Abstract
Objectives
We sought to improve upon the usefulness of artemisinins as anti-Toxoplasma agents by synthesizing new unsaturated, carba derivatives and then testing them for in vitro efficacy against three steps of the lytic cycle of Toxoplasma gondii tachyzoites.
Methods
Novel derivatives of ART were synthesized and then tested for in vitro antiparasitic activity using T. gondii tachyzoites constitutively expressing β-galactosidase and human fibroblast host cells. Compounds were evaluated for parasite growth inhibition and cytotoxicity, inhibition of replication and inhibition of parasite invasion of host cells.
Results
Five of the seven new derivatives, 3a–c, 3e and 3f, effectively inhibited T. gondii growth (IC50 = 1.0–4.4 µM); however, only three of these proved to be relatively non-cytotoxic (TD50 ≥ 200 µM). The same five derivatives also inhibited tachyzoite replication, and attachment to and invasion of host cells as effectively as or better than the parent compound ART. In addition, one of the derivatives incapable of inhibiting growth, deoxy-3a, was found to inhibit parasite invasion.
Conclusions
These new artemisinin derivatives have the ability to inhibit multiple steps of T. gondii's lytic cycle. Synthetic unsaturated, carba derivatives of ART have potential as therapeutic agents for the prevention and treatment of toxoplasmosis in humans.
Keywords: parasite, toxoplasmosis, treatment, in vitro inhibition, antiparasitic drugs
Introduction
Toxoplasma gondii is a ubiquitous intracellular protozoan parasite known to infect approximately one-third of the world’s human population.1 Infections are often asymptomatic in immunocompetent hosts, but severe toxoplasmosis can result from infection of immunocompromised hosts and foetuses.1,2
Approved medications for the prevention and treatment of toxoplasmosis are limited in efficacy and can have serious side effects.1,3 Artemisinin (ART) and several of its derivatives display in vitro efficacy against T. gondii.4,5 However, ART and some C-10 saturated acetal (oxo-linked) analogues have shown evidence of neurotoxicity in animal models, presumably due to rapid metabolism by P450 in the liver to the neurotoxic dihydroartemisinin (DHA). The C-10-carba-linked derivatives of ART are designed to be much less susceptible to P450 oxidation thereby minimizing possible neurotoxicity due to DHA.6
A principal goal of our research is to design and synthesize ART derivatives that are highly efficacious, well-tolerated by the patient and resistant to liver P450 metabolism to DHA. We have reported on the efficacy of novel, C-10 saturated, non-acetal ART derivatives against the in vitro growth of T. gondii.5 Now we report on novel C-10 unsaturated, carba-linked derivatives and their effects on several steps in the in vitro lytic cycle of Toxoplasma.
Materials and methods
Materials and reagents
ART was obtained from Bright Chemicals (Shanghai, China) and Holley Pharmaceuticals (CA, USA). Artemether was a gift from XiaoLi Hu (Kunming, China). New ART derivatives (Figure 1a) were synthesized by conventional methods as described previously.5 All reagents and control compounds for the growth inhibition assay were obtained as previously outlined.7 The T. gondii strain 2F tachyzoites (ATCC 50839), derived from strain RH, constitutively express cytoplasmic β-galactosidase (β-gal) and are routinely grown in our laboratory in human fibroblasts (HFF; ATCC CRL-2088).
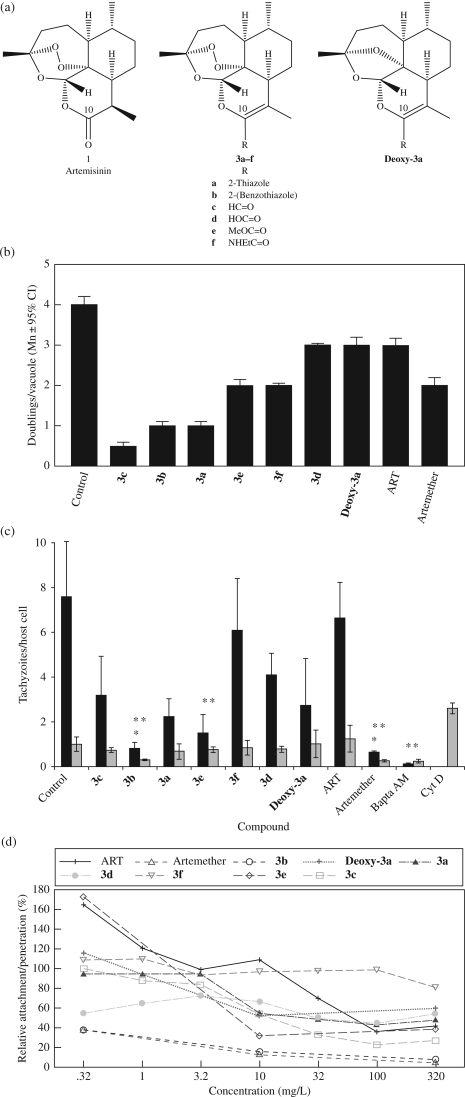
Figure 1.
