Abstract
Dopamine D2 receptor signalling is strongly implicated in the aetiology of schizophrenia. We have recently characterized the function of three DRD2 SNPs: rs12364283 in the promoter affecting total D2 mRNA expression; rs2283265 and rs1076560, respectively in introns 5 and 6, shifting mRNA splicing to two functionally distinct isoforms, the short form of D2 (D2S) and the long form (D2L). These two isoforms differentially contribute to dopamine signalling in prefrontal cortex and in striatum. We performed a case–control study to determine association of these variants and of their main haplotypes with several schizophrenia-related phenotypes. We demonstrate that the minor allele in the intronic variants is associated with reduced expression of %D2S of total mRNA in post-mortem prefrontal cortex, and with impaired working memory behavioural performance, both in patients and controls. However, the fMRI results show opposite effects in patients compared with controls: enhanced engagement of prefronto-striatal pathways in controls and reduced activity in patients. Moreover, the promoter variant is also associated with working memory activity in prefrontal cortex and striatum of patients, and less robustly with negative symptoms scores. Main haplotypes formed by the three DRD2 variants showed significant associations with these phenotypes consistent with those of the individual SNPs. Our results indicate that the three functional DRD2 variants modulate schizophrenia phenotypes possibly by modifying D2S/D2L ratios in the context of different total D2 density.
Keywords: dopamine, D2 receptor, working memory, prefrontal cortex, striatum
Introduction
Dysregulation of dopamine is centrally implicated in the pathophysiology and treatment of schizophrenia. Phenotypic expression of this brain disorder includes ‘negative symptoms’ such as affective flattening and apathy and working memory (WM) deficits. These symptoms are modulated by dopamine (Weinberger, 1987; Bertolino et al., 2004b). Furthermore, studies in animals have strongly suggested that these symptoms may be modulated by prefrontal D1 and D2 as well as by striatal D2 dopamine receptors (Seamans and Yang, 2004; Wang et al., 2004; Kellendonk et al., 2006; Drew et al., 2007). Studies in healthy humans and in patients with schizophrenia have been consistent with this animal literature (Knable et al., 1997; Okubo et al., 1997; Abi-Dargham et al., 2002; Aalto et al., 2005; Mehta et al., 2008). Moreover, brain imaging studies with radiotracers techniques in schizophrenia have also indicated increased pre-synaptic dopamine activity (Laruelle et al., 1996; Abi-Dargham et al., 2000), which is also weakly correlated with negative symptoms (McGowan et al., 2004). Finally, a recent meta-analysis has suggested that schizophrenia is associated with relatively increased density of D2 receptors (Laruelle, 1998).
D2 receptors exist in two alternatively spliced isoforms, the D2 long (D2L) and the D2 short (D2S) (Khan et al., 1998; Usiello et al., 2000). Using an animal model of D2L knockout, the function of these two isoforms has been well characterized (Usiello et al., 2000). Pre-synaptic D2S receptors are mainly found in meso-cortical projections serving to inhibit dopamine release (Rouge-Pont et al., 2002); post-synaptic D2S receptors inhibit D1 receptor responses (Usiello et al., 2000). D2L receptors are mainly post-synaptic, they are targeted by dopamine antagonists like haloperidol and work in synergy with D1 receptors (Usiello et al., 2000).
Recently, we have characterized three novel SNPs within DRD2, the gene for D2 receptors, one in the promoter, rs12364283 (T > C), and two highly linked SNPs in intron 5, rs2283265 (G > T) and intron 6, rs1076560 (G > T) (Zhang et al., 2007). The C allele of the promoter SNP is associated with enhanced total D2 mRNA expression, while the T allele of both intronic SNPs shifts splicing from D2S to D2L. The intronic T alleles are also associated with reduced WM and attention performance, together with greater (i.e. inefficient) WM cortical and subcortical activity in healthy humans (Zhang et al., 2007).
DRD2 is a logical candidate for genetic studies in schizophrenia. As schizophrenia is a multigenic complex disease, we expect penetrance of genetic factors to be greater for relatively more simple and biologically based phenotypes (Meyer-Lindenberg and Weinberger, 2006). In a case–control study, we have evaluated association of these three functional DRD2 variants and their main haplotypes with progressively more complex and distal phenotypes. Given that dopamine D2 receptor signalling modulates several phenotypes also commonly associated with schizophrenia and given our earlier data (Zhang et al., 2007), we hypothesized that the three DRD2 SNPs of proven functional relevance affect several schizophrenia-related phenotypes, including prefrontal mRNA measured in post-mortem human brain tissue, WM cortical and subcortical activity [measured with blood oxygenation level-dependent (BOLD)-functional MRI (fMRI)] and WM behavioural performance, negative symptoms and diagnosis of schizophrenia.
Material and Methods
Post-mortem human brain tissues
Seventy DNA and RNA samples (35 controls and 35 patients with schizophrenia), extracted from prefrontal cortex autopsy tissues, were obtained from The Stanley Medical Research Institute's brain collection, courtesy of Drs M.B. Knable, E.F. Torrey, M.J. Webster and R.H. Yolken (Chevy Chase, MD, USA). DNA and RNA were extracted as described in (Zhang et al., 2005). cDNA was synthesized with reverse transcriptase II (Invitrogen, Carlsbad, CA, USA) using both gene-specific primers and oligo(dT), as described in (Zhang et al., 2005).
