Abstract
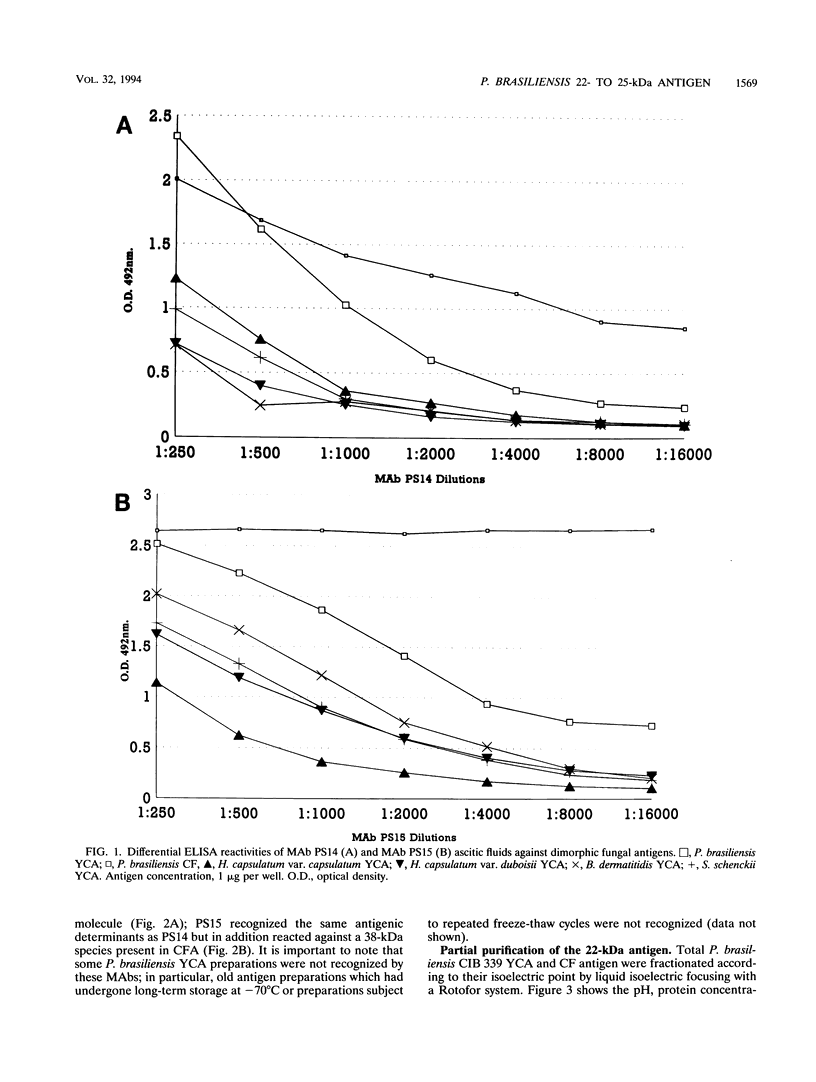
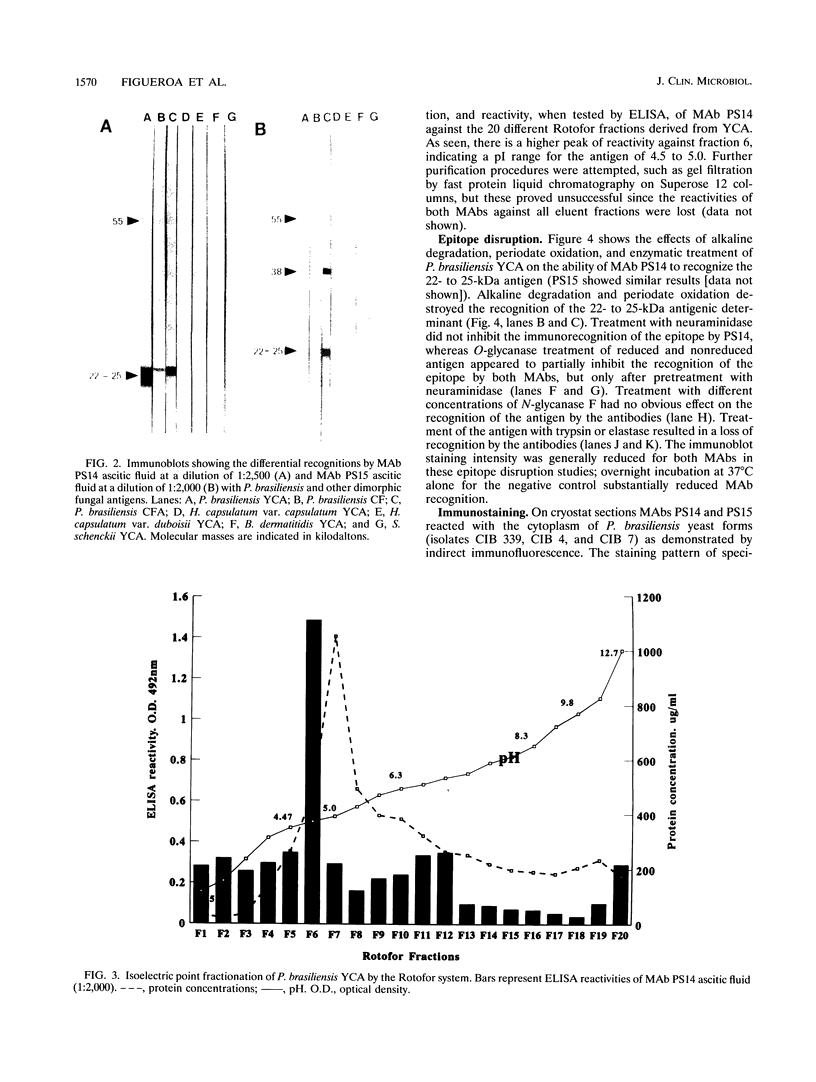
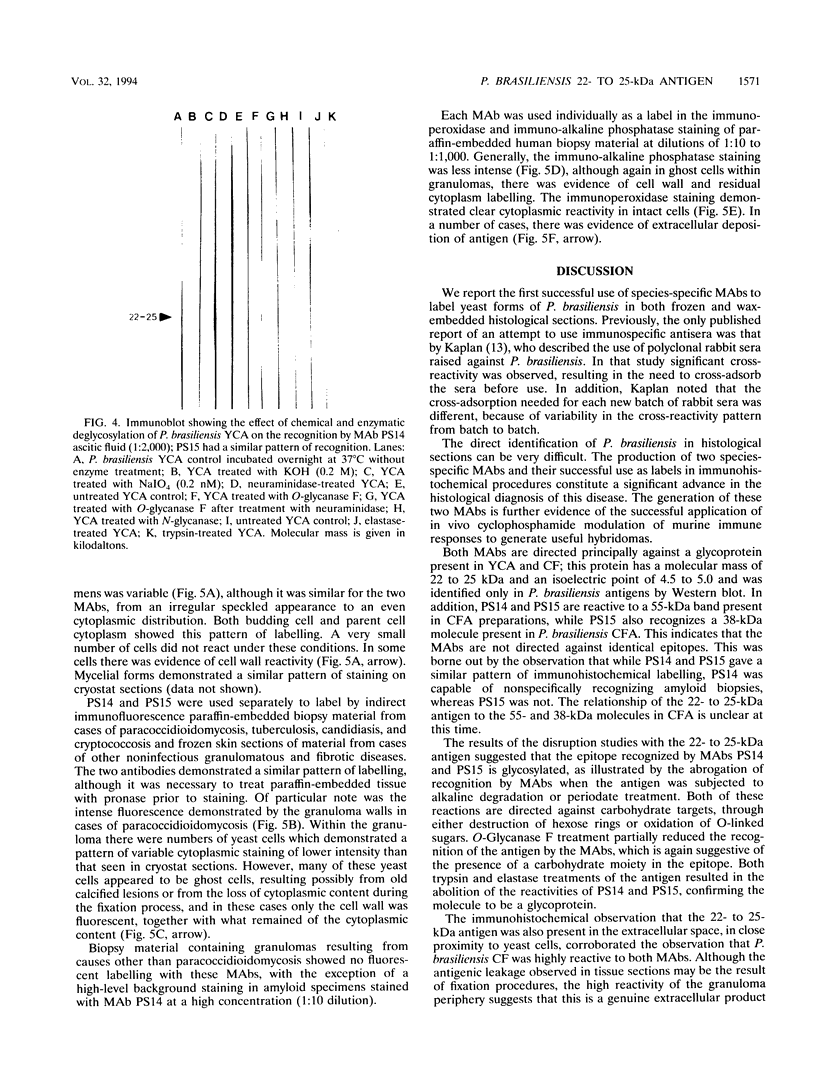
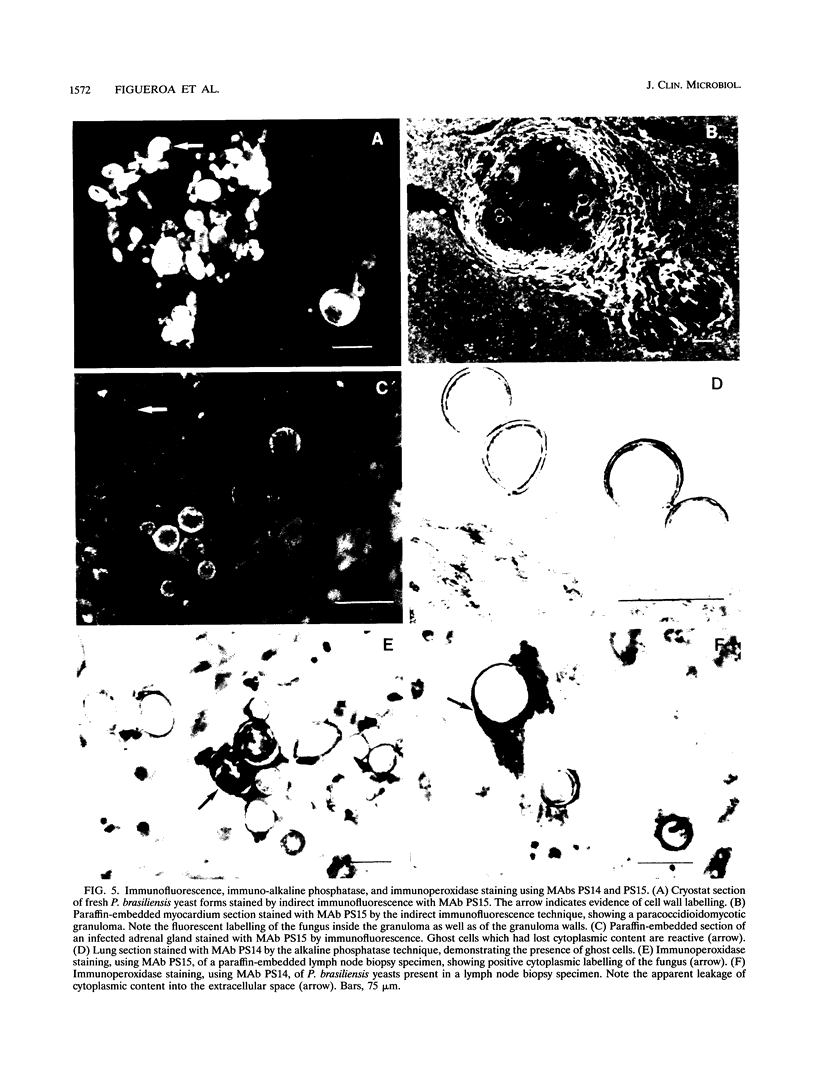
Two murine monoclonal antibodies (MAbs) specific to Paracoccidioides brasiliensis (as determined by enzyme-linked immunosorbent assay [ELISA] and Western blot [immunoblot]) were produced by using a modification of standard hybridization protocols, with cyclophosphamide included as an immunomodulator to abolish responses to highly cross-reactive immunodominant epitopes. MAbs PS14 and PS15 are two different clones which exhibit similar characteristics by ELISA and Western blot. They are directed against a 22- to 25-kDa antigen which is present in P. brasiliensis and which could not be identified in other dimorphic fungi by ELISA or Western blot. Partial purification of the antigen was accomplished by isoelectric focusing, and deglycosylation studies suggested that the 22- to 25-kDa antigen is a glycoprotein with a pI of between 4.5 and 5 and that O-linked sugars may be part of the recognized epitope. The MAbs stained the cytoplasm of P. brasiliensis yeast and hyphal cells in cryostat sections of fresh cultures of the fungus. In addition, the MAbs stained the wall of paracoccidioidomycotic granulomas, as well as the cytoplasm of the fungus, as determined by the use of immunofluorescence, immunoperoxidase, and immuno-alkaline