ART derivatives can inhibit replication of intracellular parasites as well as invasion. (a) Structures of ART and novel derivatives. Substitutions at R are as shown. (b) Replication assay (performed as described in the Materials and methods section). Compounds were tested at 10 mg/L (range 25–34 µM). All data are compiled from three independent experiments. Mn = mean. (c) Quantification of invasion inhibition using red/green assay (here shown as grey/black) performed as described in the Materials and methods section. Black (green) bars represent penetrated/intracellular parasites. Grey (red) bars depict attached/extracellular parasites. Compounds were tested at 10 mg/L (range 25–34 µM). BAPTA-AM (20 µM) and cytochalasin D (Cyt D, 2 µM) were included as positive controls for inhibition of attachment or penetration, respectively. Data are mean values ± SEM of three independent experiments, counting 10 random fields for each sample. A single asterisk indicates that tachyzoite penetration was significantly lower (P ≤ 0.05, two-tailed Student’s t-test) than the control. A double asterisk indicates a significant effect (P ≤ 0.05, one-tailed Student’s t-test) on parasite attachment relative to the control. (d) Dose–response effect of ART derivative treatment on T. gondii invasion. Cell-associated parasites (penetrated and attached) per host nuclei were counted and normalized as a percentage of untreated control parasites. Colour versions of Figure 1(c and d) are available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/).
Five day growth inhibition assay
Compounds were tested for in vitro Toxoplasma growth inhibition using published methods.7 Test and control drugs were added to HFF cells plated in 96-well plates. Beginning with 320 mg/L, the drugs were serially diluted across the plate by dilutions of 0.5 log10 ending with 0.032 mg/L. Following this, 50 T. gondii 2F tachyzoites were added to six of the eight wells in each column. Plates were incubated at 37°C/5% CO2 for 4 days after which time CPRG, the β-gal substrate, was added to Toxoplasma wells and the plates incubated for another 1 day. The cell viability reagent, CellTiter 96® AQueous One Solution Reagent (Promega Corp., WI, USA) was then added to the remaining two of the eight wells in each column and allowed to react for 3 h. Colour reactions in the wells were read in a Vmax microplate reader (Molecular Devices, CA, USA). The 50% inhibitory concentration (IC50) and the 50% cytotoxic dose (TD50) were calculated by applying the Reed and Muench8 formula. The therapeutic index (TI) was calculated by the formula TI = TD50/IC50. Data are compiled from results from three independent experiments.
Replication assay
A modification of an established procedure9 was employed. HFF monolayers in 8-well chamber slides (Nalge Nunc International, NY, USA) were inoculated with 1.6 × 105 tachyzoites/well and then incubated for 2 h. Afterwards, compounds were added to each well to final concentrations of 10 mg/L. Parasite replication was allowed to proceed for 26 h. The monolayers were then fixed, permeabilized and immunolabelled with Rb anti-p30 (SAG1) (AbD Serotec, Oxfordshire, UK) followed by goat anti-rabbit Alexa Fluoro 594 (red) (Invitrogen, CA, USA). DAPI (Invitrogen) for visualizing host cell nuclei was added to the anti-p30. The slides were visualized by phase contrast and epifluorescence using a Nikon Eclipse E800 equipped with an RT spot slider CCD camera. Data were compiled from three independent experiments, each from 10 random fields per well. The numbers of vacuoles containing 1, 2, 4, 8 or 16 parasites/vacuole were enumerated. Data are expressed as the average number of parasite doublings per vacuole.