DRD2 mRNA levels by real-time RT–PCR
RT–PCR was performed with β-actin as control, using 50 ng of cDNA, 200 nM primers (as used for SNP rs6275), SYBR-Green, and AmpliTaq Gold and AmpErase UNG on an ABI 7000 (Applied Biosystems, University Park, IL, USA) (Pinsonneault et al., 2006). Cycle thresholds of DRD2 mRNA were normalized to β-actin. Cycle thresholds were also measured for GADPH mRNA, yielding similar results compared with β-actin (r2 = 0.703).
Quantitative detection of splice isoforms
D2L and D2S were measured after PCR amplification by using a Fam-labelled exon 5 forward primer and an exon 7 reverse primer on an ABI 3730 (Applied Biosciences (ABI), Foster City, CA, USA), as described in (Wang et al., 2006). Standard curves were constructed by using varying mixtures of cloned D2L and D2S cDNA.
Subjects for genetic association with diagnosis and symptomatology
All subjects were unrelated, white Caucasians, resident in the province of Bari and provided written informed consent to participate in genetic studies. Protocols and procedures were approved by the local ethics committee and all subjects provided written informed consent to the study. Diagnoses of schizophrenia were made with the structured clinical interview for diagnosis for DSM-IV (First et al., 1996). Healthy subjects also underwent SCID. This sample included 249 healthy subjects and 196 patients with schizophrenia (see Supplementary Table S1 for demographics) who had been on stable pharmacological treatment with anti-psychotics for at least 4 weeks. One hundred and fifty-two patients were also rated with the Positive and Negative Symptoms Scale (PANSS). For association analyses between genotypes and haplotypes with symptom ratings, patients were rank-ordered based on PANSS scores and divided in two groups of high and low symptoms. All patients were receiving treatment with anti-psychotics at the time of study. Forty-four patients did not provide consent to be rated with the PANSS or to the other procedures of the study. For further demographics of patients and controls, see Supplementary Tables S1 and S4.
Subjects for N-back WM behavioural studies
A subsample of 114 healthy subjects (age mean ± SD 28.4 ± 9.6; 51 males) and 91 patients with schizophrenia (age 28.1 ± 7.9; 74 males) provided informed consent and completed WM behavioural assessment. These two groups were matched for age, socio-economical status and handedness, but not for sex (χ2 = 26.9, df 1, P < 0.0001). All subsequent analyses in this sample were covaried for gender.
Subjects for WM fMRI studies
Of the above sample of patients, 46 (age 27.4 ± 6.9; 38 males) also underwent fMRI. Moreover, of the above group of healthy subjects, 90 (age 27.2 ± 7.6; 53 males) were selected so that they would be matched with patients for age, handedness and socio-cultural status [assessed with the Hollingshead Scale, (Hollingshead and Redlich, 1958)]. Healthy subjects were also selected so that the sample would be matched for behavioural performance across genotypes. This further matching was performed so that fMRI activation changes reflect the cortical tuning efficiency phenotype previously described and not task performance per se (Mattay et al., 2003) across genotypes. In this sample, patients and controls were not matched for sex (χ2 = 4.88, df 1, P = 0.02). Therefore, all analyses in this sample were covaried for sex.
Genotype determination
Genotypes were determined as in (Zhang et al., 2007). The SNP in the promoter region (rs12364283) of DRD2 and the two intronic SNPs (rs1076560 and rs2283265) were genotyped in 70 prefrontal cortex samples and in all subjects recruited at the University of Bari. SNPs were analysed with allele-specific PCR primers as described in (Papp et al., 2003) or SNaPshot [Applied Biosciences (ABI)] (Zhang et al., 2005).
After genotype determination, the groups were divided based on DRD2 genotype (Supplementary Table S1). The allelic distribution of all SNPs was in Hardy–Weinberg equilibrium (all χ2 < 1.6, all P > 0.17).
N-back WM paradigm for behavioural performance
Briefly, ‘N-back’ refers to how far back in the sequence of stimuli the subject had to recall. The stimuli consisted of numbers (1–4) shown in random sequence and displayed at the points of a diamond-shaped box. There was a visually paced motor task which also served as a non-memory guided control condition (0-back) that simply required subjects to identify the stimulus currently seen. In the WM conditions, the task required recollection of a stimulus seen one (1-back) or two stimuli (2-back) previously while continuing to encode additionally incoming stimuli. Performance data were recorded as the number of correct responses (accuracy) and as reaction time.
Statistical analyses
Statistical associations of alleles and genotypes with diagnosis and symptomatology were evaluated with χ2. Associations with WM behavioural performance (accuracy and reaction time at 0-back, 1-back, and 2-back) were evaluated with repeated measures factorial analysis of covariance (ANCOVA; covarying for gender) using genotypes and diagnosis as independent variables. Associations with DRD2 expression were evaluated with factorial ANOVA using genotype and diagnosis as independent variables for mRNA levels. All post hoc analyses were performed with Fisher LSD.
All haplotype analyses were performed with Helixtree (http://goldenhelix.com). Haplotypes were estimated with an Expectation/Maximization algorithm. Association of haplotypes with phenotypes were calculated with step-wise logistic regression (>3% frequency) using a window with fixed size of 3. For association of haplotypes with WM performance, patients and controls were separately rank-ordered in high and low performers and diagnosis was used as a non-genetic covariate. Similarly, for association with negative symptoms scores, patients were rank-ordered in high and low scores.
