventricular remodelling after experimental MI.57 Thus, IL-1 signalling is essential for the activation of inflammatory pathways in the healing infarct.
The role of another innate immunity cytokine, TNF, has been extensively investigated after cardiac injury. In fact, TNF can mimic several symptoms of heart failure: mice overexpressing TNF develop heart failure.58,59 Systemic infusions of recombinant TNF that yielded blood concentrations of TNF seen in patients with advanced heart failure depressed left ventricular function and caused left ventricular dilatation.60 Moreover, the expression of TNF mRNA and protein is elevated in patients and in animal models with advanced heart failure due to a number of different aetiologies.50,61 Importantly, TNF has also a prognostic impact: TNF was elevated in a large portion of heart failure patients with preserved and reduced ejection fraction and was associated with a large decrease in survival.62 However, the function of TNF is much more complex than initially anticipated: double knockout mice for the TNF receptor 1 and 2 had larger infarct sizes and increased apoptosis after MI, indicating that TNF has also protective functions for the myocardium.63
3.3.2. Chemokines
Chemokines64 are small polypeptides synthesized by a number of cells of the immune system as well as by a number of non-immune cells including endothelial cells and keratinocytes. All chemokines are related in their amino acid sequences and function primarily as chemoattractants for phagocytic cells. Generally, CXC chemokines, such as RANTES, promote neutrophil migration, whereas CC chemokines, such as IL-8, mediate migration of monocytes and other cell types. A number of CXC chemokines, including IL-8 and others, appear to play a role in mediating angiogenesis. Induction of chemokines occurs in the post-infarction inflammatory response.65 IL-8 is a critical regulator of neutrophil influx in inflammatory processes. The inhibition of IL-8 in a rabbit ischaemia/reperfusion model reduced necrosis without altering inflammatory cell invasion.66 However, the role of other chemokines in cardiac wound healing has not been extensively investigated so far.
3.4. Cellular immune response
Neutrophils are important mediators of the inflammatory response. They release oxidants and proteases, secrete mediators for inflammatory cell recruitment, and phagocyte cell debris, and dead cells.
The recruitment of neutrophils to ischaemic tissue requires neutrophil–endothelial interactions that are regulated by a cascade of molecular steps. After activation, leukocytes roll along post-capillary venules, change shape, and extravasate in the tissue. The selectin family (E-, L-, P-selectin) of adhesion molecules mediates the initial capture of leukocytes. The importance of these proteins for healing is documented by the fact that E- and P-selectin knockout mice are protected from ischaemia/reperfusion injury and have reduced inflammatory cell infiltrates,67 as are feline hearts treated with an antibody against L-selectin.68
Selectin adhesion of leukocytes is not very tight. Firm adhesion and transmigration need the engagement of integrins. Integrins are a family of heterodimeric membrane glycoproteins. Consequently, integrin-related strategies have been used to mitigate ischaemia/reperfusion injury. Indeed, inhibition of integrin CD18 reduced infarct size.67
Crude depletion of neutrophils itself by leukocyte filters reduces infarct size in ischaemia–reperfusion models,69 indicating a detrimental role of neutrophils for infarct healing. The detrimental effects seem to be mediated by ICAM-1-dependent neutrophil–cardiomyocyte adhesion, a primary ligand of CD18 integrin. ICAM-1 KO mice have less myocardial injury early after MI.70 Neutrophils release various cytokines and growth factors important for healing. However, this effect is not essential for scar formation since depletion of neutrophils had no effect on granulation tissue formation in a cutaneous wound healing model.71 It is especially problematic that neutrophil effects vary depending on the stage of activation. For example, it seems that neutrophils release toxic products almost exclusively when adherent to the vascular wall, but not when they have evaded in the tissue. Thus, although multiple experiments suggest a central role of neutrophils in myocardial healing, the function of neutrophils remains unclear.
