Abstract
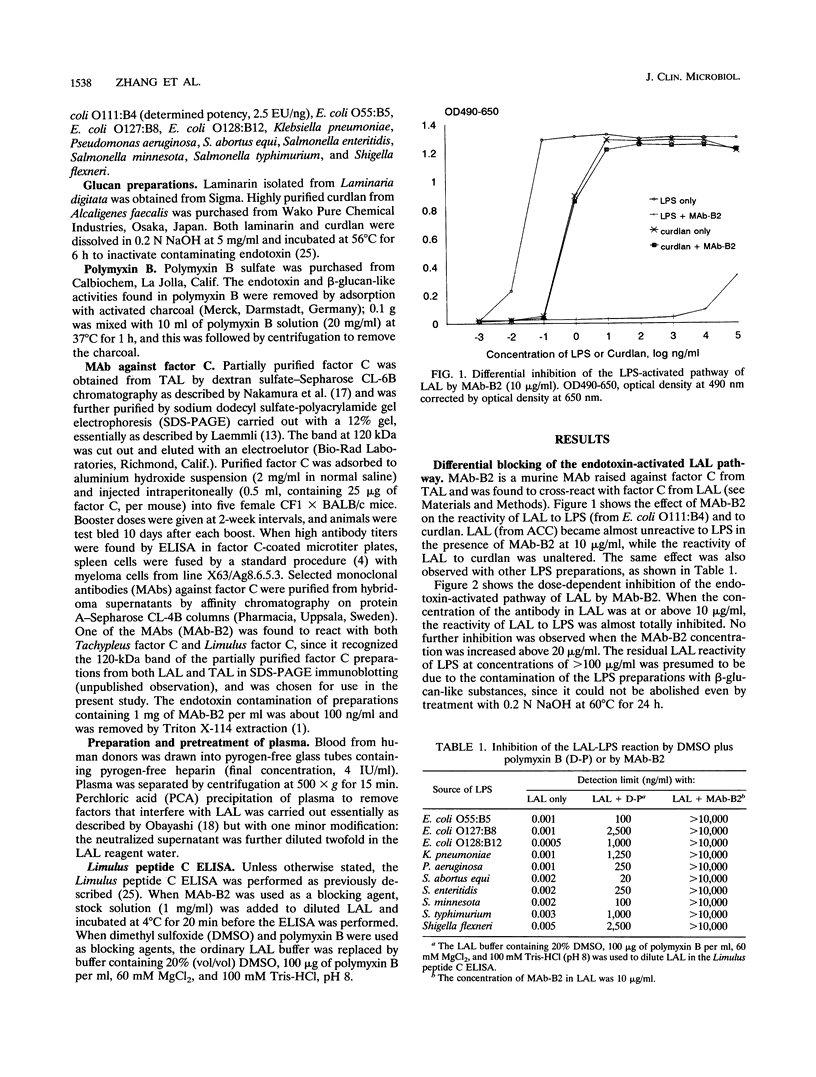
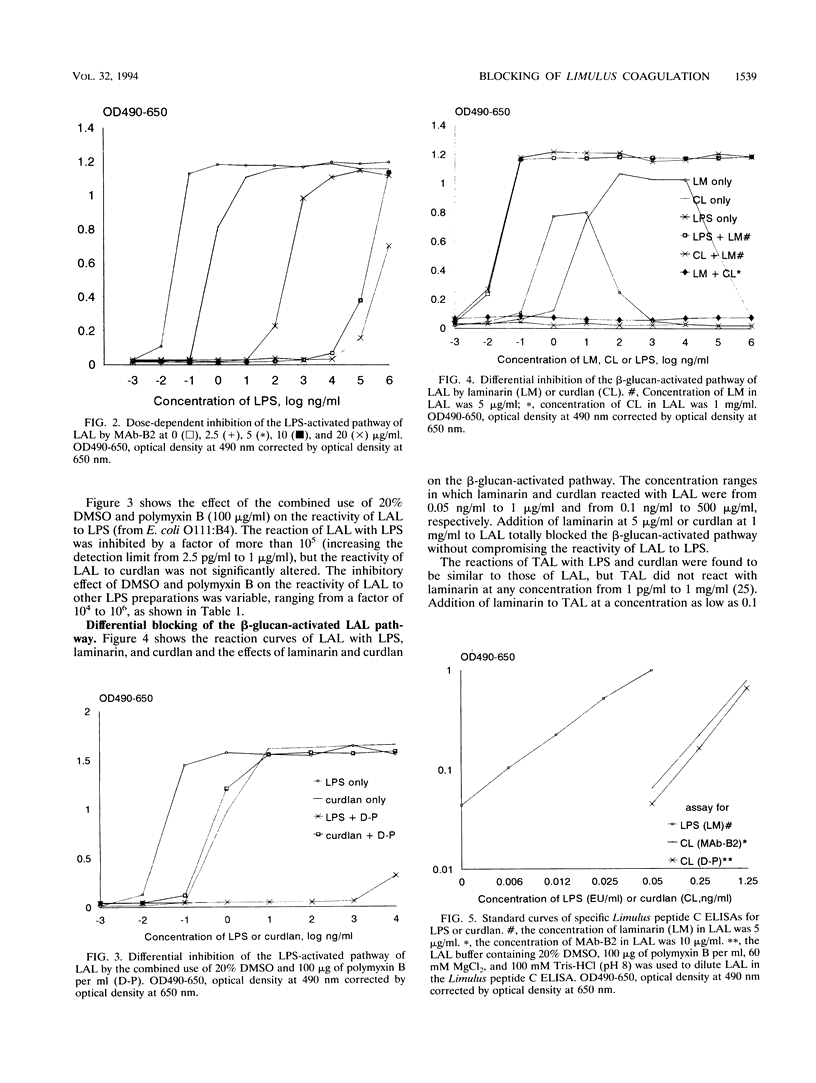
The coagulation of Limulus amebocyte lysate (LAL) can be activated through two pathways, one initiated by endotoxin and the other by beta-glucans. The two pathways join at the step of activation of the proclotting enzyme. We report here that the endotoxin-activated pathway can be differentially inhibited by two methods in a Limulus enzyme-linked immunosorbent assay (ELISA), either by the combined use of dimethyl sulfoxide and polymyxin B or by a monoclonal antibody against Limulus factor C. LAL reactivities to 10 different endotoxin preparations could be inhibited by the former method by a factor of 10(4) to 10(6) and could be blocked almost totally by the latter method, irrespective of the source of endotoxin. The sensitivity of the assay was approximately 50 pg/ml both for curdlan from Alcaligenes faecalis and for laminarin from Laminaria digitata. We also found that the beta-glucan-activated pathway could be totally blocked by laminarin (> 1 microgram/ml) without affecting the endotoxin-activated pathway, allowing endotoxin to be quantitated specifically by the Limulus ELISA with a detection limit of 0.005 endotoxin unit per ml. The use of uninhibited and differentially inhibited ELISAs demonstrated that different LAL preparations showed much greater variation in assaying beta-glucans than in assaying endotoxins. The LAL reactivity of normal human plasma was found to be due to the activation of the beta-glucan pathway, but not the endotoxin pathway, of LAL.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aida Y., Pabst M. J. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J Immunol Methods. 1990 Sep 14;132(2):191–195. doi: 10.1016/0022-1759(90)90029-u. [DOI] [PubMed] [Google Scholar]
- Baek L. New, sensitive rocket immunoelectrophoretic assay for measurement of the reaction between endotoxin and Limulus amoebocyte lysate. J Clin Microbiol. 1983 Jun;17(6):1013–1020. doi: 10.1128/jcm.17.6.1013-1020.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson L. A., Petersen N. J. LAL-reactive material associated with hemodialysis membranes. Prog Clin Biol Res. 1982;93:217–230. [PubMed] [Google Scholar]