Acquisition of the N-back WM fMRI data
Only the 0-back and the 2-back conditions were acquired during fMRI. Each subject was scanned using the same 3.0 Tesla MR scanner with a gradient-echo echo planar imaging sequence using the following parameters: 20 contiguous slices echo time = 30 ms, repetition time = 2000 ms; field of view 24 cm; matrix 64 × 64 (Bertolino et al., 2004a, b). We used a simple block design in which each block consisted of eight alternating 0-back and 2-back conditions (each lasting 30 s), obtained in 4 min and 8 s, 120 whole-brain fMRI volumes. The first four scans at the beginning of each time series were acquired to allow the signal to reach a steady state and were not included in the final analysis.
Pre-processing and statistical analyses of the fMRI data
Analysis of the fMRI data was completed using Statistical Parametric Mapping 5 (SPM5; http://www.fil.ion.ucl.ac.uk/spm). Images for each subject were realigned to the first volume in the time series to correct for head motion (<2.5 mm of translation, <1.5° rotation), spatially normalized into a standard stereotactic space (Montreal Neurological Institute, MNI, template) using a 12 parameter affine model and smoothed to minimize noise and residual differences in gyral anatomy with a Gaussian filter, set at 10 mm full-width at half-maximum. Voxel-wise signal intensities were ratio normalized to the whole-brain global mean. For each experimental condition, a box car model convolved with the haemodynamic response function (HRF) at each voxel was modelled. Pre-determined condition effects at each voxel were calculated using a t-statistic, producing a statistical image for the contrast of 2-back versus 0-back. All these individual contrast images were then used in second-level random effects models to determine task-specific regional responses at the group level for the entire sample. To detect the association between DRD2 genotypes and diagnosis with fMRI activation, the contrast images of all subjects were included in a factorial ANCOVA (covarying for gender) with SPM5. To detect association of haplotypes with fMRI activation, we performed separate multiple regression analyses for patients and controls within SPM5 using as covariate of interests the probabilities of haplotypes (rs12364283, rs2283265 and rs1076560) with frequency >3% and gender as a covariate of no interest (Meyer-Lindenberg et al., 2006). Because of our strong a priori hypothesis based on earlier fMRI studies of WM as well as on known projections of dopamine neurons (Grace, 1991; Callicott et al., 1999, 2000; Bertolino et al., 2004b, 2006a, b), a statistical threshold of P < 0.005, k = 6, with a further Family Wise Error (FWE) small volume correction for multiple comparisons (using a 12 mm radius sphere centered around the coordinates in DLPFC and in striatum published in previous studies [left middle frontal gyrus x −22, y 44, z 3 (Callicott et al., 2003); right middle frontal gyrus x 30, y 45, z 9 (Callicott et al., 2000); left striatum x −11, y 1, z 14 (Simon et al., 2002); right striatum x 20, y −4, z 18 (Tan et al., 2007)]) P = 0.05 was used to identify significant responses for all comparisons in these anatomical regions (Meyer-Lindenberg et al., 2008). Because we did not have a priori hypotheses regarding the activity of brain regions outside of DLPFC and striatum, we used a statistical threshold of P = 0.05, corrected for multiple comparisons across all voxels, for these whole-brain comparisons. All fMRI results are reported in Talairach coordinates system. Uncorrected results from non a priori regions that did not survive correction for multiple comparisons are reported in Supplementary Table S3.
Results
Association with prefrontal mRNA
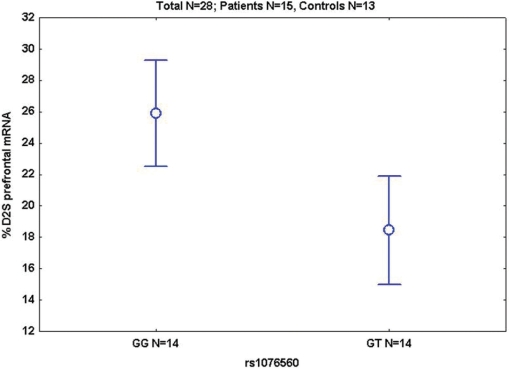
In a post-mortem sample of prefrontal cortex we found no association between total D2 mRNA and diagnosis or genotype (all P > 0.4). However, intronic SNP rs1076560 (or rs2283265, being in complete LD) was strongly associated with D2S/D2L ratios, with GG subjects showing higher % D2S mRNA in prefrontal cortex than GT subjects (P = 0.004, Fig. 1), consistent with our earlier independent results (Zhang et al., 2007). No other significant association was found (all P > 0.1). Therefore, GG genotype at the intronic SNPs is associated with greater prefrontal expression of D2S regardless of diagnosis.
Fig. 1.
Mean ± 0.95 confidence intervals (CIs) of prefrontal %D2S mRNA in patients and controls; S = short.