3.4.1. Macrophages
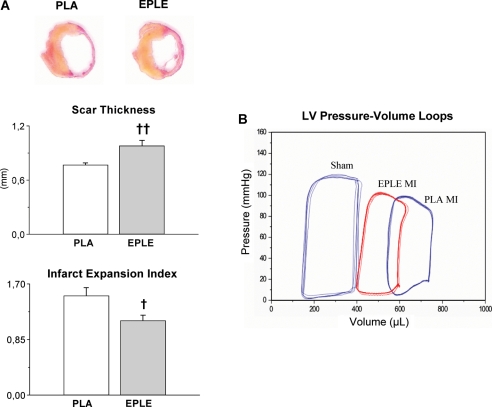
Healing of MI requires monocytes/macrophages. The mononuclear phagocytes degrade released macromolecules and scavenge dead cardiomyocytes. Infarcted hearts modulate their chemokine expression profile over time, and they sequentially and actively recruit Ly-6C(hi) and -6C(lo) monocytes. Ly-6C(hi) monocytes dominate early and exhibit phagocytic, proteolytic, and inflammatory functions. Ly-6C(lo) monocytes dominate later. Consequently, Ly-6C(hi) monocytes digest damaged tissue, whereas Ly-6C(lo) monocytes promote healing via myofibroblast accumulation, angiogenesis, and deposition of collagen.72 Depletion of macrophages in a murine cryoinjury model impaired wound healing since non-resorbed cell debris could not be discarded. This was accompanied by increased mortality. Macrophage accumulation in the healing heart is regulated by the renin–angiotensin–aldosterone system: selective mineralocorticoid receptor blockade immediately after MI improved healing (Figure 3), an effect that was blunted by macrophage depletion.45 Thus, macrophages are of central importance for adequate healing after MI.
Figure 3.
MR blockade after myocardial infarction reduced thinning and dilatation of the infarcted wall. (A) Typical sections from infarcted hearts, perfusion-fixed 7 days after myocardial infarction, scar thickness, and infarct expansion index in placebo (PLA) and eplerenone-treated rats (EPLE). Mean±SEM (n = 10 to 14). †P < 0.05, ‡P < 0.01 vs. PLA. (B) Representative LV pressure–volume loops measured in vivo with conductance catheter in sham-operated rats (sham) and in placebo- (PLA) and eplerenone-treated (EPLE) rats 7 days after MI (figure reprinted with permission of Fraccarollo et al.45).
4. Post-inflammation phase
Following the initial inflammatory phase, optimal healing requires mechanisms that inhibit cytokine release, clear the inflammatory infiltrates, and initiate collagen production to institute a solid scar.
Granulocytic infiltrates are cleared by phagocytes after granulocyte apoptosis.65 In contrast to necrosis that triggers an inflammatory response, apoptotic cells lead to the production of anti-inflammatory cytokines such as IL-10 and transforming growth factor (TGF), initiating the transition phase from inflammation to fibrosis.
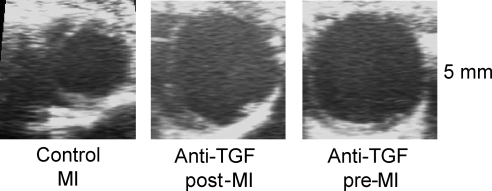
TGF-β is a locally generated cytokine and of central importance for this transition phase:73 TGF-β decreases leukocyte adhesion and stimulates fibroblast proliferation and extracellular matrix production.74 TGF-β expression is increased in the ischaemic as well as hypertrophied heart.75,76 TGF-β has an important function for healing after MI: We demonstrated recently that anti-TGF-β treatment in the first days after coronary artery ligation increases mortality and worsens left ventricular remodelling in mice with MI due to alterations in the extracellular matrix (Figure 4).77
Figure 4.
Detrimental left ventricular dilatation following myocardial infarction and inhibition of TGF. Illustrated are representative examples of two-dimensional echocardiography of mice treated with an anti-TGF-β antibody started either 1 week before (anti-TGF pre-MI) or 5 days after (anti-TGF post-MI) induction of myocardial infarction. Treatment with an anti-TGF-β antibody aggravated left ventricular dilatation (figure reprinted with permission of Frantz et al.77).
A detailed review of the post-inflammation phase is beyond the scope of the present article due to space constraints.
5. How far is the translation of experimental inflammation/healing data from the present clinical arena?
The importance of inflammation for healing processes after MI has been recognized for several years, and pathophysiological concepts have been established by experimental data. Consequently, transfer of this knowledge into standard clinical practice has been tried: for example, inflammatory markers have been used to predict mortality. Indeed, some inflammatory proteins (e.g. TNF) are associated with outcome; however, they could not be established as standard clinical utility since other parameters turned out to be more powerful. The translation of the experimental knowledge into new therapeutic modalities turned out to be a difficult task. Several treatments have been tried after acute MI.