- Hodes D. S., Heon D., Hass A., Hyatt A. C., Hodes H. L. Reaction of fungal products with amebocyte lysates of the Japanese horseshoe crab, Tachypleus tridentatus. J Clin Microbiol. 1987 Sep;25(9):1701–1704. doi: 10.1128/jcm.25.9.1701-1704.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura K., Ikegami K., Shimazu T., Yoshioka T., Sugimoto T. False-positive result in Limulus test caused by Limulus amebocyte lysate-reactive material in immunoglobulin products. J Clin Microbiol. 1989 Sep;27(9):1965–1968. doi: 10.1128/jcm.27.9.1965-1968.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga S., Miyata T., Tokunaga F., Muta T. Molecular mechanism of hemolymph clotting system in Limulus. Thromb Res. 1992 Oct 1;68(1):1–32. doi: 10.1016/0049-3848(92)90124-s. [DOI] [PubMed] [Google Scholar]
- Iwanaga S., Morita T., Harada T., Nakamura S., Niwa M., Takada K., Kimura T., Sakakibara S. Chromogenic substrates for horseshoe crab clotting enzyme. Its application for the assay of bacterial endotoxins. Haemostasis. 1978;7(2-3):183–188. doi: 10.1159/000214260. [DOI] [PubMed] [Google Scholar]
- Kakinuma A., Asano T., Torii H., Sugino Y. Gelation of Limulus amoebocyte lysate by an antitumor (1 leads to 3)-beta-D-glucan. Biochem Biophys Res Commun. 1981 Jul 30;101(2):434–439. doi: 10.1016/0006-291x(81)91278-x. [DOI] [PubMed] [Google Scholar]
- Kambayashi J., Yokota M., Sakon M., Shiba E., Kawasaki T., Mori T., Tsuchiya M., Oishi H., Matsuura S. A novel endotoxin-specific assay by turbidimetry with Limulus amoebocyte lysate containing beta-glucan. J Biochem Biophys Methods. 1991 Feb-Mar;22(2):93–100. doi: 10.1016/0165-022x(91)90022-o. [DOI] [PubMed] [Google Scholar]
- Kitagawa T., Tsuboi I., Kimura S., Sasamoto Y. Rapid method for preparing a beta-glucan-specific sensitive fraction from Limulus (Tachypleus tridentatus) amebocyte lysate. J Chromatogr. 1991 Jun 14;567(1):267–273. doi: 10.1016/0378-4347(91)80331-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levin J., Bang F. B. Clottable protein in Limulus; its localization and kinetics of its coagulation by endotoxin. Thromb Diath Haemorrh. 1968 Mar 31;19(1):186–197. [PubMed] [Google Scholar]
- Nakamura T., Morita T., Iwanaga S. Lipopolysaccharide-sensitive serine-protease zymogen (factor C) found in Limulus hemocytes. Isolation and characterization. Eur J Biochem. 1986 Feb 3;154(3):511–521. doi: 10.1111/j.1432-1033.1986.tb09427.x. [DOI] [PubMed] [Google Scholar]
