Abstract
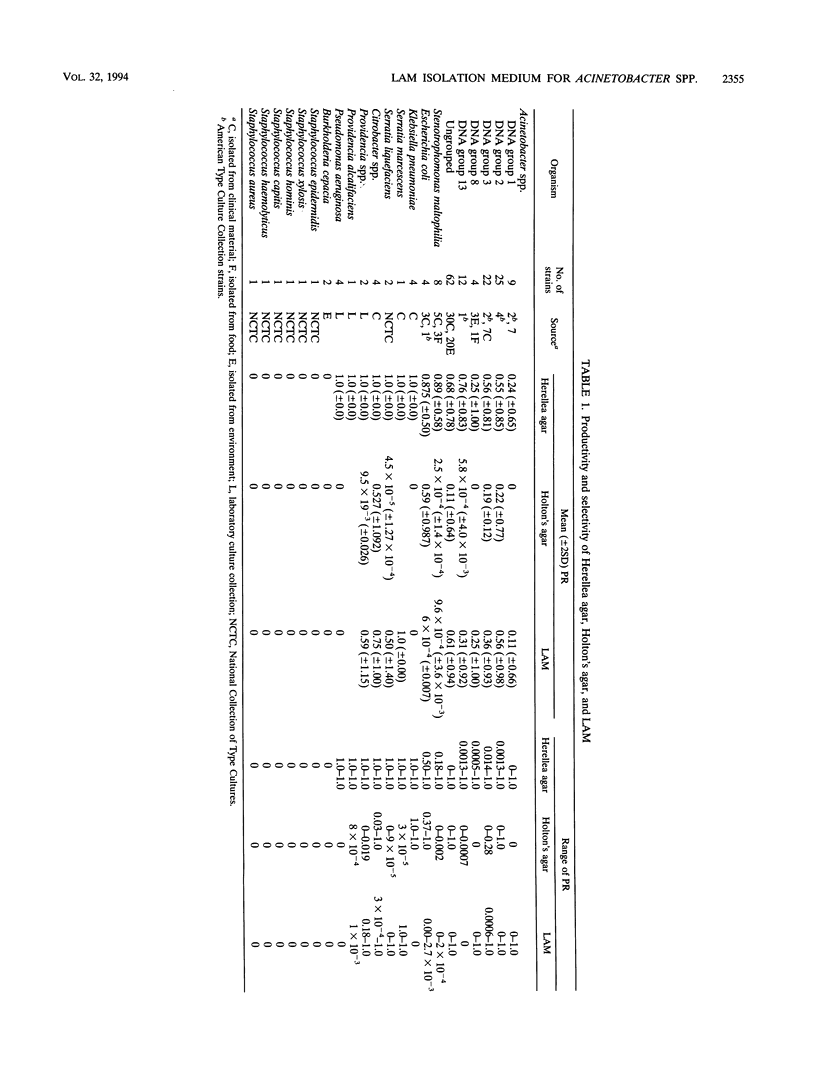
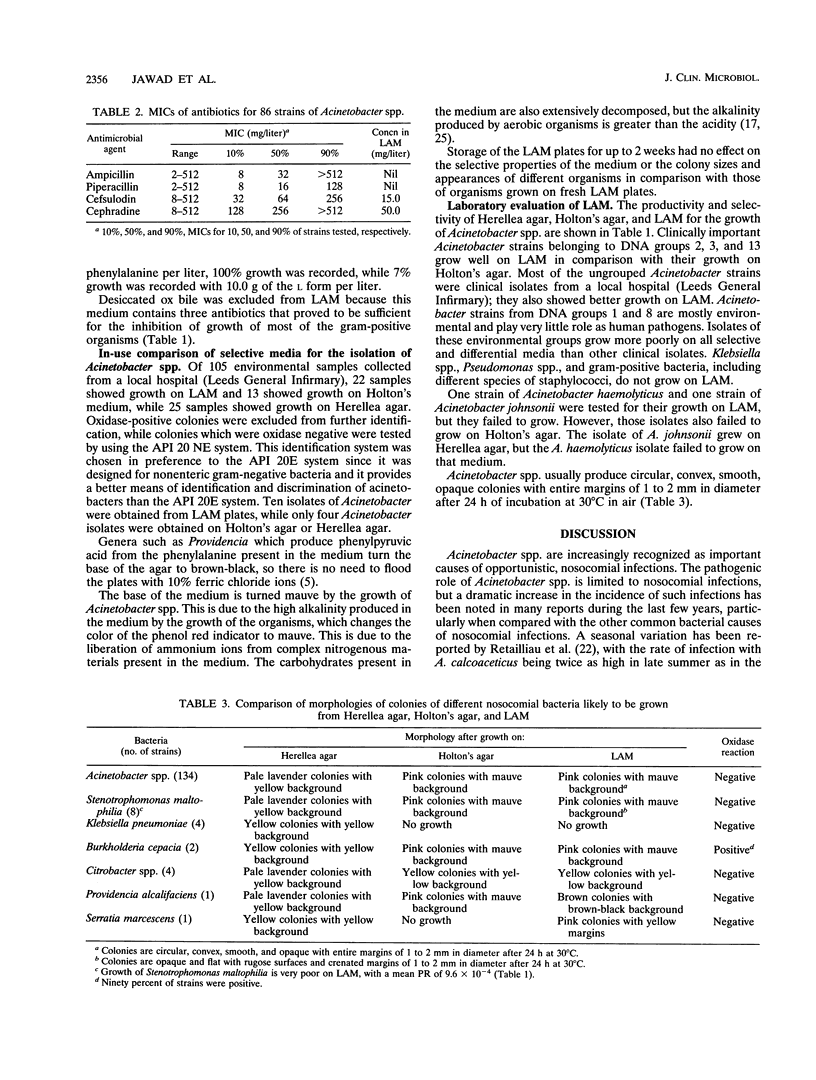
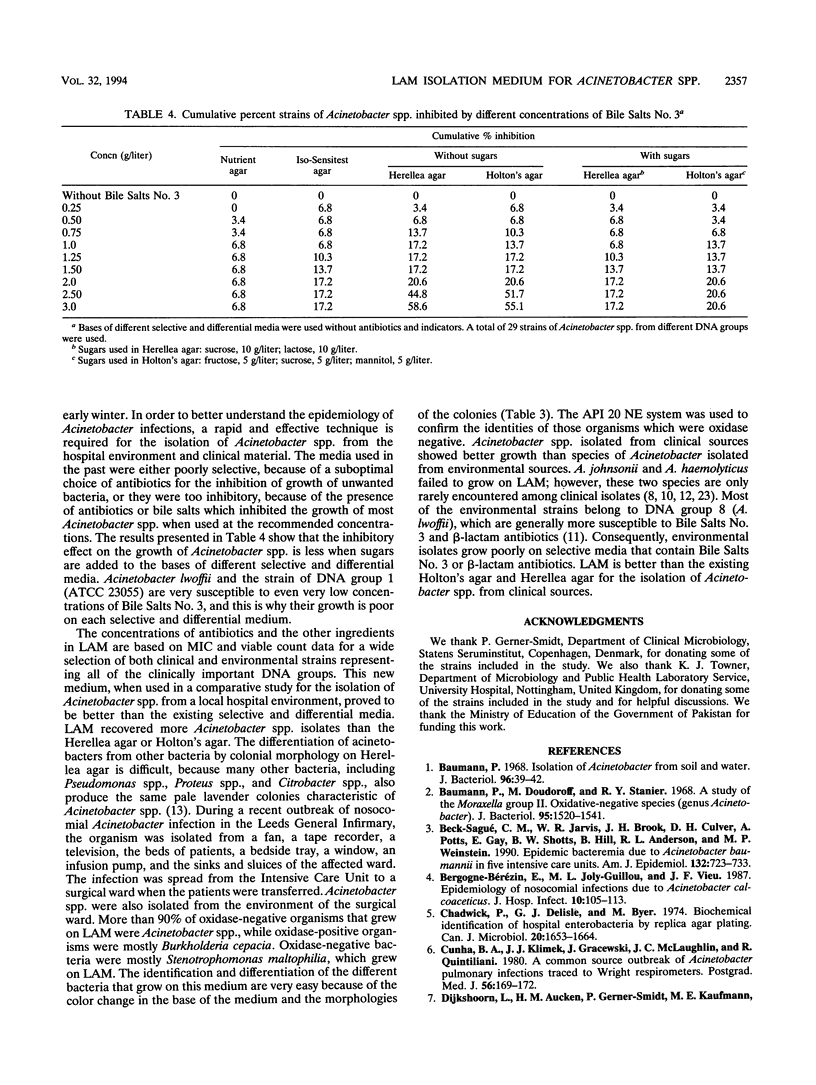
Acinetobacter spp. are responsible for an increasing number of opportunistic, nosocomial infections. They have been isolated from diverse inanimate objects in the hospital environment and are resistant to most of the commonly used antibiotics. Existing media for the isolation of Acinetobacter spp. are either nonselective, allowing the growth of unwanted bacteria, or too inhibitory, inhibiting the growth of many Acinetobacter strains. For the rapid isolation and effective control of Acinetobacter infection, a new selective and differential medium, Leeds Acinetobacter Medium (LAM), has been developed to isolate Acinetobacter spp. from clinical and environmental sources. The concentration of antibiotics and other ingredients in this medium have been determined according to the results of MIC and viable counts performed for these ingredients. LAM was compared with other selective and differential media for the isolation of Acinetobacter spp. from a local hospital environment and proved to be better in terms of recovery and selectivity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann P., Doudoroff M., Stanier R. Y. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J Bacteriol. 1968 May;95(5):1520–1541. doi: 10.1128/jb.95.5.1520-1541.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P. Isolation of Acinetobacter from soil and water. J Bacteriol. 1968 Jul;96(1):39–42. doi: 10.1128/jb.96.1.39-42.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck-Sagué C. M., Jarvis W. R., Brook J. H., Culver D. H., Potts A., Gay E., Shotts B. W., Hill B., Anderson R. L., Weinstein M. P. Epidemic bacteremia due to Acinetobacter baumannii in five intensive care units. Am J Epidemiol. 1990 Oct;132(4):723–733. doi: 10.1093/oxfordjournals.aje.a115714. [DOI] [PubMed] [Google Scholar]
