Abstract
Swine dysentery is a mucohemorrhagic diarrheal disease caused by the anaerobic spirochete Serpulina hyodysenteriae. At present, the serotyping is done by immunodiffusion testing with lipopolysaccharide (LPS) extract as antigen and rabbit hyperimmune sera produced against different serotypes of S. hyodysenteriae. Since the preparation of LPS is time-consuming and requires a large quantity of bacteria, it is desirable to use a serotyping method which does not require the extraction of LPS. In the present investigation, microagglutination was evaluated by using both formalinized whole- and boiled-cell suspensions as antigens and rabbit hyperimmune sera produced against formalinized whole-cell suspensions of reference strains of S. hyodysenteriae and S. innocens B256. Use of boiled cell suspension as antigen permitted the differentiation between isolates of S. hyodysenteriae and S. innocens as well as serotyping of S. hyodysenteriae strains accurately. A total of 18 isolates were identified as S. hyodysenteriae, and 3 isolates were identified as S. innocens. The microagglutination test was found specific, sensitive, and easy to perform; thus, it was judged suitable for routine identification and serotyping of S. hyodysenteriae isolates.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achacha M., Messier S. Identification of Treponema hyodysenteriae and Treponema innocens using two four-hour identification systems. J Vet Diagn Invest. 1991 Jul;3(3):211–214. doi: 10.1177/104063879100300304. [DOI] [PubMed] [Google Scholar]
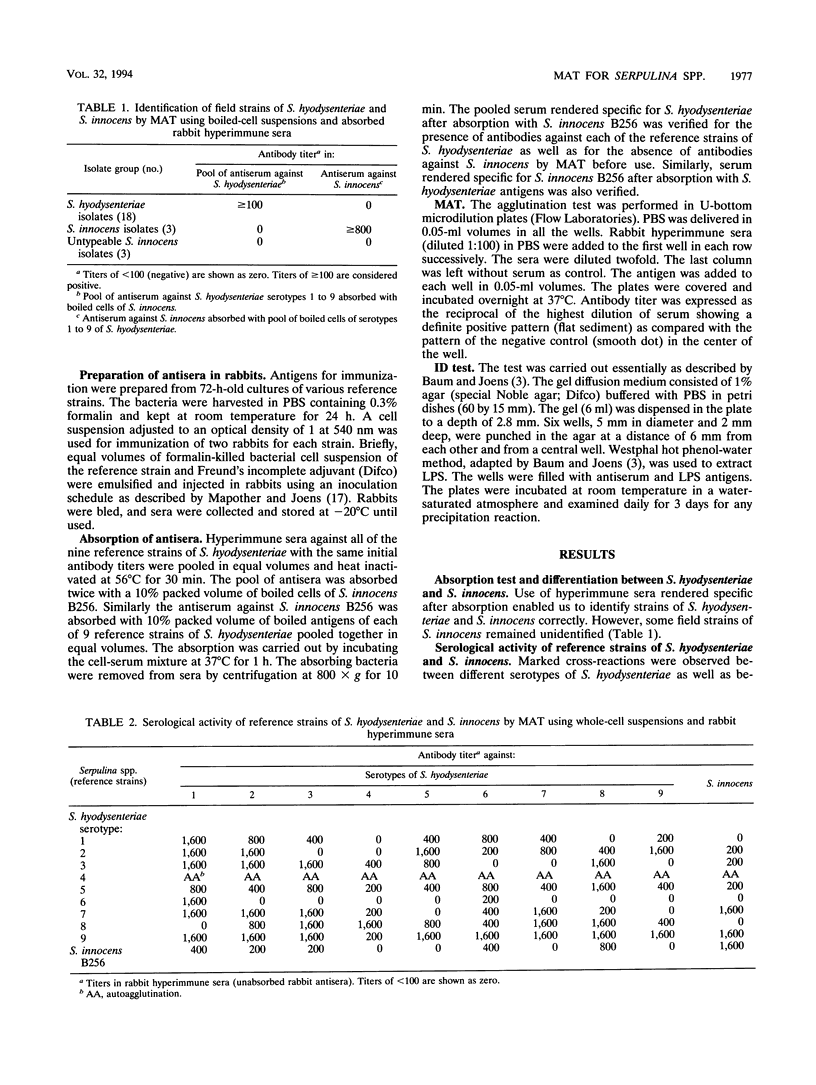
- Baum D. H., Joens L. A. Serotypes of beta-hemolytic Treponema hyodysenteriae. Infect Immun. 1979 Sep;25(3):792–796. doi: 10.1128/iai.25.3.792-796.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glock R. D., Harris D. L. Swine dysentery. II. Characterization of lesions in pigs inoculated with Treponema hyodysenteriae in pure and mixed culture. Vet Med Small Anim Clin. 1972 Jan;67(1):65–68. [PubMed] [Google Scholar]
- Hampson D. J., Mhoma J. R., Combs B. G., Lee J. I. Serological grouping of Treponema hyodysenteriae. Epidemiol Infect. 1990 Aug;105(1):79–85. doi: 10.1017/s0950268800047671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson D. J., Mhoma J. R., Combs B., Buddle J. R. Proposed revisions to the serological typing system for Treponema hyodysenteriae. Epidemiol Infect. 