Abstract
Purpose
Chronic nature of prostate cancer (PCa) growth and progression leading to metastasis provides a large window for intervention. Herein, for the first time, we investigated the effect and associated mechanisms of silibinin phosphatidylcholine (silybin-phytosome), on established prostate tumors in transgenic adenocarcinoma of the mouse prostate (TRAMP) model.
Experimental Design
Twenty-week old TRAMP male mice having palpable prostate tumor, were fed with control or 0.5% and 1%, w/w, silybin-phytosome diets for 11 weeks and then sacrificed.
Results
Dietary silibinin inhibited the growth of prostate tumors (upto 60%, P<0.001) and suppressed tumor progression from PIN (prostatic intraepithelial neoplasia) to differentiated adenocarcinoma (AC) and poorly differentiated AC, with a complete absence of poorly differentiated AC at higher dose. It also inhibited the incidence of tumor invasion of seminal vesicle (upto 81%, P<0.001) with complete absence of distant metastasis. Silibinin moderately inhibited tumor cell proliferation and induced apoptosis, but strongly suppressed tumor microvessel density (upto 60%, P<0.001), VEGF and VEGFR2 expression. Antibody array analysis of plasma showed a decrease in the circulatory levels of VEGF and bFGF. Decreased levels of MMPs, snail-1 and vimentin, and an increased level of E-cadherin were also observed, indicating the anti-epithelial-mesenchymal transition (EMT) effect of silibinin in tumors.
Conclusions
Overall, silibinin treatment of TRAMP mice bearing prostate tumor, inhibited tumor growth, progression, local invasion and distant metastasis involving suppression of tumor angiogenesis and EMT. These findings would have greater relevance for the ongoing phase II clinical trial with silibinin-phytosome in PCa patients.
Keywords: TRAMP, prostate cancer progression, angiogenesis, EMT, silibinin
Introduction
The growth and progression of prostate cancer (PCa) takes considerable amount of time which may range up to 2–3 decades from the time of initiation to the metastatic disease, and thus provide a large window for different intervention strategies. In this regard, intervention of PCa by non-toxic edible phytochemicals is an upcoming approach (1, 2). This treatment approach is advantageous for the lack of adverse health effect, being economical and human acceptability for the oral consumption of these agents. Silibinin is a natural flavanolignan and has all these above characteristics including its clinical use as an antihepatotoxic agent and dietary supplement as well as anticancer activities in various models of epithelial cancers (2–4).
Prostate tumors are heterogeneous with regard to their cellular composition, development, molecular abnormalities and clinical path (5). In the United States, although 1 in 6 men is diagnosed with PCa, only 1 in 34 dies of the metastatic disease (6). In the beginning, prostate tumor growth is androgen-dependent and treated with antiandrogen therapies; however, with time, it progresses to a hormone refractory stage (7). At the advanced stages, molecular abnormalities are involved in enhancing the angiogenic, invasive and metastatic capabilities in PCa (8, 9). In this regard, prostate tumors show overexpression of vascular endothelial growth factor (VEGF) and VEGF receptor (VEGFR) leading to the receptor activation and down-stream signaling to drive endothelial cell growth, proliferation, migration and vessel organization (8). Tumors also express high level of matrix metalloproteinases (MMPs) that degrade tissue matrix and facilitate tumor as well as endothelial cell invasion and migration (9). Furthermore, epithelial-mesenchymal-transition (EMT) has also been observed in epithelial tumor cells to facilitate these biological processes, in which tumor cells lose E-cadherin, a characteristic of epithelial cells, and express vimentin, a characteristic of mesenchymal cells (10, 11). This could be regulated by a transcription factor, called snail-1, which suppresses the expression of E-cadherin (10, 11). Therefore, antiangiogenic as well as anti-invasive strategies could be prospective and proficient approaches to intervene PCa growth, invasion and metastasis.
Silibinin has shown strong anticancer efficacy against both androgen-dependent and - independent PCa LNCaP, 22Rv1, PC-3, and DU145 cells (12–14). In PCa cell culture studies, silibinin causes cell cycle arrest by modulating cyclin-dependent kinase (CDK)-cyclin-CDK inhibitor and retinoblastoma-E2F axes, and induces differentiation morphology and growth arrest (12–14). It also inhibits epidermal growth factor receptor (EGFR) and insulin-like growth factor receptor 1 (IGF-1R) signaling in PCa cells (15, 16). In animal studies, oral silibinin suppresses the growth of DU145 and PC-3 xenografts in nude mice (17, 18). Recently, we observed the chemopreventive efficacy of silibinin in the transgenic adenocarcinoma of the mouse prostate (TRAMP) model in which mice were treated with silibinin from 4–24 weeks of age (19). In this study, silibinin modulated CDK-cyclin-CDK inhibitor axis and IGF-receptor type Iβ signaling, without affecting the transgene expression, for its antitumor efficacy against the localized prostate tumor. However, the effects and associated mechanisms of silibinin on localized prostate tumor that follows the course of invasion and metastasis in TRAMP mice have not yet been studied.
In the present study, we evaluated the efficacy of silibinin phosphatidylcholine complex (silybin-phytosome) on established prostate tumor growth, progression, invasion and distant metastasis in TRAMP mice. Additionally, we also investigated the biomarkers of antitumor effects and associated molecular mechanisms. The present findings revealed anti-invasive and anti-metastatic activities of silibinin in this in vivo tumor model, together with a strong antiangiogenic effect and inhibitory activity on EMT in silibinin-treated tumor tissues.
Materials and Methods
Animals and treatments
Heterozygous TRAMP females with C57BL/6 background were cross-bred with non-transgenic C57BL/6 breeder males. Genotyping was done by PCR-based screening assay for PB-Tag as reported (19), and routinely obtained TRAMP male mice were maintained on control diet for 20 weeks. Mice were abdominally palpated for the presence of prostate tumors. Twenty week-old TRAMP male mice, each having palpable prostate tumor, were exposed to control AIN-93M diet (n=8 mice) or 0.5%, w/w, silibinin (n=8 mice) or 1%, w/w, silibinin (n=7 mice) in AIN-93M diet for 11 weeks. Silibinin (Fig. 1A) used was silybin-phytosome which is a commercial formulation (purchased from Indena Corporation, Seattle, WA) of silibinin and phosphatidylcholine complex (approximately 1:2 ratio by weight, respectively), and has shown better bioavailability of silibinin as compared to the administration of pure silibinin (20). Twenty-week old 10 non-transgenic (C57BL/6) male mice were also fed with either control (n=5 mice) or 1%, w/w silibinin diet for 11 weeks. These diets were prepared commercially by Dyets Inc. (Bethlehem, PA). During the 11 weeks of study, mice were permitted free access to drinking water and food, and monitored for diet consumption, body weight and general health. Animal care and treatments were in accordance with Institutional guidelines and approved protocol.