Complement inhibitors have been evaluated as an adjunctive therapy to fibrinolysis or percutaneous coronary intervention (PCI) in acute MI. A humanized monoclonal antibody against complement component C5, pexelizumab, was used that specifically binds to C5 with high affinity and prevents cleavage and generation of activated C5a and C5b-9. However, although pexelizumab had been previously shown to reduce infarct size and apoptosis in rats after ischaemia and reperfusion,78 it had no effect on infarct size in patients treated with fibrinolysis (COMPLY trial79) or PCI (COMMA trial).80
In a multicentre, placebo-controlled trial, patients undergoing PCI for acute MI were treated with a blocking antibody for the CD11/CD18 integrin receptor. In 400 patients, infarct size and mortality were not improved by treatment.81
However, not all trials have been negative. For example, the data for the use of steroids after MI are not clear at the moment. Initial small and observational studies reported an increase in left ventricular rupture, i.e. impaired healing, and thus the concept was not thoroughly pursued. A recent meta-analysis (n = 2646) revealed decreased mortality in steroid-treated patients after MI, but summarized only data from small trials with no large, adequately powered study.82
Furthermore, established drugs in the treatment post-MI may play a role in inflammatory healing. For example, the early use of mineralocorticoid receptor antagonists reduces mortality after MI.83
The interpretation of clinical trials with regard to healing and inflammation is difficult due to confounding variables such as different infarct sizes, gender, age, and so on. For example, ageing is associated with decreased ability to control infection (so-called immunosenescence), alters the inflammatory response in wounds,84 and changes inflammatory markers in heart failure.85 Whereas infarct size is a major determinant of remodelling in patients younger than 65 years, in older patients, left ventricular remodelling can also occur even in the presence of small infarct sizes (PREAMI study86) potentially mediated by defects in healing.
Problematic for new anti-inflammatory approaches is that an intact immune system is necessary for many protective pathways and adequate healing in the beginning, whereas prolonged immune activation may also activate unfavourable signal cascades that drive disease progression. The major challenge of immunosuppressive drugs in the healing phase will be to limit detrimental innate immune influences while simultaneously maintaining and stimulating adequate and appropriate innate immune mechanisms. Nevertheless, there are several exciting targets such as the coagulation cascade, modulation of immune cell function, and the early use of RAS inhibitors to promote healing, improve the inflammatory response, and subsequently avoid adverse cardiac remodelling. Thus, further research is necessary to better understand the interaction of inflammation and cardiac healing. This will allow us to choose better targets at better time points for clinical intervention.
Conflict of interest: none declared.
Funding
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB 688, TP A10).
References
- 1.McKay RG, Pfeffer MA, Pasternak RC, Markis JE, Come PC, Nakao S, et al. Left ventricular remodeling after myocardial infarction: a corollary to infarct expansion. Circulation. 1986;74:693–702. doi: 10.1161/01.cir.74.4.693. [DOI] [PubMed] [Google Scholar]
- 2.Ertl G, Frantz S. Healing after myocardial infarction. Cardiovasc Res. 2005;66:22–32. doi: 10.1016/j.cardiores.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Frantz S, Bauersachs J, Kelly RA. Innate immunity and the heart. Curr Pharm Des. 2005;11:1279–1290. doi: 10.2174/1381612053507512. [DOI] [PubMed] [Google Scholar]
- 4.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 7.Frantz S, Ertl G, Bauersachs J. Mechanisms of disease: toll-like receptors in cardiovascular disease. Nat Clin Pract. 2007;4:444–454. doi: 10.1038/ncpcardio0938. [DOI] [PubMed] [Google Scholar]