Association with WM brain activity measured with fMRI
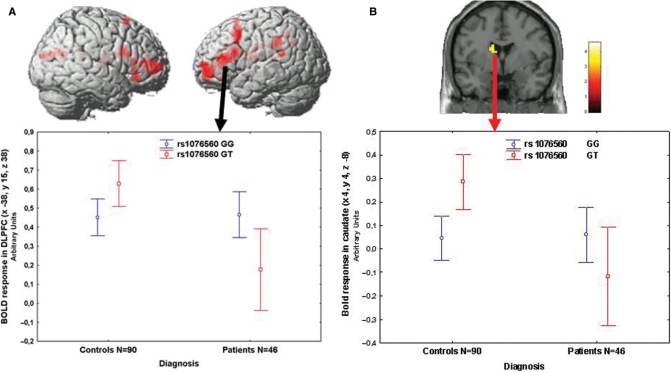
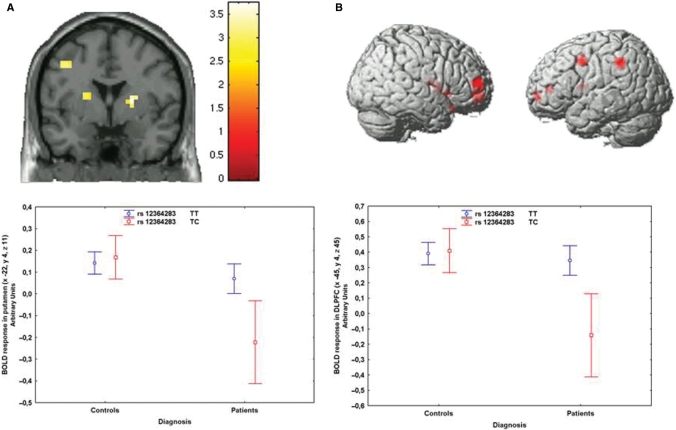
Next, we evaluated associations with brain activity during WM using the N-back task in patients and controls (2-back, all P < 0.05, Family Wise Error small volume correction for multiple comparisons). In this sample, WM behavioural performance was matched across genotypes (P > 0.5) but patients performed worse than controls (P < 0.001). ANCOVA (covarying for gender) demonstrated that patients had reduced activity in the WM network (Supplementary Tables S2 and S3), no effect of rs1076560 genotype, and an interaction between diagnosis and genotype. GT healthy subjects had greater activity in prefrontal cortex and the caudate head, while the opposite effect was evident in patients (Supplementary Table S2, Fig. 2A and B). A similar analysis with rs2283265 did not demonstrate statistically significant effects. Analysis with rs1236428 revealed an interaction between genotype and diagnosis, showing that TC patients have significantly less BOLD activity than TT patients (Fig. 3A and B).
Fig. 2.
(A) 3D rendering (above, image thresholded at P < 0.005, uncorrected) and mean ± 0.95 CIs of BOLD response (below) of the interaction between diagnosis and rs1076560 genotype on WM prefrontal activity. (B) Coronal section (above) and mean ± 0.95 CIs of BOLD response (below) of the interaction between diagnosis and rs1076560 genotype on WM activity in the left head of the caudate.
Fig. 3.
(A) Coronal section (image thresholded at P < 0.005, uncorrected) of the BOLD response of the interaction between diagnosis and rs12364283 genotype on WM activity in the striatum (above). Mean ± 0.95 CIs of the BOLD response in left putamen in patients and controls separated for rs12364283 genotype (below). (B) 3D rendering (image thresholded at P < 0.005, uncorrected) of BOLD response of the interaction between diagnosis and rs12364283 genotype on WM prefrontal activity (above). Mean ± 0.95 CIs of the BOLD response in left DLPFC in patients and controls separated for rs12364283 genotype (below).
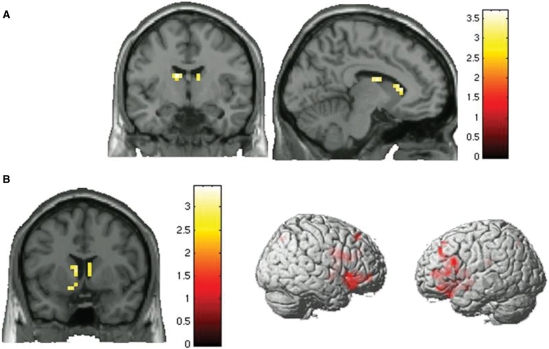
The DRD2 SNPs (rs12364283, rs2283265, rs1076560) form three haplotypes with frequency >5%: TGG, CTT, and CGG (Supplementary Table S1). Multiple regression analyses in patients demonstrated that the TGG haplotype predicted greater activity bilaterally in the caudate (Fig. 4A, Supplementary Table S2). In contrast, TGG predicted lower activity in the WM network in healthy subjects (more efficient, Fig. 4B, Supplementary Table S2). The other haplotypes had no significant effect in either group. These results reveal opposite effects of rs1076560 on brain activity in patients versus healthy subjects and additionally show an effect of promoter rs12364283 on WM pathways in patients. Consistently, the TGG haplotype had opposite effects in patients and controls.
Fig. 4.
(A) Coronal and sagittal sections (images thresholded at P < 0.005, uncorrected) of the BOLD response of the multiple regression in patients with schizophrenia. The TGG (rs12364283, rs2283265 and rs1076560) haplotype has a positive relationship with BOLD activity in caudate during WM. (B) Coronal section and 3D rendering (images thresholded at P < 0.005, uncorrected) of the BOLD response of the multiple regression in controls. The TGG (rs12364283, rs2283265 and rs1076560) haplotype has a negative relationship with BOLD activity in caudate and prefrontal cortex during WM.