1989 Feb;102(1):75–84. doi: 10.1017/s0950268800029708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson D. J. Slide-agglutination for rapid serological typing of Treponema hyodysenteriae. Epidemiol Infect. 1991 Jun;106(3):541–547. doi: 10.1017/s0950268800067601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson M. J., Alexander T. J., Lysons R. J. Diagnosis of swine dysentery: spirochaetes which may be confused with Treponema hyodysenteriae. Vet Rec. 1976 Dec 18;99(25-26):498–500. doi: 10.1136/vr.99.25-26.498. [DOI] [PubMed] [Google Scholar]
- Hunter D., Saunders C. N. Diagnosis of swine dysentery using an absorbed fluorescent antiserum. Vet Rec. 1977 Oct 8;101(15):303–304. doi: 10.1136/vr.101.15.303. [DOI] [PubMed] [Google Scholar]
- Joens L. A., Nord N. A., Kinyon J. M., Egan I. T. Enzyme-linked immunosorbent assay for detection of antibody to Treponema hyodysenteriae antigens. J Clin Microbiol. 1982 Feb;15(2):249–252. doi: 10.1128/jcm.15.2.249-252.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
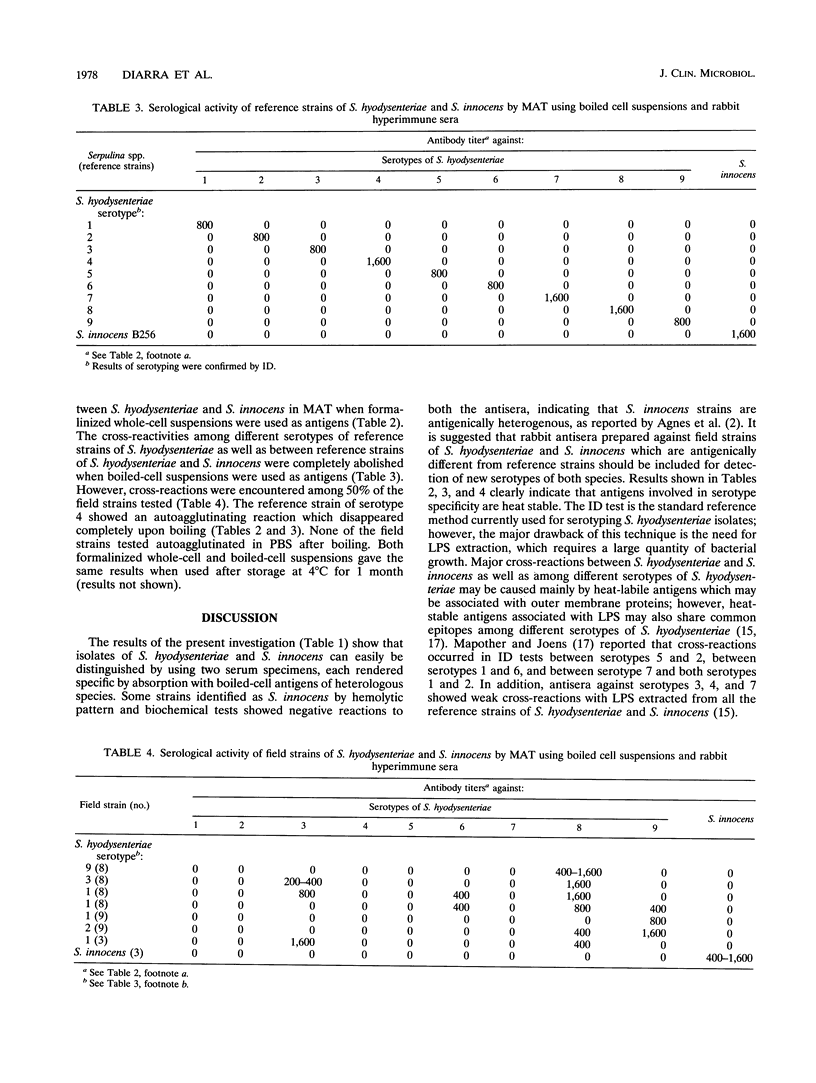
- Lau T. T., Hampson D. J. The serological grouping system for Serpulina (Treponema) hyodysenteriae. Epidemiol Infect. 1992 Oct;109(2):255–263. doi: 10.1017/s0950268800050202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. S., Bélanger M., Jacques M. Serotyping of Canadian isolates of Treponema hyodysenteriae and description of two new serotypes. J Clin Microbiol. 1991 Dec;29(12):2794–2797. doi: 10.1128/jcm.29.12.2794-2797.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysons R. J., Lemcke R. M. Swine dysentery: to isolate or to fluoresce? Vet Rec. 1983 Feb 26;112(9):203–203. doi: 10.1136/vr.112.9.203-a. [DOI] [PubMed] [Google Scholar]
- Mapother M. E., Joens L. A. New serotypes of Treponema hyodysenteriae. J Clin Microbiol. 1985 Aug;22(2):161–164. doi: 10.1128/jcm.22.2.161-164.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson L. D., Fales W. H. Comparison of stained smears and culturing for identification of Treponema hyodysenteriae. J Clin Microbiol. 1983 Oct;18(4):950–955. doi: 10.1128/jcm.18.4.950-955.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Huurne A. A., van Houten M., Koopman M. B., van der Zeijst B. A., Gaastra W. Characterization of Dutch porcine Serpulina (Treponema) isolates by restriction endonuclease analysis and DNA hybridization. J Gen Microbiol. 1992 Sep;138(9):1929–1934. doi: 10.1099/00221287-138-9-1929. [DOI] [PubMed] [Google Scholar]