- 8.Frantz S, Ertl G, Bauersachs J. Toll-like receptor signaling in the ischemic heart. Front Biosci. 2008;13:5772–5779. doi: 10.2741/3114. [DOI] [PubMed] [Google Scholar]
- 9.Frantz S, Kelly RA, Bourcier T. Role of TLR-2 in the activation of nuclear factor-kappa B by oxidative stress in cardiac myocytes. J Biol Chem. 2001;276:5197–5203. doi: 10.1074/jbc.M009160200. [DOI] [PubMed] [Google Scholar]
- 10.Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT, et al. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest. 1999;104:271–280. doi: 10.1172/JCI6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birks EJ, Felkin LE, Banner NR, Khaghani A, Barton PJ, Yacoub MH. Increased toll-like receptor 4 in the myocardium of patients requiring left ventricular assist devices. J Heart Lung Transplant. 2004;23:228–235. doi: 10.1016/S1053-2498(03)00106-2. [DOI] [PubMed] [Google Scholar]
- 12.Shishido T, Nozaki N, Yamaguchi S, Shibata Y, Nitobe J, Miyamoto T, et al. Toll-like receptor-2 modulates ventricular remodeling after myocardial infarction. Circulation. 2003;108:2905–2910. doi: 10.1161/01.CIR.0000101921.93016.1C. [DOI] [PubMed] [Google Scholar]
- 13.Riad A, Jager S, Sobirey M, Escher F, Yaulema-Riss A, Westermann D, et al. Toll-like receptor-4 modulates survival by induction of left ventricular remodeling after myocardial infarction in mice. J Immunol. 2008;180:6954–6961. doi: 10.4049/jimmunol.180.10.6954. [DOI] [PubMed] [Google Scholar]
- 14.Oyama J, Blais C, Jr, Liu X, Pu M, Kobzik L, Kelly RA, et al. Reduced myocardial ischemia–reperfusion injury in toll-like receptor 4-deficient mice. Circulation. 2004;109:784–789. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- 15.Hall G, Hasday JD, Rogers TB. Regulating the regulator: NF-kappaB signaling in heart. J Mol Cell Cardiol. 2006;41:580–591. doi: 10.1016/j.yjmcc.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Morgan EN, Boyle EM, Jr, Yun W, Griscavage-Ennis JM, Farr AL, Canty TG, Jr, et al. An essential role for NF-kappaB in the cardioadaptive response to ischemia. Ann Thorac Surg. 1999;68:377–382. doi: 10.1016/s0003-4975(99)00646-3. [DOI] [PubMed] [Google Scholar]
- 17.Chandrasekar B, Freeman GL. Induction of nuclear factor kappaB and activation protein 1 in postischemic myocardium. FEBS Lett. 1997;401:30–34. doi: 10.1016/s0014-5793(96)01426-3. [DOI] [PubMed] [Google Scholar]
- 18.Morishita R, Sugimoto T, Aoki M, Kida I, Tomita N, Moriguchi A, et al. In vivo transfection of cis element ‘decoy’ against nuclear factor-kappaB binding site prevents myocardial infarction. Nat Med. 1997;3:894–899. doi: 10.1038/nm0897-894. [DOI] [PubMed] [Google Scholar]
- 19.Brown M, McGuinness M, Wright T, Ren X, Wang Y, Boivin GP, et al. Cardiac-specific blockade of NF-kappaB in cardiac pathophysiology: differences between acute and chronic stimuli in vivo. Am J Physiol Heart Circ Physiol. 2005;289:H466–H476. doi: 10.1152/ajpheart.00170.2004. [DOI] [PubMed] [Google Scholar]
- 20.Onai Y, Suzuki J, Kakuta T, Maejima Y, Haraguchi G, Fukasawa H, et al. Inhibition of IkappaB phosphorylation in cardiomyocytes attenuates myocardial ischemia/reperfusion injury. Cardiovasc Res. 2004;63:51–59. doi: 10.1016/j.cardiores.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Frantz S, Tillmanns J, Kuhlencordt PJ, Schmidt I, Adamek A, Dienesch C, et al. Tissue-specific effects of the nuclear factor {kappa}B subunit p50 on myocardial ischemia–reperfusion injury. Am J Pathol. 2007;171:507–512. doi: 10.2353/ajpath.2007.061042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tillmanns J, Carlsen H, Blomhoff R, Valen G, Calvillo L, Ertl G, et al. Caught in the act: in vivo molecular imaging of the transcription factor NF-kappaB after myocardial infarction. Biochem Biophys Res Commun. 2006;342:773–774. doi: 10.1016/j.bbrc.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 23.Frantz S, Fraccarollo D, Wagner H, Behr TM, Jung P, Angermann CE, et al. Sustained activation of nuclear factor kappa B and activator protein 1 in chronic heart failure. Cardiovasc Res. 2003;57:749–756. doi: 10.1016/s0008-6363(02)00723-x. [DOI] [PubMed] [Google Scholar]