Association with WM behavioural performance
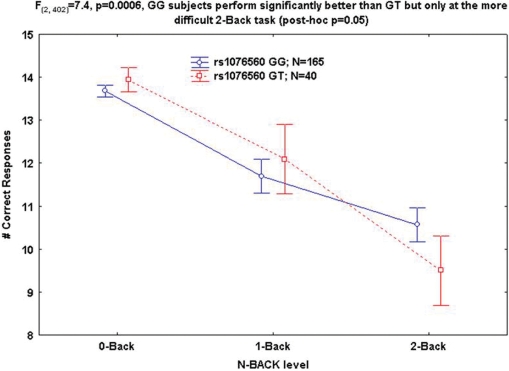
Next, we evaluated associations with WM performance using the N-back task with increasing difficulty (0-back as control, 1-back and 2-back). ANCOVA indicated an interaction between rs1076560 genotype and WM difficulty suggesting that GG subjects perform significantly better than GT but only at the more difficult 2-back task (post hoc P = 0.05, Fig. 5). No genotype by diagnosis by WM load interaction was found. rs2283265 behaved similarly [interaction between genotype and WM load: F(2,402) = 6.5, P = 0.001], while no effect was evident with rs12364283 (all P > 0.2). Moreover, the CTT haplotype was associated with low WM performance (frequency 0.06 in both groups, χ2 = 8.9, coefficient = 2.72, P = 0.002, odds ratio 15.2). These results reveal consistent effects of rs1076560 and of the CTT haplotype on WM behavioural performance at higher cognitive load.
Fig. 5.
Mean ± 0.95 CI of the interaction between WM behavioural performance and rs1076560 genotype.
Association with symptoms and diagnosis
Negative symptoms (rated with PANSS) were weakly associated with promoter rs1236428 (Supplementary Table S1, P = 0.07). This association was more pronounced for haplotype TGG (P = 0.03), showing that the T allele of rs1236428 was more frequent in patients with low negative symptoms (Supplementary Table S1). No association was found with the other symptom scores. In 249 controls and 196 patients with schizophrenia (Supplementary Table S1) genotyped for these three SNPs we did not find significant association between diagnosis and alleles, genotypes or haplotypes.
Discussion
The results of the present study indicate that DRD2 variants are associated with several schizophrenia phenotypes known to be modulated by dopamine, while no association with diagnosis was evident in this relatively small sample. GG subjects for rs1076560 and for rs2283265 have greater relative D2S mRNA in prefrontal cortex than GT subjects, regardless of diagnosis. The fMRI results demonstrate association of rs1076560 (which is strongly linked to rs2283265) with prefrontal and striatal activity during WM, with opposite effects in patients and controls. In controls, subjects heterozygous for rs1076560 have reduced performance and greater brain activity, i.e. they are inefficient. In patients, who perform worse than controls and have reduced brain activity, heterozygote subjects have reduced performance and reduced activity, i.e. they do not fully engage prefrontal–striatal resources. Thus the GG genotype is advantageous in both controls and patients. Similar analysis with rs1236428 revealed that TT patients have greater BOLD activity than TC patients. Consistent with the single SNP results, the TGG haplotype (rs12364283, rs2283265 and rs1076560) had opposite effects on brain activity in patients and controls. The single SNP and haplotype associations with WM behaviour are consistent with the fMRI data. Finally, the promoter SNP weakly predicted negative symptoms by itself and more strongly when part of a haplotype including the two intronic SNPs (again TGG). These results may further suggest that the T allele in the promoter SNP is advantageous in patients especially when in combination with the G allele from both intronic SNPs. All these results together indicate that genetically determined D2 receptor signalling modulates manifestation of several phenotypes in schizophrenia.
Dopamine modulation of WM is very well known. While a role for D1 receptors has long been recognized, more recent studies have demonstrated that D2 receptors are also involved. Behavioural and electrophysiological experiments in non-human primates indicate that D2 agonists and antagonists may, respectively, increase and decrease tuning of prefrontal neurons to the task at hand, especially during the response phase (Arnsten et al., 1995; Williams and Goldman-Rakic, 1995; Wang et al., 2004). Several studies have demonstrated that D2 agonists and antagonists, respectively, improve and deteriorate WM performance also in humans (Mehta and Riedel, 2006). Consistently, D2 agonists and antagonists, respectively, reduce and increase activity in prefrontal cortex and striatum during WM (Kimberg et al., 2001; Mehta et al., 2003), suggesting that D2 receptor modulation modifies tuning of neuronal resources. D2 receptors are found with different density and localization in prefrontal cortex and in striatum. While D2S receptors are found mainly, but not exclusively, in the pre-synaptic environment with relatively greater abundance in prefrontal cortex; D2L receptors are mainly found postsynaptically, being relatively more abundant in striatum. The effects we have measured in vivo may have resulted from prefrontal and/or striatal modulation of dopamine signalling. Earlier studies have indicated that post-synaptic D1 receptors are negatively modulated by post-synaptic D2S, while they act in synergy with post-synaptic D2L (Usiello et al., 2000). Thus, we propose that subjects homozygous for the G allele at the two intronic SNPs have a more favourable modulation of prefrontal D1 receptors based on greater D2S/D2L ratios. This interpretation is consistent with our mRNA data in prefrontal cortex suggesting that a post-synaptic mechanism may be relevant. A prefrontal pre-synaptic mechanism may also be involved with the known modulation by pre-synaptic D2 receptors of NMDA and of GABA A receptors of pyramidal neurons and interneurons (Tseng and O'Donnell, 2007; Tseng et al., 2007). Moreover, our results may also be explained by modulation of dopamine signalling in the striatum within the cortico-striato-thalamo-cortical network. Whereas the overall D2 receptor number modulates GABA-mediated inhibition of striatal neurons, inhibition of glutamate release preferentially involves the D2S variant (Centonze et al., 2003, 2004). Therefore, reduced D2S expression is expected to increase excitability of striatal medium spiny neurons. Future studies are needed to resolve the molecular/neuronal mechanisms accounting for our effects. On the other hand, the opposite effects manifested by the intronic SNPs in patients and controls, the interaction between the promoter SNP and diagnosis of schizophrenia, the opposite effects of haplotypes with brain activity during WM are consistent with the assumption that dopamine transmission in schizophrenia is disrupted (Carlsson et al., 1999). Thus, these interactions may arise from different dopamine activity between patients and controls.
