Abstract
The presence of human immunodeficiency virus type 1 (HIV-1) proviral DNA in peripheral blood mononuclear cells (PBMC) of three groups (group 1, more than 500 CD4+ T cells per microliter; group 2, between 200 and 499 CD4+ T cells per microliter; group 3, fewer than 200 CD4+ T cells per microliter) of HIV-1-infected patients, in different stages of the disease, was determined by using a newly developed flow cytometry analysis of the product of in situ PCR assay and compared with other markers of viral replication (HIV-1 p24 antigenemia and viral isolation). Results showed varied percentages of HIV-1-infected PBMC, ranging from 0.6 to 20%. Patients with more than 500 CD4+ T cells per microliter showed the lowest percentage of HIV-1-infected PBMC (2.1 +/- 1.7), compared with patients with CD4+ T-cell counts of between 200 and 499 per microliter (6.5% +/- 4.1%; P < 0.001) and patients with fewer than 200 CD4+ T cells per microliter (4.9% +/- 4.7%; P < 0.05). The difference in the percentage of HIV-1-infected PBMC between group 2 and group 3 patients may in part reflect the loss of CD4+ T lymphocytes in more advanced stages of the disease. However, the results clearly indicate a striking coincidence between the fall of the CD4+ T-cell count below 400/microliter and the sharp increase in PBMC virus loading and p24 antigenemia. Since the procedure is relatively easy to perform, it could be used to monitor the evolution of HIV-1 infection and may prove a useful adjunct in tailoring therapeutic strategies.
Full text
PDF

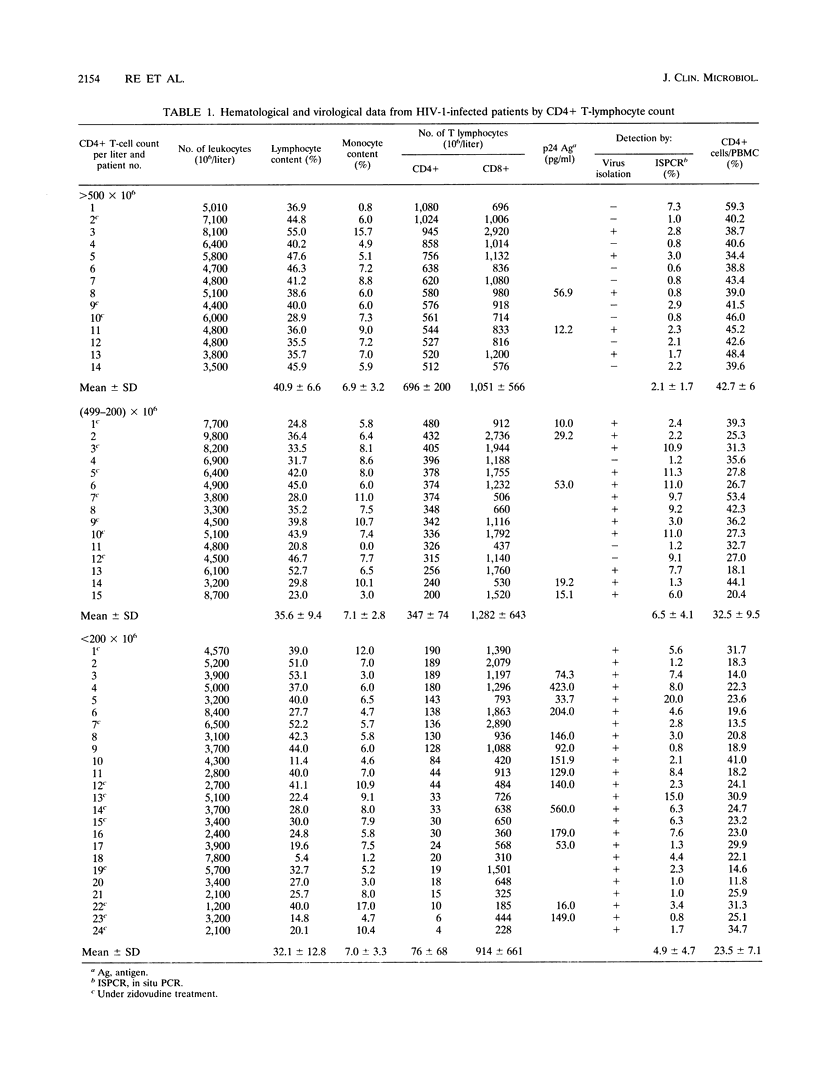
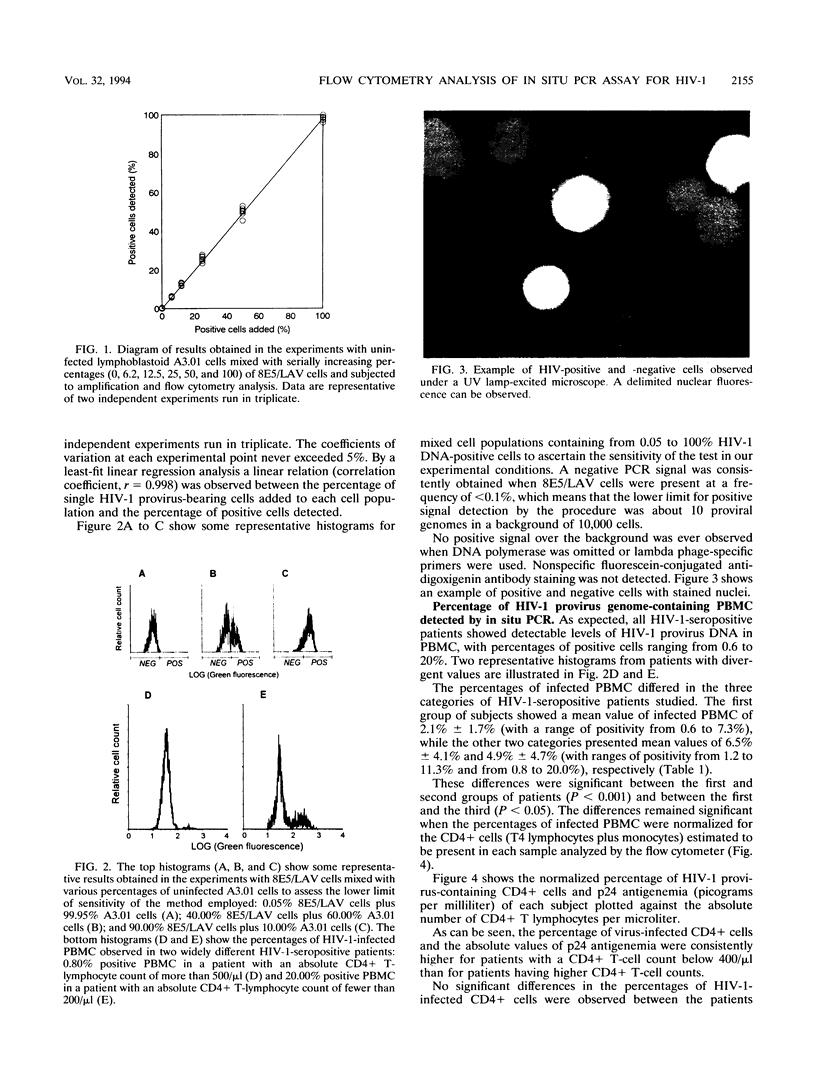
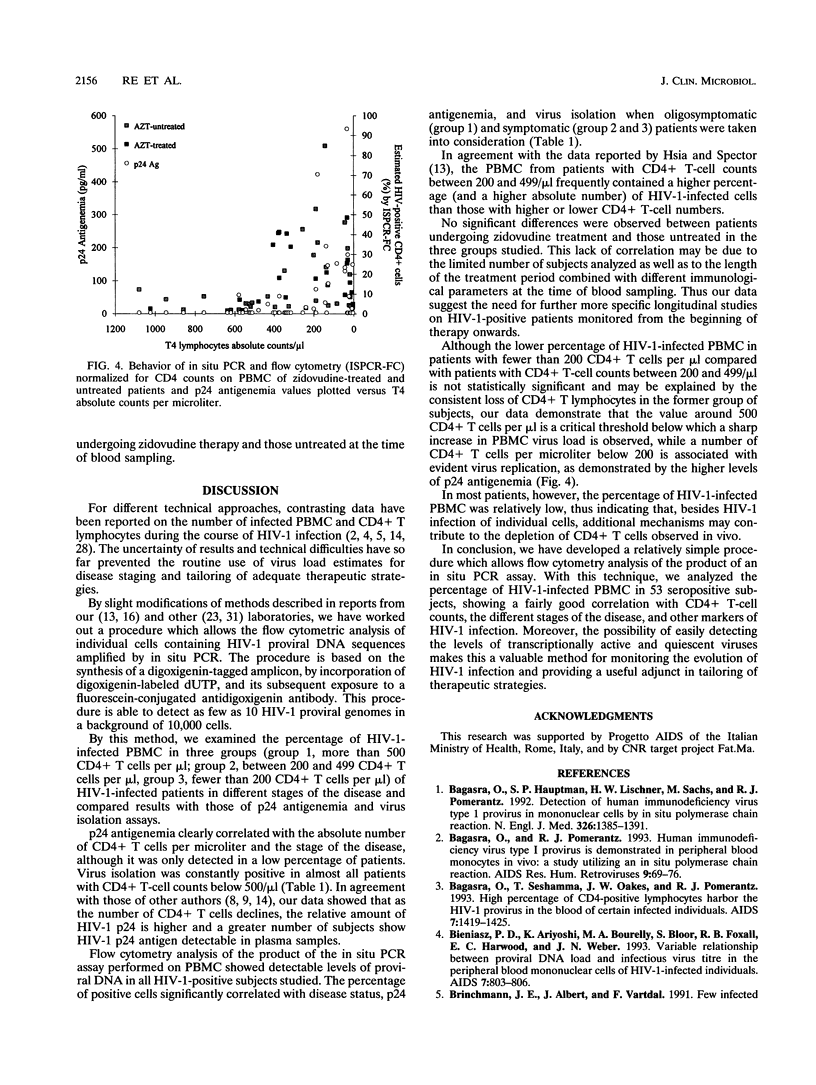

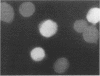
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagasra O., Hauptman S. P., Lischner H. W., Sachs M., Pomerantz R. J. Detection of human immunodeficiency virus type 1 provirus in mononuclear cells by in situ polymerase chain reaction. N Engl J Med. 1992 May 21;326(21):1385–1391. doi: 10.1056/NEJM199205213262103. [DOI] [PubMed] [Google Scholar]