- 24.Frantz S, Hu K, Bayer B, Gerondakis S, Strotmann J, Adamek A, et al. Absence of NF-kappaB subunit p50 improves heart failure after myocardial infarction. FASEB J. 2006;20:1918–1920. doi: 10.1096/fj.05-5133fje. [DOI] [PubMed] [Google Scholar]
- 25.Weisman HF, Bartow T, Leppo MK, Marsh HC, Jr, Carson GR, Concino MF, et al. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990;249:146–151. doi: 10.1126/science.2371562. [DOI] [PubMed] [Google Scholar]
- 26.Carroll MC, Prodeus AP. Linkages of innate and adaptive immunity. Curr Opin Immunol. 1998;10:36–40. doi: 10.1016/s0952-7915(98)80028-9. [DOI] [PubMed] [Google Scholar]
- 27.Tanhehco EJ, Yasojima K, McGeer PL, Washington RA, Kilgore KS, Homeister JW, et al. Preconditioning reduces tissue complement gene expression in the rabbit isolated heart. Am J Physiol. 1999;277:H2373–H2380. doi: 10.1152/ajpheart.1999.277.6.H2373. [DOI] [PubMed] [Google Scholar]
- 28.Weiser MR, Williams JP, Moore FD, Jr, Kobzik L, Ma M, Hechtman HB, et al. Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J Exp Med. 1996;183:2343–2348. doi: 10.1084/jem.183.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams JP, Pechet TT, Weiser MR, Reid R, Kobzik L, Moore FD, Jr, et al. Intestinal reperfusion injury is mediated by IgM and complement. J Appl Physiol. 1999;86:938–942. doi: 10.1152/jappl.1999.86.3.938. [DOI] [PubMed] [Google Scholar]
- 30.Zhang M, Alicot EM, Chiu I, Li J, Verna N, Vorup-Jensen T, et al. Identification of the target self-antigens in reperfusion injury. J Exp Med. 2006;203:141–152. doi: 10.1084/jem.20050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang M, Michael LH, Grosjean SA, Kelly RA, Carroll MC, Entman ML. The role of natural IgM in myocardial ischemia–reperfusion injury. J Mol Cell Cardiol. 2006;41:62–67. doi: 10.1016/j.yjmcc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Dreyer WJ, Michael LH, Nguyen T, Smith CW, Anderson DC, Entman ML, et al. Kinetics of C5a release in cardiac lymph of dogs experiencing coronary artery ischemia–reperfusion injury. Circ Res. 1992;71:1518–1524. doi: 10.1161/01.res.71.6.1518. [DOI] [PubMed] [Google Scholar]
- 33.Josephson RA, Silverman HS, Lakatta EG, Stern MD, Zweier JL. Study of the mechanisms of hydrogen peroxide and hydroxyl free radical-induced cellular injury and calcium overload in cardiac myocytes. J Biol Chem. 1991;266:2354–2361. [PubMed] [Google Scholar]
- 34.Grieve DJ, Shah AM. Oxidative stress in heart failure. More than just damage. Eur Heart J. 2003;24:2161–2163. doi: 10.1016/j.ehj.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 35.Byrne JA, Grieve DJ, Cave AC, Shah AM. Oxidative stress and heart failure. Arch Mal Coeur Vaiss. 2003;96:214–221. [PubMed] [Google Scholar]
- 36.Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, et al. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol. 2003;41:2164–2171. doi: 10.1016/s0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- 37.Frantz S, Brandes RP, Hu K, Rammelt K, Wolf J, Scheuermann H, et al. Left ventricular remodeling after myocardial infarction in mice with targeted deletion of the NADPH oxidase subunit gp91(PHOX) Basic Res Cardiol. 2006;101:127–132. doi: 10.1007/s00395-005-0568-x. [DOI] [PubMed] [Google Scholar]
- 38.Hill MF, Singal PK. Antioxidant and oxidative stress changes during heart failure subsequent to myocardial infarction in rats. Am J Pathol. 1996;148:291–300. [PMC free article] [PubMed] [Google Scholar]
- 39.Murdoch CE, Zhang M, Cave AC, Shah AM. NADPH oxidase-dependent redox signalling in cardiac hypertrophy, remodelling and failure. Cardiovasc Res. 2006;71:208–215. doi: 10.1016/j.cardiores.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 40.Shiomi T, Tsutsui H, Matsusaka H, Murakami K, Hayashidani S, Ikeuchi M, et al. Overexpression of glutathione peroxidase prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation. 2004;109:544–549. doi: 10.1161/01.CIR.0000109701.77059.E9. [DOI] [PubMed] [Google Scholar]