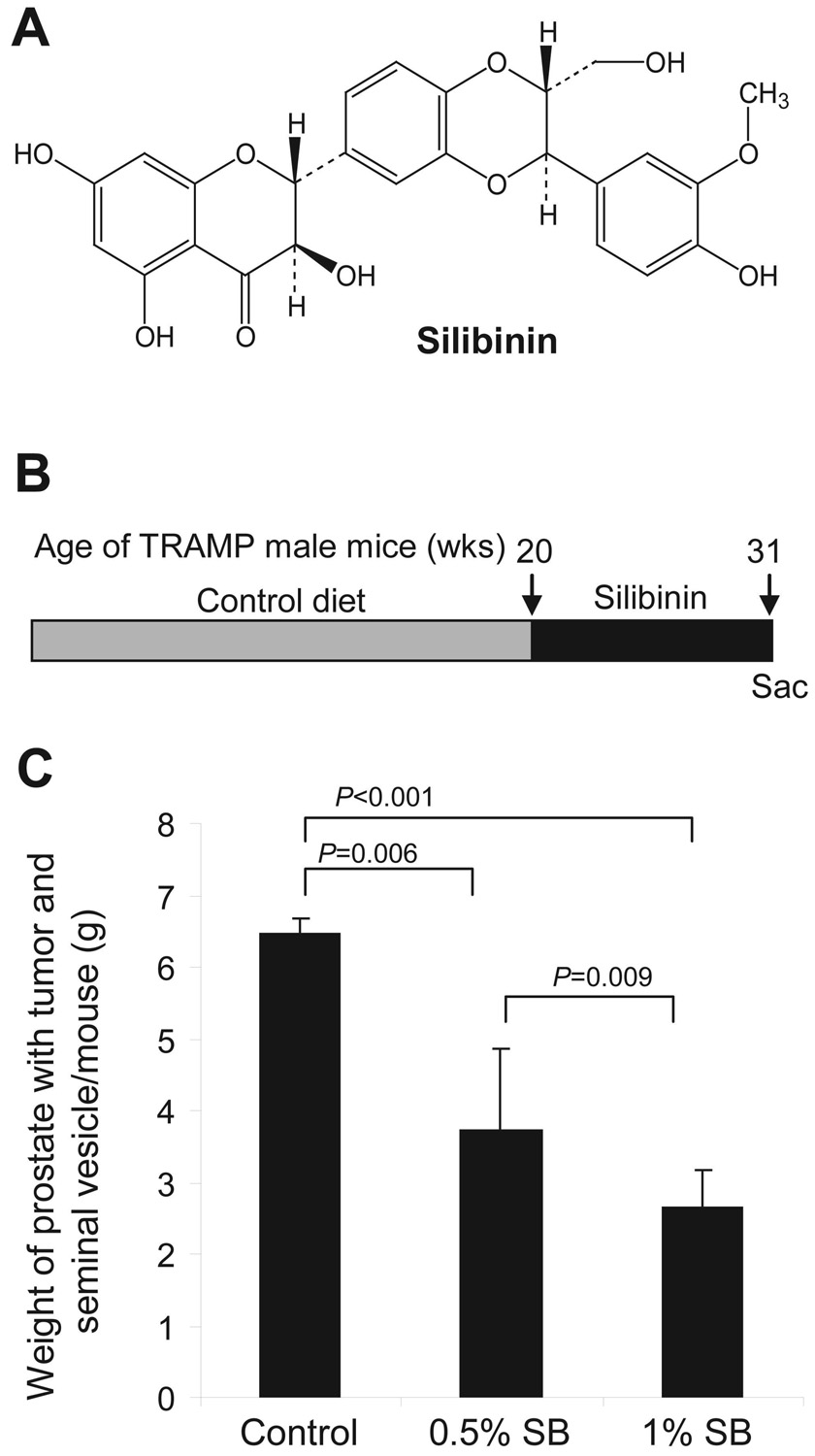
Figure 1. Inhibition of established prostate tumor growth by dietary silibinin in TRAMP mice.
A, chemical structure of the flavanolignan compound silibinin. B, experimental design, in which 20-week old TRAMP male mice were sacrificed or exposed to AIN-93M control diet or control diet containing 0.5 and 1% (w/w) silibinin for 11 weeks. C, cumulative weight of enbloc prostate with tumor and seminal vesicle (g) per mouse at the termination of the experiment (31 weeks of age). Data are represented as mean ± s.e.m. (error bars) from 7–8 mice in each group. Sac, sacrificed; SB, silibinin.
Histopathology
Animals were injected with heparin and euthanized by carbon dioxide asphyxiation. Blood was collected for harvesting plasma. At necropsy, animals were examined for gross pathology, and any evidence of edema, abnormal organ size or appearance in non-target organs such as invasion of seminal vesicle and distant metastasis. Prostate along with seminal vesicles was removed en bloc and weighed. One portion of the prostate was snap-frozen and stored at −80°C and other was fixed overnight in 10% (v/v) phosphate-buffered formalin and processed conventionally. Liver, lung, kidney, lymph nodes (inguinal, cervical and mediastinal) and bone (femur) were also collected and fixed in formalin for histopathological examination. Fixed bones were decalcified in Cal-EXR (Fisher Diagnostics, NJ) solution containing 0.03 M chelating agent sodium-EDTA and 1.35 N HCl, overnight and then washed in running water for 3–4 h followed by conventional processing for sectioning. In each case, 5µm thick sections of paraffin embedded tissues were stained with H&E for histopathological evaluation.
Immunohistochemical analysis
Paraffin-embedded tissue sections were deparaffinized and stained using specific primary antibody followed by 3, 3′-diaminobenzidine (DAB) staining, as previously described (21). Primary antibodies used were anti-PCNA (1:250 dilution; Dako, Carpinteria, CA), goat polyclonal anti-CD31 (1:200 dilution; Santa Cruz Biotechnology Inc., Santa Cruz, CA) and rabbit polyclonal anti-VEGF (1:100 dilution, Santa Cruz Biotechnology). Biotinylated secondary antibodies used were rabbit anti-mouse IgG (1:200; Dako) and goat anti-rabbit IgG (1:200; Santa Cruz). Apoptotic cells were identified by TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) staining using Dead End Colorometric TUNEL System (Promega Corp., Madison, WI) as published (17, 18). PCNA- and TUNEL-positive cells were quantified by counting brown-stained cells within total number of cells at 10 randomly selected fields at x400 magnification. Tumor microvessel density was quantified by counting the CD31-positive cells at 10 randomly selected fields at 400x magnification. Branching vessels (i.e. a group of CD31-positive cells connected with each other) were considered as one count. For VEGF, immunoreactivity (represented by intensity of brown staining) was scored as 0 (no staining), +1 (non-uniform and very weak), +2 (non-uniform and weak), +3 (uniform and moderate) and +4 (uniform and strong) as reported (21). In all immunohistochemical staining, negative staining controls (and positive staining control for TUNEL by incubation with nuclease to generate nicks) were employed, where sections were incubated with N-Universal Negative Control-mouse or rabbit antibody (Dako) under identical conditions.