The weak association with negative symptoms of the promoter SNP also may suggest that genetically determined total number of D2 receptors may be clinically relevant, especially in the context of homozygosity for the G allele in the two intronic SNPs. Once again, the T allele of the promoter SNP and the G allele of the intronic SNPs seem to be beneficial in that they are associated with lower negative symptoms. These data are consistent with some evidence in earlier cross-sectional and longitudinal studies (Himei et al., 2002; Lane et al., 2004) indicating association between DRD2 polymorphisms and negative symptoms. On the other hand, in this case–control study we did not find any evidence for association of the three variants with diagnosis of schizophrenia. A recent meta-analysis of all case–control studies investigating the DRD2 Ser311Cys (rs1801028) variant has indicated association with schizophrenia with an odds ratio of 1.3 for the Cys allele (Glatt et al., 2003). As rs1801028 is in strong LD with the intron 5/6 SNPs, we propose that the main functional effects may be exerted by their effect on splicing, rather than the imputed amino acid change caused by the non-synonymous SNP. This meta-analysis also suggested that the effect of this DRD2 polymorphism on schizophrenia risk is reliable and uniform across populations, although the magnitude of its effect is small. Similarly, another recent meta-analysis also suggested that rs1801028 may be implicated in risk for schizophrenia (Allen et al., 2008). Moreover, another recent family-based sample in Han-Chinese has indicated association of DRD2 SNPs and haplotypes with schizophrenia involving the intron 5 SNP rs2283265 by itself and as part of a haplotype block as well as a haplotype block including rs1801028 (Glatt et al., 2008). Of note, these two SNPs are in high LD. Finally, DRD2 has also been implicated in linkage areas suggested in the genome-wide linkage meta-analysis (Lewis et al., 2003). Thus, these previous studies and the LD structure of the gene suggest that DRD2 may be implicated in risk for schizophrenia and that our inability to demonstrate statistically significant association with diagnosis is related to the relatively small number of cases and controls.
A limitation of the present study has to be acknowledged. Since all our patients were treated with anti-psychotic drugs, we cannot definitively exclude an effect of treatment on the measured phenotypes. However, several factors indicate that the effect of treatment is not a confounder of our results. First, genotype groups did not differ in terms of chlorpromazine equivalents (all P > 0.1). Second, all patients had been on stable treatment for at least 1 month. Third, there was no correlation between WM performance or brain activity with chlorpromazine equivalents or between lifetime exposure to anti-psychotics and %D2S mRNA (data not shown, all P > 0.3). Fourth, our prefrontal post-mortem data demonstrate unchanged total D2 mRNA. Previous studies have indicated that treatment with anti-psychotics increases total D2 mRNA thus suggesting that pharmacological treatment is unlikely to account for our results (Martres et al., 1992; D'Souza et al., 1997; Lidow et al., 1997).
In conclusion, our results indicate associations of three DRD2 variants with prefronto-striatal phenotypes of relevance to schizophrenia, showing that endo-phenotypes may help identify genetic variants affecting disease presentation (Hall et al., 2006). The potential utility of these genetic variants as biomarkers in schizophrenia therapy requires further study.
Supplementary material
Supplementary material is available at Brain online.
Funding
National Institute of Health Research (DA022199 and DA021620 to W.S.).
Supplementary Material
Acknowledgements
We would like to acknowledge Riccarda Lomuscio, BA, Rita Masellis, BA, Miriam Rizzo, MD, Luca Ursini, MD, Apostolos Papazacharias, MD, Luciana Lobianco, MD, for data acquisition. We also would like to express our thanks to all patients and their families for having participated to the study as well as to our colleagues who referred them to us.