phosphatase staining techniques in paraffin-embedded sections of human biopsy material, and they failed to stain granulomas resulting from other clinical conditions. These findings suggest that these MAbs have potential use in the immunohistochemical identification of P. brasiliensis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biagioni L., Souza M. J., Chamma L. G., Mendes R. P., Marques S. A., Mota N. G., Franco M. Serology of paracoccidioidomycosis. II. Correlation between class-specific antibodies and clinical forms of the disease. Trans R Soc Trop Med Hyg. 1984;78(5):617–621. doi: 10.1016/0035-9203(84)90220-7. [DOI] [PubMed] [Google Scholar]
- Blotta M. H., Camargo Z. P. Immunological response to cell-free antigens of Paracoccidioides brasiliensis: relationship with clinical forms of paracoccidioidomycosis. J Clin Microbiol. 1993 Mar;31(3):671–676. doi: 10.1128/jcm.31.3.671-676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano L. E., Restrepo A. Predictive value of serologic tests in the diagnosis and follow-up of patients with paracoccidioidomycosis. Rev Inst Med Trop Sao Paulo. 1987 Sep-Oct;29(5):276–283. doi: 10.1590/s0036-46651987000500003. [DOI] [PubMed] [Google Scholar]
- Figueroa J. I., Hamilton A. J., Bartholomew M. A., Harada T., Fenelon L., Hay R. J. Preparation of species-specific murine monoclonal antibodies against the yeast phase of Paracoccidioides brasiliensis. J Clin Microbiol. 1990 Aug;28(8):1766–1769. doi: 10.1128/jcm.28.8.1766-1769.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco M., Montenegro M. R., Mendes R. P., Marques S. A., Dillon N. L., Mota N. G. Paracoccidioidomycosis: a recently proposed classification of its clinical forms. Rev Soc Bras Med Trop. 1987 Apr-Jun;20(2):129–132. doi: 10.1590/s0037-86821987000200012. [DOI] [PubMed] [Google Scholar]
- Freitas-da-Silva G., Roque-Barreira M. C. Antigenemia in paracoccidioidomycosis. J Clin Microbiol. 1992 Feb;30(2):381–385. doi: 10.1128/jcm.30.2.381-385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini M. J., Bueno J. P., Shikanai-Yasuda M. A., Stolf A. M., Masuda A., Amato Neto V., Ferreira A. W. Antibody response to the 43 kDa glycoprotein of Paracoccidioides brasiliensis as a marker for the evaluation of patients under treatment. Am J Trop Med Hyg. 1990 Aug;43(2):200–206. doi: 10.4269/ajtmh.1990.43.200. [DOI] [PubMed] [Google Scholar]
- Hamilton A. J., Bartholomew M. A., Fenelon L. E., Figueroa J., Hay R. J. A murine monoclonal antibody exhibiting high species specificity for Histoplasma capsulatum var. capsulatum. J Gen Microbiol. 1990 Feb;136(2):331–335. doi: 10.1099/00221287-136-2-331. [DOI] [PubMed] [Google Scholar]