Western immunoblot analysis
Dorsolateral prostate samples from control and silibinin-fed groups of mice were analyzed by immunoblotting as previously described (19). Randomly, three tumors were selected from each group (control, 0.5% and 1% silibinin groups) and homogenized in non-denaturing lysis buffer and centrifuged at 14,000 × g followed by protein concentration determination in supernatants. Equal protein per lysate was resolved on Tris-glycine gel, transferred on to nitrocellulose membrane and blocked for 1 h with 5% non-fat dry milk. Membranes were incubated with specific primary antibodies including anti-VEGF (sc-152), anti-VEGF-R2 (sc-6251), anti-MMP-3 (sc-31074), anti-E-cadherin (sc-7870), anti-vimentin (sc-7557) from Santa Cruz Biotechnology; anti-MMP-2 (ab19167) from Chemicon International (Temecula, CA) and anti-snail-1 (ab17332) from Abcam (Cambridge, MA), overnight at 4° C and then with appropriate horseradish peroxidase-conjugated secondary antibody followed by ECL detection. Blots were scanned with Adobe Photoshop 6.0 with minimum background. Densitometric analysis was done with Scion image software (NIH, Bethesda, MD) and data are shown as fold change of the control value. Membranes were stripped and reprobed with anti-β-actin antibody for loading control.
Mouse angiogenesis antibody array
Plasma samples collected at the end of the experiment after 11 weeks of silibinin treatment were used to analyze the levels of circulating angiogenesis-related molecules using a Mouse Angiogenesis Antibody Array (RayBiotech, Inc., Norcross, GA) as recently reported by us (21). A representative plasma sample (100µL/assay) from control and 1% silibinin-treated groups was analyzed according to the manufacturer’s assay protocol. Each protein expression was represented by a pair of dots (duplex-dots) on the membrane. Duplex-dots identifying each protein were scanned by Photoshop Adobe and quantitated by ScionImage program (NIH, Bethesda, MD). The mean value of the intensity of the two dots in arbitrary unit was determined for inter-group comparison.
Statistical and microscopic analyses
All statistical analyses were carried out with Sigma Stat software version 2.03 (Jandel Scientific, San Rafael, CA) and P values <0.05 were considered significant. Fisher’s Exact test was used to compare incidence of PIN, adenocarcinoma, invasion of seminal vesicle and metastatic lesions in (31-week) control versus other groups. For other data, the difference between control and silibinin-fed groups was analyzed by Student’s t-test. All microscopic histopathological and immunohistochemical analyses were done by Zeiss Axioscope 2 microscope (Carl Zeiss, Inc., Jena, Germany) and photomicrographs were captured by Carl Zeiss AxioCam MrC5 camera and shown at 400x magnification.
Results
Silibinin inhibits palpable prostate tumor growth in TRAMP mice
TRAMP male mice at 20 weeks of age, each having palpable prostate tumor, were fed with purified AIN-93M diet (control) or the diet containing 0.5% or 1%, w/w silibinin (Fig. 1B). The efficacy of the treatment was measured (weekly) by the absence/presence of a palpable tumor, till 31 weeks of the age, which indicated slower increase in tumor mass in mice fed with silibinin diets compared to control. Animal health, body weight and diet consumption were also monitored bi-weekly, which did not show any change due to silibinin supplementation in diet (data not shown). At 31 weeks of age, silibinin-diets significantly and dose-dependently decreased (up to 60%) the weight of prostate together with tumor and seminal vesicle which were 3.7 ± 0.5 (P<0.006) and 2.6 ± 0.8 (P<0.001) g/mouse in lower and higher doses of silibinin groups as compared to 6.5 ± 1.2 g/mouse in control group, respectively (Fig. 1C). Corresponding weight of prostate with seminal vesicle in 31-week-old wild-type mice fed with control diet was 2.0 ± 0.2 g/mouse that did not show any considerable change due to 11 weeks of 1% silibinin consumption in diet (data not shown). These observations suggest that silibinin feeding at these doses is non-toxic, and that it selectively as well as dose-dependently suppresses the aberrant or neoplastic growth of prostate tissue. It is likely that silibinin, in addition to inhibition, may also cause the reversal of the disease progression in this animal model. This is supported by the earlier studies in which approximately more than half of TRAMP mice at 20 weeks of age have shown the different stages of AC (22). Next, prostate tissue samples were analyzed for histopathological alterations.
Silibinin inhibits the progression of established prostate tumor in TRAMP mice
H&E-stained tissue sections were microscopically examined for the presence of PIN showing the characteristics of foci with two or more layers of atypical cells with elongated hyper chromatic nuclei filling or almost filling the lumen of the ducts with distorted duct and cribriform structures and intact or enlarged gland profiles; well differentiated AC showing invasion of basement membrane, loss of intraductal spaces and increased quantity of small glands; and poorly differentiated AC showing total loss of intraductal spaces and presence of poorly differentiated cells with leftovers of trapped glands with fairly solid growth (19, 23). Histopathological analysis of prostate showed a marked difference for the incidence of PIN, well differentiated AC and poorly differentiated AC between the 31-week control and both silibinin-treated groups. Also, the portions of the prostate with these progressive neoplastic stages were decreased by silibinin treatments. At 31 weeks of age, there was complete absence of PIN with 12% and 88% incidences of well differentiated AC and poorly differentiated AC, respectively, in control mice (Fig. 2). Silibinin treatments at 0.5% and 1% doses inhibited the progression of PIN into the advanced stages of PCa by 50% and 86% (P=0.001), respectively. More importantly, silibinin also suppressed the severity of adenocarcinoma with the increase in its doses, showing 50% incidence of poorly differentiated AC at 0.5% silibinin dose which was completely absent at 1% silibinin dose that showed only 14% incidence for well differentiated AC (Fig. 2). In age-matched non-transgenic mice at 31 weeks, no difference was observed in the prostate histopathology between the control and silibinin-treated groups (data not shown). These results suggest that the beginning of silibinin treatment to TRAMP mice bearing tumor was dose-dependently effective in arresting the tumor progression at PIN stage, and thereby reducing the incidence of AC. Further, silibinin also decreased the aggressiveness and severity of AC.
Figure 2. Inhibition of tumor progression by dietary silibinin in TRAMP mice with established prostate tumors.