Invasion assay
The red/green invasion assay was performed using modified published methods.10 Briefly, 5 × 106 2F tachyzoites were mixed with each compound to final concentrations ranging from 0.32 to 320 mg/L or with DMSO and allowed to sit at room temperature for 20 min before being added to HFF monolayers in 8-well chamber slides. After 1 h (37°C, 5% CO2) the chamber was removed, the slide was rinsed with PBS to remove unattached parasites and then fixed. Attached/extracellular parasites were detected using Rb anti-p30 followed by Alexa Fluoro 594 (red). After permeabilization, penetrated/intracellular parasites were stained with MAb 9e11 anti-SAG1 (Argene Inc., NY, USA) followed by goat anti-mouse Alexa Fluoro 488 (green) (Invitrogen). DAPI was added to the secondary antibody. Slides were visualized as described earlier. Numbers of green and red tachyzoites per host cell nucleus were counted.
Results
All of the newly synthesized compounds, except the non-peroxidic deoxy-3a, inhibited the growth of Toxoplasma during the course of the 5 day growth inhibition assay with varying degrees of efficacy (IC50 = 1–40.3 µM) (Table 1) producing sigmoidal inhibition curves over the concentration range tested [see Figure S1 available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/)]. The lowest IC50 value (1 µM) was seen in 3c and 3b. However, due to drug cytotoxicity, the corresponding TIs were relatively low. The highest TI values, 975 and 210, were seen in 3a and 3e, respectively. When compared with the IC50s of trimethoprim (19 µM) and ART (8 µM)5, only 3d required a higher concentration to achieve 50% inhibition. Three of the derivatives, 3a, 3d and deoxy-3a, had no measurable cytotoxic effects at the highest concentration tested (TD50 > 900 µM).
Table 1.
Growth inhibition of T. gondii by ART derivatives
| Compounda | IC50 |
TD50 |
TI | ||
|---|---|---|---|---|---|
| mg/L (SEM)b | μM | mg/L | µM | ||
| 3c (aldehyde) | 0.3 (0.02) | 1.0 | 26 | 89 | 92 |
| 3b (benzothiazole) | 0.4 (0.12) | 1.0 | 9 | 23 | 28 |
| 3a (2-thiazole) | 0.6 (0.09) | 1.7 | ≥320c | 916 | 975 |
| 3e (methyl ester) | 0.9 (0.15) | 2.9 | 177 | 545 | 210 |
| 3f (ethyl amide) | 1.5 (0.32) | 4.4 | 72 | 215 | 52 |
| 3d (carboxylic acid) | 12.5 (3.60) | 40.3 | ≥320c | 1031 | 60 |
| Deoxy-3a | 280.6 | 841.5 | ≥320c | 960 | 2 |
| Trimethoprim | 5.5 | 19 | 60 | 207 | 11 |
aC-10 unsaturated ART derivatives are listed in decreasing order of efficacy according to concentrations in mg/L. Chemical nature of derivative shown in parentheses.
bData were compiled from the results of three independent experiments; standard error of the mean (SEM) is shown in parentheses.
cToxicity endpoint was not reached; a value 1/4 log10 greater than the concentration tested was used to compute the TI.
We next tested the derivatives for efficacy in a replication assay wherein Toxoplasma is allowed to establish an infection before drugs are added. ART and artemether served as controls for the parent compound and C-10 derivatives, respectively. The results are shown in Figure 1(b) with the derivatives listed in the same order as in Table 1. At a concentration of 10 mg/L (25–33.5 µM), all of the compounds inhibited Toxoplasma replication with the same relative efficacy as in the 5 day growth inhibition assay (Figure 1b and Table 1). It is of note that neither ART nor artemether proved as effective at inhibiting replication in this assay as they were in the 5 day growth inhibition assay [IC50 = 2.3 mg/L (8 µM) and 0.2 mg/L (0.7 µM), respectively].5 Fluorescence images representative of those from which the numerical data for Figure 1(b) were generated can be viewed in Figure S2 [available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/)].