Glossary
Abbreviations
- BOLD
blood oxygenation level-dependent
- D2L
D2 long
- D2S
D2 short
- PANSS
Positive and Negative Symptoms Scale
- WM
working memory
References
- Aalto S, Bruck A, Laine M, Nagren K, Rinne JO. Frontal and temporal dopamine release during working memory and attention tasks in healthy humans: a positron emission tomography study using the high-affinity dopamine D2 receptor ligand [11C]FLB 457. J Neurosci. 2005;25:2471–7. doi: 10.1523/JNEUROSCI.2097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22:3708–19. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA. 2000;97:8104–9. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NC, Bagade S, McQueen MB, Ioannidis JP, Kavvoura FK, Khoury MJ, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40:827–34. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Steere JC, Goldman-Rakic PS. Dopamine D2 receptor mechanisms contribute to age-related cognitive decline: the effects of quinpirole on memory and motor performance in monkeys. J Neurosci. 1995;15:3429–39. doi: 10.1523/JNEUROSCI.15-05-03429.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Blasi G, Caforio G, Latorre V, De Candia M, Rubino V, et al. Functional lateralization of the sensorimotor cortex in patients with schizophrenia: effects of treatment with olanzapine. Biol Psychiatry. 2004a;56:190–7. doi: 10.1016/j.biopsych.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Blasi G, Latorre V, Rubino V, Rampino A, Sinibaldi L, et al. Additive effects of genetic variation in dopamine regulating genes on working memory cortical activity in human brain. J Neurosci. 2006a;26:3918–22. doi: 10.1523/JNEUROSCI.4975-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Caforio G, Blasi G, De Candia M, Latorre V, Petruzzella V, et al. Interaction of COMT Val108/158 Met genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. Am J Psychiatry. 2004b;161:1798–805. doi: 10.1176/ajp.161.10.1798. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Caforio G, Petruzzella V, Latorre V, Rubino V, Dimalta S, et al. Prefrontal dysfunction in schizophrenia controlling for COMT Val(158)Met genotype and working memory performance. Psychiatry Res. 2006b;147:221–6. doi: 10.1016/j.pscychresns.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–92. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–6. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160:2209–15. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Carlsson ML. Neurotransmitter interactions in schizophrenia–therapeutic implications. Biol Psychiatry. 1999;46:1388–95. doi: 10.1016/s0006-3223(99)00117-1. [DOI] [PubMed] [Google Scholar]
- Centonze D, Grande C, Usiello A, Gubellini P, Erbs E, Martin AB, et al. Receptor subtypes involved in the presynaptic and postsynaptic actions of dopamine on striatal interneurons. J Neurosci. 2003;23:6245–54. doi: 10.1523/JNEUROSCI.23-15-06245.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Gubellini P, Usiello A, Rossi S, Tscherter A, Bracci E, et al. Differential contribution of dopamine D2S and D2L receptors in the modulation of glutamate and GABA transmission in the striatum. Neuroscience. 2004;129:157–66. doi: 10.1016/j.neuroscience.2004.07.043. [DOI] [PubMed] [Google Scholar]
- D'Souza U, McGuffin P, Buckland PR. Antipsychotic regulation of dopamine D1, D2 and D3 receptor mRNA. Neuropharmacology. 1997;36:1689–96. doi: 10.1016/s0028-3908(97)00163-9. [DOI] [PubMed] [Google Scholar]
- Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S, et al. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J Neurosci. 2007;27:7731–9. doi: 10.1523/JNEUROSCI.1736-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. New York: Biometrics Research; 1996. User's guide for the structured interview for DSM-IV axis I disorders-Research version. [Google Scholar]
- Glatt SJ, Faraone SV, Lasky-Su JA, Kanazawa T, Hwu HG, Tsuang MT. Family-based association testing strongly implicates DRD2 as a risk gene for schizophrenia in Han Chinese from Taiwan. Mol Psychiatry. 2008 Mar 11; doi: 10.1038/mp.2008.30. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt SJ, Faraone SV, Tsuang MT. Meta-analysis identifies an association between the dopamine D2 receptor gene and schizophrenia. Mol Psychiatry. 2003;8:911–5. doi: 10.1038/sj.mp.4001321. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Hall J, Whalley HC, Job DE, Baig BJ, McIntosh AM, Evans KL, et al. A neuregulin 1 variant associated with abnormal cortical function and psychotic symptoms. Nat Neurosci. 2006;9:1477–8. doi: 10.1038/nn1795. [DOI] [PubMed] [Google Scholar]
- Himei A, Koh J, Sakai J, Inada Y, Akabame K, Yoneda H. The influence on the schizophrenic symptoms by the DRD2Ser/Cys311 and -141C Ins/Del polymorphisms. Psychiatry Clin Neurosci. 2002;56:97–102. doi: 10.1046/j.1440-1819.2002.00935.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB, Redlich FC. Social class and mental illness. New York: Wiley; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–15. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Khan ZU, Mrzljak L, Gutierrez A, de la Calle A, Goldman-Rakic PS. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci USA. 1998;95:7731–6. doi: 10.1073/pnas.95.13.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberg DY, Aguirre GK, Lease J, D’Esposito M. Cortical effects of bromocriptine, a D-2 dopamine receptor agonist, in human subjects, revealed by fMRI. Hum Brain Mapp. 2001;12:246–57. doi: 10.1002/1097-0193(200104)12:4<246::AID-HBM1019>3.0.CO;2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knable MB, Egan MF, Heinz A, Gorey J, Lee KS, Coppola R, et al. Altered dopaminergic function and negative symptoms in drug-free patients with schizophrenia. [123I]-iodobenzamide SPECT study. Br J Psychiatry. 1997;171:574–7. doi: 10.1192/bjp.171.6.574. [DOI] [PubMed] [Google Scholar]