The prostate tissue samples from the experiment detailed in Figure 1, were histopathologically analyzed for various stages of prostate tumor progression including prostatic intraepithelial neoplasia (PIN), well differentiated and poorly differentiated adenocarcinoma (AC) as described in Materials and Methods. The incidence of each stage is shown from 7–8 mice in each group and values in parentheses indicate percent incidence for that group. Fisher’s exact test was done to compare control group with silibinin-treated groups for each stage of PCa.
Silibinin inhibits tumor invasion and metastasis in TRAMP mice
During the tumor progression in TRAMP, prostate tumor can invade surrounding tissues such as seminal vesicle, and at advanced stages, it metastasizes to the distant organs. At necropsy, in 31-week control group 6 out of 8 (75%) mice had invasive tumor showing local invasion of seminal vesicles (Table 1). In lower and higher doses of silibinin groups, 4 out of 8 (50%) and 1 out of 7 (14%, P=0.04 versus 31-week control) mice had the incidence of prostate tumor invasion of seminal vesicles, respectively, all of which were confirmed by histopathology (Table 1). A phenomenal anti-metastatic effect of silibinin was also observed with no secondary tumors in distant organs (including liver, kidney, lung, lymph node and bone) whereas 31-week control group showed three cases of metastatic lesions, 2 in liver and 1 in kidney, in which one mouse had lesions in both liver and kidney (Table 1). These findings suggest the anti-invasive and anti-metastatic effects of silibinin during PCa progression.
Table 1.
Inhibitory effect of silibinin on prostate tumor invasion of seminal vesicle and distant metastasis in TRAMP mice*
| Treatment groups for TRAMP (20–31 wk) |
Tumor invasion of seminal vesicle |
Secondary tumor in liver |
Secondary tumor in kidney |
|---|---|---|---|
| Control (n=8) | 6 (75%) | 2 (25%) | 1 (12%) |
| 0.5% silibinin (n=8) | 4 (50%) | ||
| 1.0% silibinin (n=7) | 1 (14%) P=0.04 |
The incidence of tumor invasion of seminal vesicle and distant metastasis in liver and kidney are from the study detailed in Figure 1, with each group having 7–8 mice. Values in parentheses show percent incidence for that group. Fisher’s exact test was done to compare control group with silibinin-treated groups for each event of PCa development.
Silibinin inhibits cell proliferation, survival and angiogenesis in established prostate tumor in TRAMP mice
The immunohistochemical analysis of prostate tumors showed inhibition of cell proliferation (up to 40%, P=0.015) and an induction of apoptosis (up to 2-fold, P=0.006) by silibinin (data not shown) which is consistent with its similar effects in other tumor models including PCa (17–20). During PCa progression, the intraductal microvessel density (MVD) increases as a function of tumor grade with the transition from PIN to differentiated and poorly differentiated AC stages, which has been observed in clinical PCa specimens (24, 25). TRAMP mice on control diet for 11 weeks showed prostate tumors with enriched vasculature (Fig. 3A, left) which was strongly reduced by silibinin-diets (Fig. 3A, right). The quantification of MVD showed 12 ± 1 (P<0.01) and 8 ± 2 (P<0.001) microvessels (CD31-positive cells) per field in lower and higher doses of silibinin groups as compared to 20 ± 2 in control group, respectively, which accounts for 40–60% decrease in tumor MVD (Fig. 3B). These results suggest that antiangiogenic effect of silibinin, together with antiproliferative and proapoptotic activities, possibly plays a major role in its inhibitory potential on PCa growth and progression. We further explored the potential molecular antiangiogenic targets of silibinin in tumors and circulating mouse plasma.
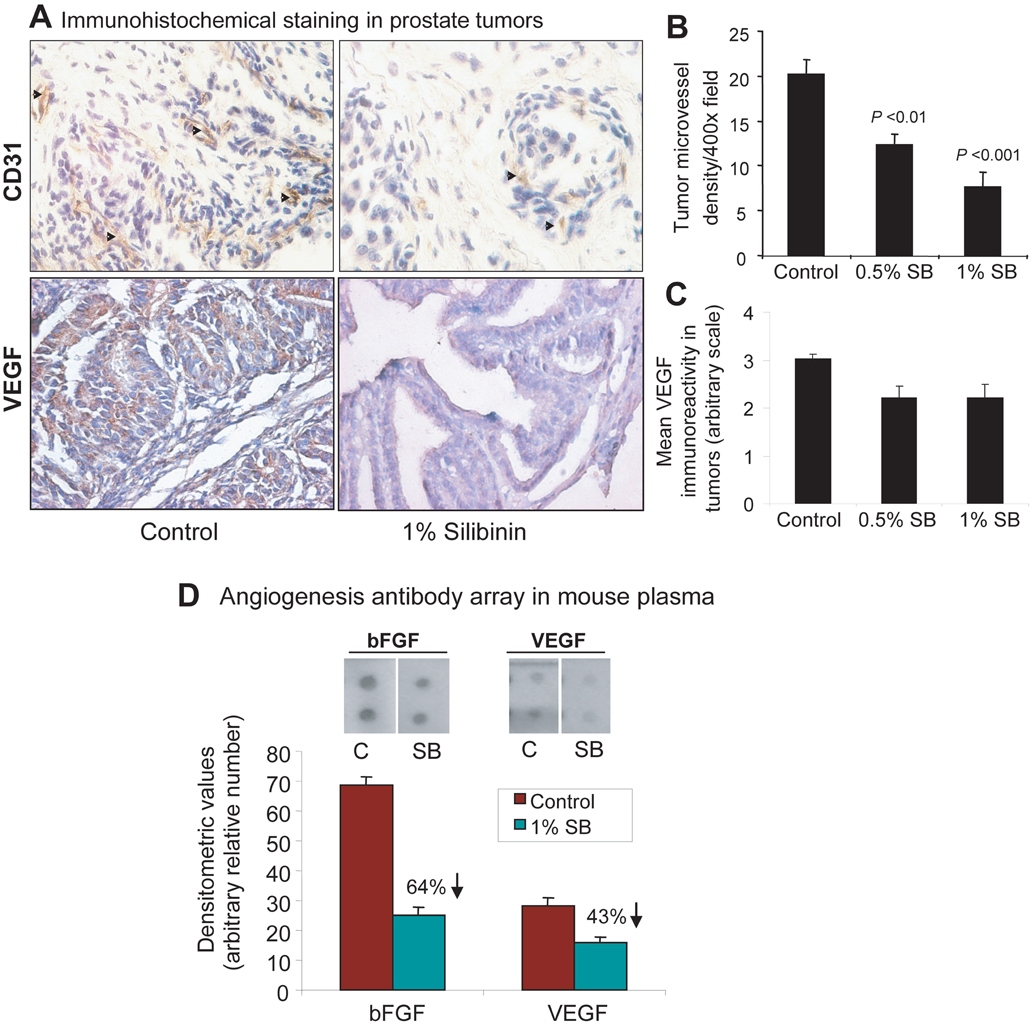
Figure 3. Inhibitory effect of silibinin on tumor angiogenesis in TRAMP mice with established prostate tumors.