Invasion of host cells by T. gondii is the first step in the lytic growth cycle of the tachyzoite in the intermediate host9 and is initiated by attachment of the tachyzoite to the host cell followed by active penetration. Using the red/green invasion assay (shown as grey/black, respectively), we tested the ability of the derivatives to inhibit the attachment and penetration steps of cell invasion. BAPTA-AM (20 µM) and cytochalasin D (2 µM) were added as controls for defects in attachment and penetration, respectively. As shown in Figure 1(c) for treatment at 10 mg/L, two compounds, 3b and artemether, caused significant (single asterisk) reductions in the number of penetrated parasites. Treatment with 3c, 3a and 3e also resulted in a >50% reduction of penetration. Of note, deoxy-3a, virtually inactive in growth inhibition assays, caused a reduction of penetrated parasites approximately equal to that of the highly effective derivative 3c (Table 1 and Figure 1c). Conversely, ART showed little or no effect on the invasion process. A decrease in the combined numbers of attached and penetrated parasites relative to control is highly indicative of a direct effect on the parasite’s ability to attach to host cells.11 Seven ART derivatives showed a moderate (3a, 3c, 3d, deoxy-3a) to significant (3b, 3e, artemether) (double asterisk) inhibitory effect on tachyzoite attachment to the cells relative to DMSO-treated tachyzoites (Figure 1c). Fluorescence images representative of those from which the numerical data for Figure 1(c) were generated can be viewed in Figure S3 [available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/)].
We investigated the dose–response relationship of the derivatives on invasion by treating parasites with varying concentrations (0.32–320 mg/L) of ART or derivatives and then evaluating the inhibition using the red/green invasion assay. The total number of cell-associated tachyzoites, attached, penetrating and penetrated per cell nuclei were normalized to that of the DMSO-treated control (that being 100%). Artemether and 3b showed nearly identical profiles of inhibition, effectively inhibiting invasion over the entire range of concentrations, never allowing more than 40% relative attachment/penetration (Figure 1d). We observed a gradual decrease in the number of attached/penetrated parasites as the concentration increased with most of the other derivatives and ART (50% inhibition reached between 7 and 32 mg/L) with the exception of 3f, which did not inhibit more than 20% of the invading tachyzoites at any concentration tested.
Discussion
We have demonstrated that C-10 unsaturated, carba-linked derivatives of ART can be effective in the inhibition of infection by T. gondii. Furthermore, this inhibition occurs at more than one step in the parasite’s lytic cycle.
In the 5 day growth inhibition assay, all of the new derivatives except deoxy-3a demonstrated higher TIs than trimethoprim, a drug used for the treatment of Toxoplasma infections. In addition, these same compounds, with the exception of 3d, displayed lower IC50s relative to ART. Treatment with 3a resulted in the highest TI, 975, which compares favourably with other C-10 ART derivatives that we have synthesized and tested, as well as to artemether, one of the medications used for the treatment of human malaria.5 The virtual lack of growth inhibitory activity of deoxy-3a indicates that the peroxide bridge of ART is required for this inhibitory property of the derivatives.
The 5 day growth inhibition assay tests for activity against extracellular and intracellular parasites. Conversely, the replication assay tests for activity only against intracellular parasites. All of the new derivatives effectively inhibited the intracellular parasites, again except deoxy-3a. Interestingly, both ART and artemether, which are highly effective in our growth inhibition assay, were not as effective in the replication assay even at concentrations of 4× and 50×, respectively, higher than their established IC50s (Figure 1b).5 These results are somewhat different from those of Ou-Yang et al.4 who reported that both ART and artemether, when added to an established infection, successfully inhibited the formation of T. gondii plaques. The reason for this difference could be related to a combination of factors. Ou-Yang et al.4 added drugs to previously infected cells, similar to our replication assay, but then unlike our assay, allowed the infection/treatment to proceed for 5 days before plaque quantification. Thus by design, the tachyzoites were allowed to undergo at least 5× the number of doublings as in our replication assay and consequently they were exposed to the drugs 5× longer. This longer exposure in vitro might be required for ART and artemether to show efficacy. Such delayed onset of action has been described for the anti-Toxoplasma drug clindamycin.12
We have also documented that treatment of extracellular tachyzoites with these new derivatives results in an inhibition of parasite invasion (Figure 1c and d), 3f being the only exception. Deoxy-3a, essentially inactive in growth inhibition assays, demonstrated efficient inhibition of tachyzoite invasion with an IC50 = 10 mg/L. This inhibitory effect shown by the majority of the derivatives might be due to a mobilization of intracellular calcium within the parasites [Ca2+]i. Such an increase in [Ca2+]i results in a premature secretion of the parasite’s microneme protein MIC2, a protein essential for efficient invasion of host cells.10 This effect on [Ca2+]i has recently been reported for ART.13
Our results indicate that C-10 unsaturated, carba-linked derivatives of ART have anti-Toxoplasma activity and that these derivatives affect the proliferation of T. gondii at several steps in the life cycle of the tachyzoite. In addition, our data show that inhibition of one step is not necessarily linked to inhibition of another step. Furthermore, even though these derivatives did not have higher TIs than artemether (TI = 1100)5, three, 3a–c, were more efficient at inhibition of replication and 3b was comparable in invasion inhibition. Most importantly, these derivatives, due to a carbon linkage rather than an oxygen linkage at C-10 as in ART and artemether, are resistant to the oxidation by cytochrome P450 to the reportedly neurotoxic DHA6 and thus represent promising compounds for development. Further studies will be performed to define the molecular mechanisms of the Toxoplasma inhibitory activity of the different compounds and to design additional active compounds.