- 41.Opal SM, Esmon CT. Bench-to-bedside review: functional relationships between coagulation and the innate immune response and their respective roles in the pathogenesis of sepsis. Crit Care. 2003;7:23–38. doi: 10.1186/cc1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nahrendorf M, Hu K, Frantz S, Jaffer FA, Tung CH, Hiller KH, et al. Factor XIII deficiency causes cardiac rupture, impairs wound healing, and aggravates cardiac remodeling in mice with myocardial infarction. Circulation. 2006;113:1196–1202. doi: 10.1161/CIRCULATIONAHA.105.602094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nahrendorf M, Aikawa E, Figueiredo JL, Stangenberg L, van den Borne SW, Blankesteijn WM, et al. Transglutaminase activity in acute infarcts predicts healing outcome and left ventricular remodelling: implications for FXIII therapy and antithrombin use in myocardial infarction. Eur Heart J. 2008;29:445–454. doi: 10.1093/eurheartj/ehm558. [DOI] [PubMed] [Google Scholar]
- 44.Gemmati D, Federici F, Campo G, Tognazzo S, Serino ML, De Mattei M, et al. Factor XIIIA-V34L and factor XIIIB-H95R gene variants: effects on survival in myocardial infarction patients. Mol Med. 2007;13:112–120. doi: 10.2119/2006-00049.Gemmati. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fraccarollo D, Galuppo P, Schraut S, Kneitz S, van Rooijen N, Ertl G, et al. Immediate mineralocorticoid receptor blockade improves myocardial infarct healing by modulation of the inflammatory response. Hypertension. 2008;51:905–914. doi: 10.1161/HYPERTENSIONAHA.107.100941. [DOI] [PubMed] [Google Scholar]
- 46.Hasdai D, Scheinowitz M, Leibovitz E, Sclarovsky S, Eldar M, Barak V. Increased serum concentrations of interleukin-1 beta in patients with coronary artery disease. Heart. 1996;76:24–28. doi: 10.1136/hrt.76.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guillen I, Blanes M, Gomez-Lechon MJ, Castell JV. Cytokine signaling during myocardial infarction: sequential appearance of IL-1 beta and IL-6. Am J Physiol. 1995;269:R229–R235. doi: 10.1152/ajpregu.1995.269.2.R229. [DOI] [PubMed] [Google Scholar]
- 48.Han R, Ray P, Baughman K, Feldman A. Detection of interleukin 1 and interleukin-1-receptor mRNA in human heart by polymerase chain reaction. Biochem Biophys Res Commun. 1991;181:520–523. doi: 10.1016/0006-291x(91)91219-3. [DOI] [PubMed] [Google Scholar]
- 49.Francis SE, Holden H, Holt CM, Duff GW. Interleukin-1 in myocardium and coronary arteries of patients with dilated cardiomyopathy. J Mol Cell Cardiol. 1998;30:215–223. doi: 10.1006/jmcc.1997.0592. [DOI] [PubMed] [Google Scholar]
- 50.Testa M, Yeh M, Lee P, Fanelli R, Loperfido F, Berman JW, et al. Circulating levels of cytokines and their endogenous modulators in patients with mild to severe congestive heart failure due to coronary artery disease or hypertension. J Am Coll Cardiol. 1996;28:964–971. doi: 10.1016/s0735-1097(96)00268-9. [DOI] [PubMed] [Google Scholar]
- 51.Herskowitz A, Choi S, Ansari AA, Wesselingh S. Cytokine mRNA expression in postischemic/reperfused myocardium. Am J Pathol. 1995;146:419–428. [PMC free article] [PubMed] [Google Scholar]
- 52.Brown JM, Anderson BO, Repine JE, Shanley PF, White CW, Grosso MA, et al. Neutrophils contribute to TNF induced myocardial tolerance to ischaemia. J Mol Cell Cardiol. 1992;24:485–495. doi: 10.1016/0022-2828(92)91838-v. [DOI] [PubMed] [Google Scholar]
- 53.Brown JM, White CW, Terada LS, Grosso MA, Shanley PF, Mulvin DW, et al. Interleukin 1 pretreatment decreases ischemia/reperfusion injury. Proc Natl Acad Sci USA. 1990;87:5026–5030. doi: 10.1073/pnas.87.13.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nogae C, Makino N, Hata T, Nogae I, Takahashi S, Suzuki K, et al. Interleukin 1 alpha-induced expression of manganous superoxide dismutase reduces myocardial reperfusion injury in the rat. J Mol Cell Cardiol. 1995;27:2091–2099. doi: 10.1016/s0022-2828(95)91155-3. [DOI] [PubMed] [Google Scholar]