A, the prostate tissue samples from the experiment detailed in Figure 1 (at 31 weeks of mice age), were immunohistochemically analyzed for CD31-positive microvessels and VEGF immunoreactivity as detailed in Materials and Methods. The representative pictograph (400x ) for CD31-positive brown-stained endothelial cells (arrow heads) and VEGF expression (brown color) are shown from control and 1% silibinin-treated groups. Quantitative data for (B) microvessel density per 400x microscopic field and (C) level of VEGF expression in prostate tumor are shown as mean ± s.e.m. (error bars) from 7–8 mice in each group. D, a representative plasma sample each from control and 1% silibinin-treated groups was analyzed by angiogenic cytokine array for the circulating levels of angiogenic factors as detailed in Materials and Methods. The marked inhibitory effect of silibinin was observed for bFGF and VEGF as shown in the scanned array for these proteins. The array was quantified by densitometry and represented by the mean ± s.e.m. (error bars) of duplicate dots for each molecule shown in the figure. C, control (31 week); SB, silibinin; CD31, cluster of differentiation 31, and endothelial cell marker; VEGF, vascular endothelial growth factor; bFGF, basic fibroblast growth factor.
Silibinin suppresses the levels of angiogenic growth factors and receptor
Immunohistochemical analysis of tumors showed decreased levels of VEGF immunoreactivity in silibinin-treated group as compared to the control group (Fig. 3A, bottom) which was evident in quantitative data for the same (Fig. 3C). These results were confirmed by immunoblot analysis of tumor samples which showed a marked dose-dependent decrease in the expression levels of VEGF as well as VEGFR-2 by silibinin (Fig. 4A). Further, we did angiogenic cytokine array in mouse plasma from control and 1% silibinin-treated group and found that silibinin strikingly decreases circulating levels of basic fibroblast growth factor (bFGF) by 64% and VEGF by 43% as compared to that of control group (Fig. 3D). These results indicated the predominant antiangiogenic effect of silibinin as a potential mechanism to suppress prostate tumor growth, progression and metastasis. Another important observation was made in this study where approximately 3-fold higher levels of bFGF were noted when compared with the level of VEGF at 31-week age of TRAMP mice which was associated with the advanced and metastatic disease phenotypes.
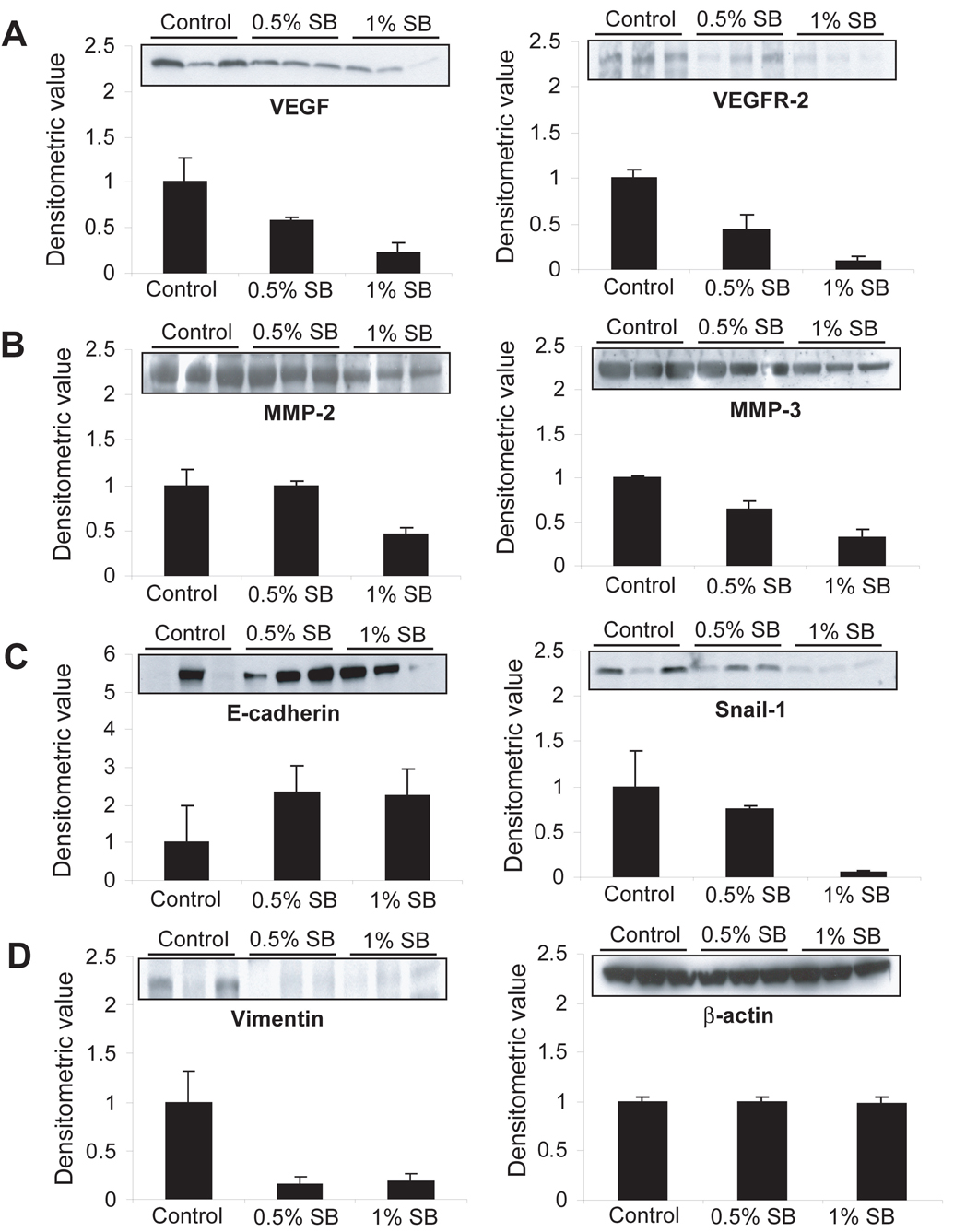
Figure 4. Effect of silibinin on molecular events related to angiogenesis, invasion and epithelial-mesenchymal transition in established prostate tumor in TRAMP mice.