The availability of compounds with selective activity at different steps of the tachyzoite life cycle could lead to new pharmacological methods for the prevention and treatment of Toxoplasma infection in humans on a worldwide basis. This potential for global use will depend upon the safety and efficacy of the compounds as well as on the possible effects of these compounds on concomitant anti-malaria programmes that employ similar compounds.
Funding
Financial support was provided by the National Institutes of Health (grant AI 34885) to G. H. P. and the Stanley Medical Research Institute to G. H. P. and R. Y.
Transparency declarations
None to declare.
Supplementary data
Supplementary Material
Acknowledgements
We thank Drs Chuck Long (JHU NMR) and Phil Mortimer (JHU Mass Spec) for their valuable assistance and Dr Vern Carruthers (U. Michigan) for critical reading of the manuscript.
References
- 1.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–76. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 2.Vogel N, Kirisits M, Michael E, et al. Congenital toxoplasmosis transmitted from an immunologically competent mother infected before conception. Clin Infect Dis. 1996;23:1055–60. doi: 10.1093/clinids/23.5.1055. [DOI] [PubMed] [Google Scholar]
- 3.Georgiev VS. Management of toxoplasmosis. Drugs. 1994;48:179–88. doi: 10.2165/00003495-199448020-00005. [DOI] [PubMed] [Google Scholar]
- 4.Ou-Yang K, Krug EC, Marr JJ, et al. Inhibition of growth of Toxoplasma gondii by qinghaosu and derivatives. Antimicrob Agents Chemother. 1990;34:1961–5. doi: 10.1128/aac.34.10.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones-Brando L, D’Angelo J, Posner GH, et al. In vitro inhibition of Toxoplasma gondii by four new derivatives of artemisinin. Antimicrob Agents Chemother. 2006;50:4206–8. doi: 10.1128/AAC.00793-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Neill PM, Searle NL, Kan K-W, et al. Novel, potent, semisynthetic antimalarial carba analogues of the first-generation 1,2,4-trioxane artemether. J Med Chem. 1999;42:5487–93. doi: 10.1021/jm9903545. [DOI] [PubMed] [Google Scholar]
- 7.Jones-Brando L, Torrey EF, Yolken RH. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr Res. 2003;62:237–44. doi: 10.1016/s0920-9964(02)00357-2. [DOI] [PubMed] [Google Scholar]
- 8.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–9. [Google Scholar]
- 9.Silverman JA, Hayes ML, Luft BJ, et al. Characterization of anti-Toxoplasma activity of SDZ 215-918, a cyclosporin derivative lacking immunosuppressive and peptidyl-prolyl-isomerase-inhibiting activity: possible role of a P glycoprotein in Toxoplasma physiology. Antimicrob Agents Chemother. 1997;41:1859–66. doi: 10.1128/aac.41.9.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huynh M-H, Carruthers V. Toxoplasma MIC2 is a major determinant of invasion and virulence. PLoS Pathog. 2006;2:753–62. doi: 10.1371/journal.ppat.0020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huynh M-H, Rabenau KE, Harper JM, et al. Rapid invasion of host cells by Toxoplasma requires secretion of the MIC2-M2AP adhesive protein complex. EMBO J. 2003;22:2082–90. doi: 10.1093/emboj/cdg217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfefferkorn ER, Nothnagel RF, Borotz SE. Parasiticidal effect of clindamycin on Toxoplasma gondii grown in cultured cells and selection of a drug-resistant mutant. Antimicrob Agents Chemother. 1992;36:1091–6. doi: 10.1128/aac.36.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagamune K, Beatty WL, Sibley LD. Artemisinin induces calcium-dependent protein secretion in the protozoan parasite Toxoplasma gondii. Eukaryot Cell. 2007;6:2147–56. doi: 10.1128/EC.00262-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.