- Obayashi T. Addition of perchloric acid to blood samples for colorimetric limulus test using chromogenic substrate: comparison with conventional procedures and clinical applications. J Lab Clin Med. 1984 Sep;104(3):321–330. [PubMed] [Google Scholar]
- Obayashi T., Tamura H., Tanaka S., Ohki M., Takahashi S., Arai M., Masuda M., Kawai T. A new chromogenic endotoxin-specific assay using recombined limulus coagulation enzymes and its clinical applications. Clin Chim Acta. 1985 Jun 30;149(1):55–65. doi: 10.1016/0009-8981(85)90273-6. [DOI] [PubMed] [Google Scholar]
- Roslansky P. F., Novitsky T. J. Sensitivity of Limulus amebocyte lysate (LAL) to LAL-reactive glucans. J Clin Microbiol. 1991 Nov;29(11):2477–2483. doi: 10.1128/jcm.29.11.2477-2483.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Aketagawa J., Takahashi S., Shibata Y., Tsumuraya Y., Hashimoto Y. Inhibition of high-molecular-weight-(1-->3)-beta-D-glucan-dependent activation of a limulus coagulation factor G by laminaran oligosaccharides and curdlan degradation products. Carbohydr Res. 1993 May 21;244(1):115–127. doi: 10.1016/0008-6215(93)80008-3. [DOI] [PubMed] [Google Scholar]
- Tokunaga F., Nakajima H., Iwanaga S. Further studies on lipopolysaccharide-sensitive serine protease zymogen (factor C): its isolation from Limulus polyphemus hemocytes and identification as an intracellular zymogen activated by alpha-chymotrypsin, not by trypsin. J Biochem. 1991 Jan;109(1):150–157. doi: 10.1093/oxfordjournals.jbchem.a123337. [DOI] [PubMed] [Google Scholar]
- Yoshioka T., Ikegami K., Ikemura K., Shiono S., Uenishi M., Sugimoto H., Sugimoto T. A study on limulus amebocyte lysate (LAL) reactive material derived from dialyzers. Jpn J Surg. 1989 Jan;19(1):38–41. doi: 10.1007/BF02471564. [DOI] [PubMed] [Google Scholar]
- Zhang G. H., Baek L., Koch C. New microassay for quantitation of endotoxin using Limulus amebocyte lysate combined with enzyme-linked immunosorbent assay. J Clin Microbiol. 1988 Aug;26(8):1464–1470. doi: 10.1128/jcm.26.8.1464-1470.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G. H., Baek L., Nielsen P. E., Buchardt O., Koch C. Sensitive quantitation of endotoxin by enzyme-linked immunosorbent assay with monoclonal antibody against Limulus peptide C. J Clin Microbiol. 1994 Feb;32(2):416–422. doi: 10.1128/jcm.32.2.416-422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]