At the end of (31 weeks of mice age) the experiment, three tumors were randomly selected from each control, 0.5% and 1% silibinin-treated groups and analyzed by western immunoblotting for the molecules related to angiogenesis, invasion and metastasis. A marked decrease in expression of (A) VEGF, VEGFR-2, (B) MMP-2, MMP-3, (C, left) snail-1 and (D, left) vimentin, and (C, right) an increase in E-cadherin expression were noted. Membranes were stripped and reprobed for (D, right) beta-actin as loading control, a representative blot is shown. In each case, densitometric analysis of the bands were carried out and data are presented as mean ± s.e.m. (error bars) from three samples for each group below the blot. SB, silibinin; VEGF, vascular endothelial growth factor; VEGFR-2, VEGF receptor-2; MMP, matrix metalloproteinase.
Silibinin suppresses molecular events driving invasion and EMT in prostate tumor
Further, we examined the potential molecular targets of silibinin for its anti-invasive and anti-metastatic activities. At the end of 11 weeks of treatment, three tumor samples from each group were randomly selected for the western immunoblot analysis. As matrix metalloproteinases (MMPs) degrade tissue matrix and facilitate tumor invasion, first we analyzed their levels wherein MMP-2 (P<0.05) and MMP-3 (P<0.01) levels were decreased in tumors by silibinin treatments, specifically at higher dose (Fig. 4B). The loss of E-cadherin and corresponding increase in vimentin causes EMT which is a critical stage in invasion and migration of tumor cells of epithelial origin (10). Snail-1 is known as master regulator of EMT as well as a transcriptional repressor of E-cadherin (11). Silibinin treatments showed increased level of E-cadherin in parallel with a decrease in vimentin, and also decreased the level of snail-1 in tumors (Fig. 4C–D). All the blots were subjected to densitometric analysis of the bands which was adjusted with the level of β-actin that did not show any change for all the samples (Fig. 4D). These results suggest that silibinin could target the expression of MMPs as well as EMT via snail-1-mediated regulation of E-cadherin and vimentin for its anti-invasive and anti-metastatic activities in PCa.
Discussion
The genetically engineered mouse models of cancer have widely been used to understand the mechanisms of multistage carcinogenesis in tissue microenvironment. The TRAMP model closely mimics human PCa growth and progression, and provides an excellent opportunity for the intervention studies (26–28). In this model, androgen-regulated and probasin-driven expression of SV40 large T antigen abrogates p53 and Rb function to spontaneously develop the progressive stages of PCa from PIN to the advanced stages of AC that subsequently leads to metastatic dissemination of tumor cells to distant organs (22, 28–30). Although in humans, PCa is mostly diagnosed at advanced stages; efforts are being made for its early detection through screening for different biomarkers. There could be a clinical situation in which prostate tumor is diagnosed at PIN stage to provide enough time to decide for the treatment options. In the present preclinical intervention study, all mice had palpable tumor when the silibinin treatment started. Therefore, our present findings of antitumor efficacy of silibinin in TRAMP mice with established tumors could have potential clinical significance.
The antitumor effect of silibinin on established prostate tumor was observed in terms of (a) reduction of tumor mass, (b) inhibition of tumor progression from PIN stage with a concomitant decrease in the incidence of AC, (c) reduced severity of AC i.e. well differentiated versus poorly differentiated AC, (d) decreased incidence of tumor invasion of seminal vesicle, and (e) reduced incidence of distant metastasis; these effects were mostly silibinin dose-dependent. We have recently reported that TRAMP mice fed with silibinin diets for 20 weeks, starting at 4 weeks of age where these mice have normal prostatic epithelium (as tumor initiation is hormonally regulated and starts when mice reach at puberty), show suppression of prostate tumor progression with moderate effect on the tumor mass (19). In the present study with established tumor, silibinin suppressed the growth of tumor mass as well as inhibited tumor progression from PIN to the advanced AC stages. Silibinin (at 1% dose) showed absolute absence of poorly differentiated AC while 88% of control mice showed this stage of PCa. These findings suggest that, in addition to its efficacy at early stages of PCa development (19), silibinin could be useful for the intervention of PCa diagnosed at relatively advanced stages. This suggestion is also supported by the fact that silibinin inhibits the growth of human prostate carcinoma DU145 and PC3 tumor xenografts in athymic nude mice model (17, 18).
While exploring the biomarkers of antitumor effects of silibinin in the present study, we observed moderate effects of silibinin in inhibiting tumor cell proliferation and apoptosis induction. At molecular level, silibinin could target IGF-1-IGFBP-3 axis, CDK-cyclin-CDKI and EGF receptor signaling to inhibit cell proliferation; and activate caspase-pathway to induce apoptosis as observed in different studies (4, 17–19). Our next focus was to examine its antiangiogenic effect as we observed its strong inhibitory effects on tumor growth as well as progression, and invasion and metastasis. There are studies that establish the potential role as well as diagnostic and prognostic values of angiogenesis in PCa progression and metastasis (24, 31, 32). Silibinin has shown its direct inhibitory effect on endothelial cells proliferation, survival, migration and capillary organization in cell culture (33). Immunohistochemical analysis of tumors for microvessel showed a significant dose-dependent decrease in MVD by silibinin, which was accompanied by the decrease in VEGF expression. Since circulating levels of angiogenic factor could also relate with the angiogenic stage of the tumor, an array analysis was done for the same in mouse plasma. Two significant observations were made in which control mouse having advanced PCa showed higher levels of bFGF as compared to VEGF, and secondly the levels of both these endothelial cell mitogens were strongly decreased by silibinin treatment. The VEGFR-2, also known as kinase insert domain-containing receptor/fetal liver kinase-1 (KDR/Flk1), is constitutively expressed in endothelial cells and also expressed on tumor cells, which mediates endothelial cell proliferation, differentiation and survival, and modulation of developmental angiogenesis through the interaction with VEGF (34, 35). VEGF binding with VEGFR-2 causes its dimerization and oligomerization, which activates its intrinsic tyrosine kinase activity resulting in activation of down-stream signaling cascades for different biological effects (35, 36). Therefore, we also analyzed the tumor level of VEGFR-2 which was decreased by silibinin treatment similar to its inhibitory effect on VEGF. These findings suggest that silibinin, in addition to lowering the level of bFGF, potentially targets VEGF-VEFGR-2 axis for its antiangiogenic effect in prostate tumors.
Furthermore, we investigated the anti-invasive and anti-metastatic activities of silibinin in an in vivo tumor model. Dietary silibinin showed an inhibition of invasiveness of growing prostate tumors to the seminal vesicle. In the control mice, gross observation showed that most parts of the seminal vesicles were encroached by tumor tissues. Additionally, most of them were also reddish in color indicating the higher level of blood circulation. Such appearances of tumor growth in adjacent seminal vesicle indicated the coupling of invasion with angiogenesis in which the latter can facilitate the former for the expansion of tumor mass. Moreover, angiogenesis and invasion potentially lead to metastatic spread of cancer cells (29–31). At the termination of the experiment, three cases of metastatic lesions were observed in distant organs in control mice which were completely absent in silibinin-treated mice. While analyzing the mechanistic events underlying the invasive and metastatic potential of these prostate tumors and their modulation by silibinin, first we observed higher levels of MMP-2 and MMP-3 in control group which were decreased by silibinin (at higher dose). The mechanisms, by which silibinin decreases the levels of MMPs and VEGFR-2 are yet to be investigated, however, we anticipate that inhibition of mitogenic and angiogenic signaling by silibinin could mediate these effects.