- Bagasra O., Pomerantz R. J. Human immunodeficiency virus type I provirus is demonstrated in peripheral blood monocytes in vivo: a study utilizing an in situ polymerase chain reaction. AIDS Res Hum Retroviruses. 1993 Jan;9(1):69–76. doi: 10.1089/aid.1993.9.69. [DOI] [PubMed] [Google Scholar]
- Bagasra O., Seshamma T., Oakes J. W., Pomerantz R. J. High percentages of CD4-positive lymphocytes harbor the HIV-1 provirus in the blood of certain infected individuals. AIDS. 1993 Nov;7(11):1419–1425. doi: 10.1097/00002030-199311000-00003. [DOI] [PubMed] [Google Scholar]
- Bieniasz P. D., Ariyoshi K., Bourelly M. A., Bloor S., Foxall R. B., Harwood E. C., Weber J. N. Variable relationship between proviral DNA load and infectious virus titre in the peripheral blood mononuclear cells of HIV-1-infected individuals. AIDS. 1993 Jun;7(6):803–806. doi: 10.1097/00002030-199306000-00007. [DOI] [PubMed] [Google Scholar]
- Chou Q., Russell M., Birch D. E., Raymond J., Bloch W. Prevention of pre-PCR mis-priming and primer dimerization improves low-copy-number amplifications. Nucleic Acids Res. 1992 Apr 11;20(7):1717–1723. doi: 10.1093/nar/20.7.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Ribas J. L., Burke A., Racz P., Tenner-Racz K., Haase A. T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993 Mar 25;362(6418):359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Powell D., Lightfoote M., Koenig S., Fauci A. S., Benn S., Rabson A., Daugherty D., Gendelman H. E., Hoggan M. D. Biological and biochemical characterization of a cloned Leu-3- cell surviving infection with the acquired immune deficiency syndrome retrovirus. J Exp Med. 1986 Jul 1;164(1):280–290. doi: 10.1084/jem.164.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks T., Benn S., Rabson A., Theodore T., Hoggan M. D., Martin M., Lightfoote M., Sell K. Characterization of a continuous T-cell line susceptible to the cytopathic effects of the acquired immunodeficiency syndrome (AIDS)-associated retrovirus. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4539–4543. doi: 10.1073/pnas.82.13.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia K., Spector S. A. Human immunodeficiency virus DNA is present in a high percentage of CD4+ lymphocytes of seropositive individuals. J Infect Dis. 1991 Sep;164(3):470–475. doi: 10.1093/infdis/164.3.470. [DOI] [PubMed] [Google Scholar]