- 55.Frantz S, Ducharme A, Sawyer D, Rohde LE, Kobzik L, Fukazawa R, et al. Targeted deletion of caspase-1 reduces early mortality and left ventricular dilatation following myocardial infarction. J Mol Cell Cardiol. 2003;35:685–694. doi: 10.1016/s0022-2828(03)00113-5. [DOI] [PubMed] [Google Scholar]
- 56.Merkle S, Frantz S, Schon MP, Bauersachs J, Buitrago M, Frost RJ, et al. A role for caspase-1 in heart failure. Circ Res. 2007;100:645–653. doi: 10.1161/01.RES.0000260203.55077.61. [DOI] [PubMed] [Google Scholar]
- 57.Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, et al. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;173:57–67. doi: 10.2353/ajpath.2008.070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, et al. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res. 1997;81:627–635. doi: 10.1161/01.res.81.4.627. [DOI] [PubMed] [Google Scholar]
- 59.Bryant D, Becker L, Richardson J, Shelton J, Franco F, Peshock R, et al. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation. 1998;97:1375–1381. doi: 10.1161/01.cir.97.14.1375. [DOI] [PubMed] [Google Scholar]
- 60.Bozkurt B, Kribbs SB, Clubb FJ, Jr, Michael LH, Didenko VV, Hornsby PJ, et al. Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation. 1998;97:1382–1391. doi: 10.1161/01.cir.97.14.1382. [DOI] [PubMed] [Google Scholar]
- 61.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 1996;27:1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 62.Dunlay SM, Weston SA, Redfield MM, Killian JM, Roger VL. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625–631. doi: 10.1161/CIRCULATIONAHA.107.759191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kurrelmeyer KM, Michael LH, Baumgarten G, Taffet GE, Peschon JJ, Sivasubramanian N, et al. Endogenous tumor necrosis factor protects the adult cardiac myocyte against ischemic-induced apoptosis in a murine model of acute myocardial infarction. Proc Natl Acad Sci USA. 2000;97:5456–5461. doi: 10.1073/pnas.070036297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001;69:513–521. [PubMed] [Google Scholar]
- 65.Frangogiannis NG. The mechanistic basis of infarct healing. Antioxid Redox Signal. 2006;8:1907–1939. doi: 10.1089/ars.2006.8.1907. [DOI] [PubMed] [Google Scholar]
- 66.Boyle EM, Jr, Kovacich JC, Hebert CA, Canty TG, Jr, Chi E, Morgan EN, et al. Inhibition of interleukin-8 blocks myocardial ischemia–reperfusion injury. J Thorac Cardiovasc Surg. 1998;116:114–121. doi: 10.1016/S0022-5223(98)70249-1. [DOI] [PubMed] [Google Scholar]
- 67.Jones SP, Trocha SD, Strange MB, Granger DN, Kevil CG, Bullard DC, et al. Leukocyte and endothelial cell adhesion molecules in a chronic murine model of myocardial reperfusion injury. Am J Physiol. 2000;279:H2196–H2201. doi: 10.1152/ajpheart.2000.279.5.H2196. [DOI] [PubMed] [Google Scholar]
- 68.Ma XL, Weyrich AS, Lefer DJ, Buerke M, Albertine KH, Kishimoto TK, et al. Monoclonal antibody to L-selectin attenuates neutrophil accumulation and protects ischemic reperfused cat myocardium. Circulation. 1993;88:649–658. doi: 10.1161/01.cir.88.2.649. [DOI] [PubMed] [Google Scholar]
- 69.Litt MR, Jeremy RW, Weisman HF, Winkelstein JA, Becker LC. Neutrophil depletion limited to reperfusion reduces myocardial infarct size after 90 min of ischemia. Evidence for neutrophil-mediated reperfusion injury. Circulation. 1989;80:1816–1827. doi: 10.1161/01.cir.80.6.1816. [DOI] [PubMed] [Google Scholar]
- 70.Metzler B, Mair J, Lercher A, Schaber C, Hintringer F, Pachinger O, et al. Mouse model of myocardial remodelling after ischemia: role of intercellular adhesion molecule-1. Cardiovasc Res. 2001;49:399–407. doi: 10.1016/s0008-6363(00)00261-3. [DOI] [PubMed] [Google Scholar]