Recently, EMT has been observed to play a critical role in invasion and metastasis of epithelial tumors (10, 11). This process is mainly coordinated by the disappearance of E-cadherin with the concomitant appearance of vimentin (11, 37, 38). Loss of E-cadherin function or expression is implicated in cancer progression and metastasis which leads to decreased strength of cellular adhesion within the tissue, resulting in an increase in cellular motility and invasiveness (37). Subsequently, this could allow cancer cells to cross the basement membrane and invade surrounding tissues as observed in the present study, in which 75% of control mice showed tumor invasion of seminal vesicle. Snail-1 is a transcription factor that is known to transcriptionally repress E-cadherin during the process of EMT (11, 39). Snail-1 can also target MMPs for up-regulation in tumors (40). In the present study, silibinin treatment showed the reappearance of E-cadherin with a concomitant strong decrease in the level of vimentin. As anticipated, silibinin also decreased the level of snail-1, suggesting a possible transcriptional up-regulation of E-cadherin and a concomitant down-regulation of vimentin by silibinin. Overall, these findings showed that silibinin may target E-cadherin-vimentin equilibrium via down-regulation of snail-1 to inhibit EMT and consequently suppress invasion and metastasis of prostate tumor.
In summary, these findings suggest that silibinin’s moderate antiproliferative and proapoptotic effects together with strong antiangiogenic and anti-EMT effects could inhibit prostate tumor growth, progression, invasion and metastasis. Antiangiogenic effect of silibinin could involve the down-regulation of VEGF, VEGFR-2 and bFGF, whereas anti-EMT effect could be mediated via modulating MMPs, E-cadherin and vimentin through snail-1. These findings could have clinical relevance to control human PCa. In this regard, we have recently completed phase I clinical trial with silibinin in PCa patients (41), and a phase II clinical trial is ongoing, where present findings may find a vital practical and greater translational relevance.
Acknowledgments
Grant support: This work was supported by NCI RO1 grant CA102514.
Abbreviations
- PCa
prostate cancer
- TRAMP
transgenic adenocarcinoma of the mouse prostate
- PIN
prostatic intraepithelial neoplasia
- AC
adenocarcinoma
- PCNA
proliferation cell nuclear antigen
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick-end labeling
- VEGF
vascular endothelial growth factor
- VEGFR-2 (KDR/Flk-1)
VEGF receptor-2 (kinase insert domain receptor/fetal liver kinase-1)
- bFGF
basic fibroblast growth factor
- EMT
epithelial-mesenchymal-transition
Footnotes
Statement of Clinical Relevance
The relevance of this study is that silibinin is currently in clinical trials for prostate cancer (PCa) and yet its antitumor effects and mechanisms are not completely understood. Herein, we observed that silibinin suppresses (i) tumor growth by 60%, (ii) progression from PIN to adenocarcinoma (zero incidence of poorly differentiated adenocarcinoma at higher dose), (iii) invasion of seminal vesicle by 80% and (iv) metastasis (zero incidence) in a preclinical transgenic mouse model of prostate cancer (TRAMP) which closely mimics the human PCa progression. Specifically, the drug was administered to mice with palpable prostate tumors (at 20-week age), a similar situation with many clinical cases of human PCa at the time of diagnosis. Antiproliferative, proapoptotic, antiangiogenic and anti-invasive effects of silibinin satisfactorily accounted for why tumors remain smaller, and did not invade the adjacent tissues and metastasize. Silibinin (a) reduced VEGF and VEGFR2 expression in tumors, and circulating titre of VEGF and bFGF, and (b) altered the expression of MMPs as well as snail-1, vimentin and E-cadherin to suppress epithelial-mesenchymal transition (EMT) in tumors. Overall, these results are of clinical relevance and would provide impetus to widen the ongoing clinical trials for prostate cancer intervention with silibinin.
References
- 1.Stoner GD, Wang LS, Zikri N, et al. Cancer prevention with freeze-dried berries and berry components. Semin Cancer Biol. 2007;17:403–410. doi: 10.1016/j.semcancer.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh RP, Agarwal R. Mechanisms of action of novel agents for prostate cancer chemoprevention. Endocr Relat Cancer. 2006;13:751–778. doi: 10.1677/erc.1.01126. [DOI] [PubMed] [Google Scholar]
- 3.Wellington K, Jarvis B. Silymarin: a review of its clinical properties in the management of hepatic disorders. BioDrugs. 2001;15:465–489. doi: 10.2165/00063030-200115070-00005. [DOI] [PubMed] [Google Scholar]
- 4.Singh RP, Agarwal R. Prostate cancer chemoprevention by silibinin: bench to bedside. Mol Carcinog. 2006;45:436–442. doi: 10.1002/mc.20223. [DOI] [PubMed] [Google Scholar]
- 5.Joshua AM, Evans A, Van der Kwast T, et al. Prostatic preneoplasia and beyond. Biochim Biophys Acta. 2008;1785:156–181. doi: 10.1016/j.bbcan.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Jemal R, Siegel E, Ward T, Murray J, Xu MJ. Thun. Cancer Statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 7.Hadaschik BA, Gleave ME. Therapeutic options for hormone-refractory prostate cancer in 2007. Urol Oncol. 2007;25:413–419. doi: 10.1016/j.urolonc.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Neri D, Bicknell R. Tumour vascular targeting. Nat Rev Cancer. 2005;5:436–446. doi: 10.1038/nrc1627. [DOI] [PubMed] [Google Scholar]
- 9.Overall CM, Kleifeld O. Tumour microenvironment - opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 10.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 11.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 12.Zi X, Agarwal R. Silibinin decreases prostate-specific antigen with cell growth inhibition via G1 arrest, leading to differentiation of prostate carcinoma cells: implications for prostate cancer intervention. Proc Natl Acad Sci USA. 1999;96:7490–7495. doi: 10.1073/pnas.96.13.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deep G, Singh RP, Agarwal C, Kroll DJ, Agarwal R. Silymarin and silibinin cause G1 and G2-M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells: a comparison of flavanone silibinin with flavanolignan mixture silymarin. Oncogene. 2006;25:1053–1069. doi: 10.1038/sj.onc.1209146. [DOI] [PubMed] [Google Scholar]