- Bergogne-Bérézin E., Joly-Guillou M. L., Vieu J. F. Epidemiology of nosocomial infections due to Acinetobacter calcoaceticus. J Hosp Infect. 1987 Sep;10(2):105–113. doi: 10.1016/0195-6701(87)90135-6. [DOI] [PubMed] [Google Scholar]
- Chadwick P., Delisle G. J., Byer M. Biochemical identification of hospital enterobacteria by replica agar plating. Can J Microbiol. 1974 Dec;20(12):1653–1664. doi: 10.1139/m74-257. [DOI] [PubMed] [Google Scholar]
- Cunha B. A., Klimek J. J., Gracewski J., McLaughlin J. C., Quintiliani R. A common source outbreak of Acinetobacter pulmonary infections traced to Wright respirometers. Postgrad Med J. 1980 Mar;56(653):169–172. doi: 10.1136/pgmj.56.653.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkshoorn L., Aucken H. M., Gerner-Smidt P., Kaufmann M. E., Ursing J., Pitt T. L. Correlation of typing methods for Acinetobacter isolates from hospital outbreaks. J Clin Microbiol. 1993 Mar;31(3):702–705. doi: 10.1128/jcm.31.3.702-705.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer M., Przondo-Mordarska A. Wystepowanie gatunków rodzaju Acinetobacter w materiale od chorych i w środowisku szpitalnym. Med Dosw Mikrobiol. 1993;45(2):213–217. [PubMed] [Google Scholar]
- GARRISON R. G. THE OCCURRENCE OF BACTERIUM ANITRATUM IN SECRETIONS OF PULMONARY ORIGIN. Am J Clin Pathol. 1963 Sep;40:260–262. doi: 10.1093/ajcp/40.3.260. [DOI] [PubMed] [Google Scholar]
- Gennari M., Lombardi P. Comparative characterization of Acinetobacter strains isolated from different foods and clinical sources. Zentralbl Bakteriol. 1993 Nov;279(4):553–564. doi: 10.1016/s0934-8840(11)80428-7. [DOI] [PubMed] [Google Scholar]