- Lane HY, Lee CC, Chang YC, Lu CT, Huang CH, Chang WH. Effects of dopamine D2 receptor Ser311Cys polymorphism and clinical factors on risperidone efficacy for positive and negative symptoms and social function. Int J Neuropsychopharmacol. 2004;7:461–70. doi: 10.1017/S1461145704004389. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging dopamine transmission in schizophrenia. A review and meta-analysis. Q J Nucl Med. 1998;42:211–21. [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D'Souza CD, Erdos J, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA. 1996;93:9235–40. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: schizophrenia. Am J Hum Genet. 2003;73:34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS, Elsworth JD, Goldman-Rakic PS. Down-regulation of the D1 and D5 dopamine receptors in the primate prefrontal cortex by chronic treatment with antipsychotic drugs. J Pharmacol Exp Ther. 1997;281:597–603. [PubMed] [Google Scholar]
- Martres MP, Sokoloff P, Giros B, Schwartz JC. Effects of dopaminergic transmission interruption on the D2 receptor isoforms in various cerebral tissues. J Neurochem. 1992;58:673–9. doi: 10.1111/j.1471-4159.1992.tb09770.x. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100:6186–91. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan S, Lawrence AD, Sales T, Quested D, Grasby P. Presynaptic dopaminergic dysfunction in schizophrenia: a positron emission tomographic [18F]fluorodopa study. Arch Gen Psychiatry. 2004;61:134–42. doi: 10.1001/archpsyc.61.2.134. [DOI] [PubMed] [Google Scholar]
- Mehta MA, McGowan SW, Lawrence AD, Aitken MR, Montgomery AJ, Grasby PM. Systemic sulpiride modulates striatal blood flow: relationships to spatial working memory and planning. Neuroimage. 2003;20:1982–94. doi: 10.1016/j.neuroimage.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Montgomery AJ, Kitamura Y, Grasby PM. Dopamine D2 receptor occupancy levels of acute sulpiride challenges that produce working memory and learning impairments in healthy volunteers. Psychopharmacology. 2008;196:157–65. doi: 10.1007/s00213-007-0947-0. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Riedel WJ. Dopaminergic enhancement of cognitive function. Curr Pharm Des. 2006;12:2487–500. doi: 10.2174/138161206777698891. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Nichols T, Callicott JH, Ding J, Kolachana B, Buckholtz J, et al. Impact of complex genetic variation in COMT on human brain function. Mol Psychiatry. 2006;11:867–77, . doi: 10.1038/sj.mp.4001860. 797. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Nicodemus KK, Egan MF, Callicott JH, Mattay V, Weinberger DR. False positives in imaging genetics. Neuroimage. 2008;40:655–61. doi: 10.1016/j.neuroimage.2007.11.058. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–27. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, et al. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385:634–6. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- Papp AC, Pinsonneault JK, Cooke G, Sadee W. Single nucleotide polymorphism genotyping using allele-specific PCR and fluorescence melting curves. Bio Techniques. 2003;34:1067–72. doi: 10.2144/03345dd03. [DOI] [PubMed] [Google Scholar]
- Pinsonneault JK, Papp AC, Sadee W. Allelic mRNA expression of X-linked monoamine oxidase a (MAOA) in human brain: dissection of epigenetic and genetic factors. Hum Mol Genet. 2006;15:2636–49. doi: 10.1093/hmg/ddl192. [DOI] [PubMed] [Google Scholar]
- Rouge-Pont F, Usiello A, Benoit-Marand M, Gonon F, Piazza PV, Borrelli E. Changes in extracellular dopamine induced by morphine and cocaine: crucial control by D2 receptors. J Neurosci. 2002;22:3293–301. doi: 10.1523/JNEUROSCI.22-08-03293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Simon SR, Meunier M, Piettre L, Berardi AM, Segebarth CM, Boussaoud D. Spatial attention and memory versus motor preparation: premotor cortex involvement as revealed by fMRI. J Neurophysiol. 2002;88:2047–57. doi: 10.1152/jn.2002.88.4.2047. [DOI] [PubMed] [Google Scholar]
- Tan HY, Chen Q, Goldberg TE, Mattay VS, Meyer-Lindenberg A, Weinberger DR, et al. Catechol-O-methyltransferase Val158Met modulation of prefrontal-parietal-striatal brain systems during arithmetic and temporal transformations in working memory. J Neurosci. 2007;27:13393–401. doi: 10.1523/JNEUROSCI.4041-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Lipska BK, O’Donnell P. Post-pubertal disruption of medial prefrontal cortical dopamine-glutamate interactions in a developmental animal model of schizophrenia. Biol Psychiatry. 2007;62:730–8. doi: 10.1016/j.biopsych.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. D2 dopamine receptors recruit a GABA component for their attenuation of excitatory synaptic transmission in the adult rat prefrontal cortex. Synapse. 2007;61:843–50. doi: 10.1002/syn.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, LeMeur M, et al. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- Wang D, Papp AC, Binkley PF, Johnson JA, Sadee W. Highly variable mRNA expression and splicing of L-type voltage-dependent calcium channel alpha subunit 1C in human heart tissues. Pharmacogenet Genomics. 2006;16:735–45. doi: 10.1097/01.fpc.0000230119.34205.8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–6. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–9. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–5. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R, et al. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci USA. 2007;104:20552–7. doi: 10.1073/pnas.0707106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang D, Johnson AD, Papp AC, Sadee W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem. 2005;280:32618–24. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.