- 14.Tyagi A, Agarwal C, Agarwal R. Inhibition of retinoblastoma protein (Rb) phosphorylation at serine sites and an increase in Rb-E2F complex formation by silibinin in androgen-dependent human prostate carcinoma LNCaP cells: role in prostate cancer prevention. Mol Cancer Ther. 2002;1:525–532. [PubMed] [Google Scholar]
- 15.Zi X, Grasso AW, Kung HJ, Agarwal R. A flavonoid antioxidant, silymarin, inhibits activation of erbB1 signaling and induces cyclin-dependent kinase inhibitors, G1 arrest, and anticarcinogenic effects in human prostate carcinoma DU145 cells. Cancer Res. 1998;58:1920–1929. [PubMed] [Google Scholar]
- 16.Zi X, Zhang J, Agarwal R, Pollak M. Silibinin up-regulates insulin-like growth factor-binding protein 3 expression and inhibits proliferation of androgen-independent prostate cancer cells. Cancer Res. 2000;60:5617–5620. [PubMed] [Google Scholar]
- 17.Singh RP, Dhanalakshmi S, Tyagi AK, Chan DC, Agarwal C, Agarwal R. Dietary feeding of silibinin inhibits advance human prostate carcinoma growth in athymic nude mice and increases plasma insulin-like growth factor-binding protein-3 levels. Cancer Res. 2002;62:3063–3069. [PubMed] [Google Scholar]
- 18.Singh RP, Deep G, Blouin MJ, Pollak MN, Agarwal R. Silibinin suppresses in vivo growth of human prostate carcinoma PC-3 tumor xenograft. Carcinogenesis. 2007;28:2567–2574. doi: 10.1093/carcin/bgm218. [DOI] [PubMed] [Google Scholar]
- 19.Raina K, Blouin MJ, Singh RP, et al. Dietary feeding of silibinin inhibits prostate tumor growth and progression in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2007;67:11083–11091. doi: 10.1158/0008-5472.CAN-07-2222. [DOI] [PubMed] [Google Scholar]
- 20.Singh RP, Gu M, Agarwal R. Silibinin inhibits colorectal cancer growth by inhibiting tumor cell proliferation and angiogenesis. Cancer Res. 2008;68:2043–2050. doi: 10.1158/0008-5472.CAN-07-6247. [DOI] [PubMed] [Google Scholar]
- 21.Singh RP, Deep G, Chittezhath M, et al. Effect of silibinin on the growth and progression of primary lung tumors in mice. J Natl Cancer Inst. 2006;98:846–855. doi: 10.1093/jnci/djj231. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan-Lefko PJ, Chen TM, Ittmann MM, et al. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate. 2003;55:219–237. doi: 10.1002/pros.10215. [DOI] [PubMed] [Google Scholar]
- 23.Shappell SB, Thomas GV, Roberts RL, et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64:2270–2305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- 24.Hrouda D, Nicol DL, Gardiner RA. The role of angiogenesis in prostate development and the pathogenesis of prostate cancer. Urol Res. 2003;30:347–355. doi: 10.1007/s00240-002-0287-9. [DOI] [PubMed] [Google Scholar]
- 25.Bostwick DG, Aquilina JW. Prostatic intraepithelial neoplasia (PIN) and other prostatic lesions as risk factors and surrogate endpoints for cancer chemoprevention trials. J Cell Biochem Suppl. 1996;25:156–164. [PubMed] [Google Scholar]
- 26.Gingrich JR, Greenberg NM. A transgenic mouse prostate cancer model. Toxicol Pathol. 1996;24:502–504. doi: 10.1177/019262339602400414. [DOI] [PubMed] [Google Scholar]
- 27.Gingrich JR, Barrios RJ, Kattan MW, Nahm HS, Finegold MJ, Greenberg NM. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res. 1997;57:4687–4691. [PubMed] [Google Scholar]
- 28.Greenberg NM, DeMayo FJ, Sheppard PC, et al. The rat probasin gene promoter directs hormonally and developmentally regulated expression of a heterologous gene specifically to the prostate in transgenic mice. Mol Endocrinol. 1994;8:230–239. doi: 10.1210/mend.8.2.8170479. [DOI] [PubMed] [Google Scholar]
- 29.Gingrich JR, Barrios RJ, Foster BA, Greenberg NM. Pathologic progression of autochthonous prostate cancer in the TRAMP model. Prostate Cancer Prostatic Dis. 1999;2:70–75. doi: 10.1038/sj.pcan.4500296. [DOI] [PubMed] [Google Scholar]
- 30.Gingrich JR, Barrios RJ, Morton RA, et al. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56:4096–4102. [PubMed] [Google Scholar]
- 31.Huss WJ, Hanrahan CF, Barrios RJ, Simons JW, Greenberg NM. Angiogenesis and prostate cancer: identification of a molecular progression switch. Cancer Res. 2001;61:2736–2743. [PubMed] [Google Scholar]
- 32.Isayeva T, Chanda D, Kallman L, Eltoum IE, Ponnazhagan S. Effects of sustained antiangiogenic therapy in multistage prostate cancer in TRAMP model. Cancer Res. 2007;67:5789–5797. doi: 10.1158/0008-5472.CAN-06-3637. [DOI] [PubMed] [Google Scholar]
- 33.Singh RP, Dhanalakshmi S, Agarwal C, Agarwal R. Silibinin strongly inhibits growth and survival of human endothelial cells via cell cycle arrest and downregulation of survivin, Akt and NF-kappaB: implications for angioprevention and antiangiogenic therapy. Oncogene. 2005;24:1188–1202. doi: 10.1038/sj.onc.1208276. [DOI] [PubMed] [Google Scholar]
- 34.Khosravi Shahi P, Fernández Pineda I. Tumoral angiogenesis: review of the literature. Cancer Invest. 2008;26:104–108. doi: 10.1080/07357900701662509. [DOI] [PubMed] [Google Scholar]
- 35.Shibuya M. Vascular endothelial growth factor (VEGF)-Receptor2: its biological functions, major signaling pathway, and specific ligand VEGF-E. Endothelium. 2006;13:63–69. doi: 10.1080/10623320600697955. [DOI] [PubMed] [Google Scholar]
- 36.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 37.Frixen UH, Behrens J, Sachs M, et al. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991;113:173–185. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lang SH, Hyde C, Reid IN, et al. Enhanced expression of vimentin in motile prostate cell lines and in poorly differentiated and metastatic prostate carcinoma. Prostate. 2002;52:253–263. doi: 10.1002/pros.10088. [DOI] [PubMed] [Google Scholar]
- 39.Batlle E, Sancho E, Francí C, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 40.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 41.Flaig TW, Gustafson DL, Su LJ, et al. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest New Drugs. 2007;25:139–146. doi: 10.1007/s10637-006-9019-2. [DOI] [PubMed] [Google Scholar]