- Lafeuillade A., Tamalet C., Pellegrino P., Tourres C., Yahi N., Vignoli C., Quilichini R., de Micco P. High viral burden in lymph nodes during early stages of HIV-1 infection. AIDS. 1993 Nov;7(11):1527–1528. doi: 10.1097/00002030-199311000-00019. [DOI] [PubMed] [Google Scholar]
- Lalli E., Gibellini D., Santi S., Facchini A. In situ hybridization in suspension and flow cytometry as a tool for the study of gene expression. Anal Biochem. 1992 Dec;207(2):298–303. doi: 10.1016/0003-2697(92)90015-y. [DOI] [PubMed] [Google Scholar]
- Levy J. A. HIV pathogenesis and long-term survival. AIDS. 1993 Nov;7(11):1401–1410. doi: 10.1097/00002030-199311000-00001. [DOI] [PubMed] [Google Scholar]
- Nuovo G. J., Margiotta M., MacConnell P., Becker J. Rapid in situ detection of PCR-amplified HIV-1 DNA. Diagn Mol Pathol. 1992 Jun;1(2):98–102. [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Butini L., Pizzo P. A., Schnittman S. M., Kotler D. P., Fauci A. S. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9838–9842. doi: 10.1073/pnas.88.21.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Demarest J. F., Butini L., Montroni M., Fox C. H., Orenstein J. M., Kotler D. P., Fauci A. S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993 Mar 25;362(6418):355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Fauci A. S. New concepts in the immunopathogenesis of human immunodeficiency virus infection. N Engl J Med. 1993 Feb 4;328(5):327–335. doi: 10.1056/NEJM199302043280508. [DOI] [PubMed] [Google Scholar]
- Patterson B. K., Till M., Otto P., Goolsby C., Furtado M. R., McBride L. J., Wolinsky S. M. Detection of HIV-1 DNA and messenger RNA in individual cells by PCR-driven in situ hybridization and flow cytometry. Science. 1993 May 14;260(5110):976–979. doi: 10.1126/science.8493534. [DOI] [PubMed] [Google Scholar]
- Poznansky M. C., Walker B., Haseltine W. A., Sodroski J., Langhoff E. A rapid method for quantitating the frequency of peripheral blood cells containing HIV-1 DNA. J Acquir Immune Defic Syndr. 1991;4(4):368–373. [PubMed] [Google Scholar]