- Gerner-Smidt P., Frederiksen W. Acinetobacter in Denmark: I. Taxonomy, antibiotic susceptibility, and pathogenicity of 112 clinical strains. APMIS. 1993 Nov;101(11):815–825. doi: 10.1111/j.1699-0463.1993.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Gospodarek E., Kania I. Wystepowanie gatunków z rodzaju Acinetobacter w materiale klinicznym i innych źródłach. Med Dosw Mikrobiol. 1992;44(1-2):41–48. [PubMed] [Google Scholar]
- Grehn M., von Graevenitz A. Search for Acinetobacter calcoaceticus subsp. anitratus: enrichment of fecal samples. J Clin Microbiol. 1978 Sep;8(3):342–343. doi: 10.1128/jcm.8.3.342-343.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGH R., LEIFSON E. The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram negative bacteria. J Bacteriol. 1953 Jul;66(1):24–26. doi: 10.1128/jb.66.1.24-26.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen S. D. Moraxella, Acinetobacter, and the Mimeae. Bacteriol Rev. 1973 Dec;37(4):522–561. doi: 10.1128/br.37.4.522-561.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton J. A note on the preparation and use of a selective differential medium for the isolation of Acinetobacter spp. from clinical sources. J Appl Bacteriol. 1983 Feb;54(1):141–142. doi: 10.1111/j.1365-2672.1983.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Juni E. Genetics and physiology of Acinetobacter. Annu Rev Microbiol. 1978;32:349–371. doi: 10.1146/annurev.mi.32.100178.002025. [DOI] [PubMed] [Google Scholar]