- 71.Simpson DM, Ross R. The neutrophilic leukocyte in wound repair: a study with antineutrophil serum. J Clin Invest. 1972;51:2009–2023. doi: 10.1172/JCI107007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 74.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004;63:423–432. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 75.Deten A, Holzl A, Leicht M, Barth W, Zimmer HG. Changes in extracellular matrix and in transforming growth factor beta isoforms after coronary artery ligation in rats. J Mol Cell Cardiol. 2001;33:1191–1207. doi: 10.1006/jmcc.2001.1383. [DOI] [PubMed] [Google Scholar]
- 76.Kuwahara F, Kai H, Tokuda K, Kai M, Takeshita A, Egashira K, et al. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation. 2002;106:130–135. doi: 10.1161/01.cir.0000020689.12472.e0. [DOI] [PubMed] [Google Scholar]
- 77.Frantz S, Hu K, Adamek A, Wolf J, Sallam A, Maier SK, et al. Transforming growth factor beta inhibition increases mortality and left ventricular dilatation after myocardial infarction. Basic Res Cardiol. 2008;103:485–492. doi: 10.1007/s00395-008-0739-7. [DOI] [PubMed] [Google Scholar]
- 78.Vakeva AP, Agah A, Rollins SA, Matis LA, Li L, Stahl GL. Myocardial infarction and apoptosis after myocardial ischemia and reperfusion: role of the terminal complement components and inhibition by anti-C5 therapy. Circulation. 1998;97:2259–2267. doi: 10.1161/01.cir.97.22.2259. [DOI] [PubMed] [Google Scholar]
- 79.Mahaffey KW, Granger CB, Nicolau JC, Ruzyllo W, Weaver WD, Theroux P, et al. Effect of pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to fibrinolysis in acute myocardial infarction: the COMPlement inhibition in myocardial infarction treated with thromboLYtics (COMPLY) trial. Circulation. 2003;108:1176–1183. doi: 10.1161/01.CIR.0000087404.53661.F8. [DOI] [PubMed] [Google Scholar]
- 80.Granger CB, Mahaffey KW, Weaver WD, Theroux P, Hochman JS, Filloon TG, et al. Pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to primary percutaneous coronary intervention in acute myocardial infarction: the COMplement inhibition in Myocardial infarction treated with Angioplasty (COMMA) trial. Circulation. 2003;108:1184–1190. doi: 10.1161/01.CIR.0000087447.12918.85. [DOI] [PubMed] [Google Scholar]
- 81.Faxon DP, Gibbons RJ, Chronos NA, Gurbel PA, Sheehan F. The effect of blockade of the CD11/CD18 integrin receptor on infarct size in patients with acute myocardial infarction treated with direct angioplasty: the results of the HALT-MI study. J Am Coll Cardiol. 2002;40:1199–1204. doi: 10.1016/s0735-1097(02)02136-8. [DOI] [PubMed] [Google Scholar]
- 82.Giugliano GR, Giugliano RP, Gibson CM, Kuntz RE. Meta-analysis of corticosteroid treatment in acute myocardial infarction. Am J Cardiol. 2003;91:1055–1059. doi: 10.1016/s0002-9149(03)00148-6. [DOI] [PubMed] [Google Scholar]
- 83.Greenberg B, Zannad F, Pitt B. Role of aldosterone blockade for treatment of heart failure and post-acute myocardial infarction. Am J Cardiol. 2006;97:34F–40F. doi: 10.1016/j.amjcard.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 84.Ashcroft GS, Horan MA, Ferguson MW. Aging is associated with reduced deposition of specific extracellular matrix components, an upregulation of angiogenesis, and an altered inflammatory response in a murine incisional wound healing model. J Invest Dermatol. 1997;108:430–437. doi: 10.1111/1523-1747.ep12289705. [DOI] [PubMed] [Google Scholar]
- 85.von Haehling S, Genth-Zotz S, Sharma R, Bolger AP, Doehner W, Barnes PJ, et al. The relationship between age and production of tumour necrosis factor-alpha in healthy volunteers and patients with chronic heart failure. Int J Cardiol. 2003;90:197–204. doi: 10.1016/s0167-5273(02)00566-1. [DOI] [PubMed] [Google Scholar]
- 86.Ferrari R. Effects of angiotensin-converting enzyme inhibition with perindopril on left ventricular remodeling and clinical outcome: results of the randomized Perindopril and Remodeling in Elderly with Acute Myocardial Infarction (PREAMI) study. Arch Intern Med. 2006;166:659–666. doi: 10.1001/archinte.166.6.659. [DOI] [PubMed] [Google Scholar]