- Re M. C., Furlini G., La Placa M. Rapid detection of HIV-1 in clinical samples by co-culture with heat-shocked cells. J Virol Methods. 1989 Dec;26(3):313–317. doi: 10.1016/0166-0934(89)90113-4. [DOI] [PubMed] [Google Scholar]
- Rosenberg Z. F., Fauci A. S. The immunopathogenesis of HIV infection. Adv Immunol. 1989;47:377–431. doi: 10.1016/s0065-2776(08)60665-3. [DOI] [PubMed] [Google Scholar]
- Saksela K., Stevens C., Rubinstein P., Baltimore D. Human immunodeficiency virus type 1 mRNA expression in peripheral blood cells predicts disease progression independently of the numbers of CD4+ lymphocytes. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1104–1108. doi: 10.1073/pnas.91.3.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittman S. M., Greenhouse J. J., Psallidopoulos M. C., Baseler M., Salzman N. P., Fauci A. S., Lane H. C. Increasing viral burden in CD4+ T cells from patients with human immunodeficiency virus (HIV) infection reflects rapidly progressive immunosuppression and clinical disease. Ann Intern Med. 1990 Sep 15;113(6):438–443. doi: 10.7326/0003-4819-113-6-438. [DOI] [PubMed] [Google Scholar]
- Schnittman S. M., Psallidopoulos M. C., Lane H. C., Thompson L., Baseler M., Massari F., Fox C. H., Salzman N. P., Fauci A. S. The reservoir for HIV-1 in human peripheral blood is a T cell that maintains expression of CD4. Science. 1989 Jul 21;245(4915):305–308. doi: 10.1126/science.2665081. [DOI] [PubMed] [Google Scholar]
- Simmonds P., Balfe P., Peutherer J. F., Ludlam C. A., Bishop J. O., Brown A. J. Human immunodeficiency virus-infected individuals contain provirus in small numbers of peripheral mononuclear cells and at low copy numbers. J Virol. 1990 Feb;64(2):864–872. doi: 10.1128/jvi.64.2.864-872.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Yang G., Garhwal S., Olson J. C., Vyas G. N. Flow cytometric immunodetection of human immunodeficiency virus type 1 proviral DNA by heminested PCR and digoxigenin-labeled probes. Clin Diagn Lab Immunol. 1994 Jan;1(1):26–31. doi: 10.1128/cdli.1.1.26-31.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]