- MANDEL A. D., WRIGHT K., MCKINNON J. M. SELECTIVE MEDIUM FOR ISOLATION OF MIMA AND HERELLEA ORGANISMS. J Bacteriol. 1964 Nov;88:1524–1525. doi: 10.1128/jb.88.5.1524-1525.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. E., Vecchio J., Pantelick E. L., Farrel P., Mazon D., Zervos M. J., Hierholzer W. J., Jr Association of contaminated gloves with transmission of Acinetobacter calcoaceticus var. anitratus in an intensive care unit. Am J Med. 1991 Nov;91(5):479–483. doi: 10.1016/0002-9343(91)90183-x. [DOI] [PubMed] [Google Scholar]
- Retailliau H. F., Hightower A. W., Dixon R. E., Allen J. R. Acinetobacter calcoaceticus: a nosocomial pathogen with an unusual seasonal pattern. J Infect Dis. 1979 Mar;139(3):371–375. doi: 10.1093/infdis/139.3.371. [DOI] [PubMed] [Google Scholar]
- Seifert H., Baginski R., Schulze A., Pulverer G. Antimicrobial susceptibility of Acinetobacter species. Antimicrob Agents Chemother. 1993 Apr;37(4):750–753. doi: 10.1128/aac.37.4.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert H., Baginski R., Schulze A., Pulverer G. The distribution of Acinetobacter species in clinical culture materials. Zentralbl Bakteriol. 1993 Nov;279(4):544–552. doi: 10.1016/s0934-8840(11)80427-5. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Towner K. J., Chopade B. A. Biotyping of Acinetobacter calcoaceticus using the API 2ONE system. J Hosp Infect. 1987 Sep;10(2):145–151. doi: 10.1016/0195-6701(87)90140-x. [DOI] [PubMed] [Google Scholar]
- Vila J., Marcos A., Marco F., Abdalla S., Vergara Y., Reig R., Gomez-Lus R., Jimenez de Anta T. In vitro antimicrobial production of beta-lactamases, aminoglycoside-modifying enzymes, and chloramphenicol acetyltransferase by and susceptibility of clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother. 1993 Jan;37(1):138–141. doi: 10.1128/aac.37.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]