- Hamilton A. J., Bartholomew M. A., Fenelon L., Figueroa J., Hay R. J. Preparation of monoclonal antibodies that differentiate between Histoplasma capsulatum variant capsulatum and H. capsulatum variant duboisii. Trans R Soc Trop Med Hyg. 1990 May-Jun;84(3):425–428. doi: 10.1016/0035-9203(90)90347-h. [DOI] [PubMed] [Google Scholar]
- Hamilton A. J., Goodley J. Purification of the 115-kilodalton exoantigen of Cryptococcus neoformans and its recognition by immune sera. J Clin Microbiol. 1993 Feb;31(2):335–339. doi: 10.1128/jcm.31.2.335-339.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew W. D., Sandrock A. W., Jr Cyclophosphamide treatment used to manipulate the immune response for the production of monoclonal antibodies. J Immunol Methods. 1987 Jun 26;100(1-2):73–82. doi: 10.1016/0022-1759(87)90174-8. [DOI] [PubMed] [Google Scholar]
- Mendes-Giannini M. J., Bueno J. P., Shikanai-Yasuda M. A., Ferreira A. W., Masuda A. Detection of the 43,000-molecular-weight glycoprotein in sera of patients with paracoccidioidomycosis. J Clin Microbiol. 1989 Dec;27(12):2842–2845. doi: 10.1128/jcm.27.12.2842-2845.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Thomas W. A. Production of monoclonal antibodies selective for aggregation-competent chick neural retina cells. An immunosuppressive approach. J Immunol Methods. 1987 Mar 12;97(2):237–243. doi: 10.1016/0022-1759(87)90465-0. [DOI] [PubMed] [Google Scholar]
- Tsang V. C., Peralta J. M., Simons A. R. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–391. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]