Abstract
Retinoic acid (RA) is a potent signaling molecule that is essential for many biological processes, and its levels are tightly regulated by mechanisms that are only partially understood. The synthesis of RA from its precursor retinol (vitamin A) is an important regulatory mechanism. Therefore, the esterification of retinol with fatty acyl moieties to generate retinyl esters, the main storage form of retinol, may also regulate RA levels. Here we show that the neutral lipid synthesis enzyme acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1) functions as the major acyl-CoA:retinol acyltransferase (ARAT) in murine skin. When dietary retinol is abundant, DGAT1 deficiency results in elevated levels of RA in skin and cyclical hair loss; both are prevented by dietary retinol deprivation. Further, DGAT1-deficient skin exhibits enhanced sensitivity to topically administered retinol. Deletion of the enzyme specifically in the epidermis causes alopecia, indicating that the regulation of RA homeostasis by DGAT1 is autonomous in the epidermis. These findings show that DGAT1 functions as an ARAT in the skin, where it acts to maintain retinoid homeostasis and prevent retinoid toxicity. Our findings may have implications for human skin or hair disorders treated with agents that modulate RA signaling.
Regulation of cellular proliferation and differentiation of epithelial tissues is crucial in embryonic development and in adult homeostasis. Retinoic acid (RA)2 is a major regulator of these processes (1) through its ability to serve as a ligand for RA nuclear receptors (RARs) (2). Since RA is such a potent signaling molecule, its levels must be tightly controlled. Indeed, excess RA is highly teratogenic during embryonic development and may be toxic to adult tissues (3). Further, RA is used therapeutically for skin disorders, such as acne and psoriasis, and certain cancers (4), but its uses are often limited by local and systemic toxicity. Thus, understanding how RA levels are regulated has important biological and clinical relevance.
The synthesis of RA from its precursor retinol, or vitamin A, is a major node in the regulation of RA levels (5). To generate RA, retinol is oxidized in two sequential reactions, catalyzed by retinol and retinal dehydrogenases (5), whose activities regulate RA homeostasis. We hypothesized that the availability of retinol for these reactions may also be regulated by the balance between retinol and retinyl esters. Indeed, the majority of retinol in the body is stored as retinyl esters, which are concentrated in cytosolic lipid droplets of cells and serve as a local source of retinol. Retinyl esters are also stored in major organs, such as liver and white adipose tissue (WAT), from which retinol can be mobilized to supply other tissues during increased demand. Thus, retinol esterification may participate in regulating the retinol pool available for RA synthesis.
Retinol esterification is carried out by two distinct enzymatic activities. One is mediated by lecithin:retinol acyltransferase (LRAT), which catalyzes the covalent joining of a fatty acyl moiety from lecithin (phosphatidylcholine) to retinol that is bound to cellular retinol-binding protein (CRBP) (6, 7). LRAT activity is crucial for maintaining tissue retinol stores. LRAT-null (Lrat-/-) mice have severe reductions in hepatic and lung retinyl ester levels (8–10), which are accompanied by testicular hypoplasia/atrophy (9) and blindness (8). Retinyl ester levels are normal in WAT and several other tissues, indicating alternative mechanisms for retinol esterification (9, 10). This esterification is probably mediated in part by acyl CoA:retinol acyltransferase (ARAT) enzymes, which use fatty acyl-CoA and unbound retinol as substrates (11). Although many tissues exhibit ARAT activity (12), attempts to purify and clone an ARAT gene were unsuccessful, and thus molecular tools to study ARAT activity have been lacking. However, the enzyme encoded by Dgat1, an acyl CoA:diacylglycerol acyltransferase (DGAT), was recently reported to catalyze the ARAT reaction in vitro (13, 14). Moreover, several tissues of Dgat1-/- mice had reduced ARAT activity, and retinol esterification was reduced in cultured murine embryonic fibroblasts lacking DGAT1 (14). Most recently, a study of Dgat1-/- mice demonstrated a role for the enzyme in retinol absorption in the small intestine (15). Thus, accumulating evidence indicates that the retinol esterification activity of DGAT1 is of biological, and possibly clinical, importance.
In the current study, we investigated whether retinol esterification by DGAT1 is important in murine skin. Dgat1-/- mice exhibit a pleiotropic phenotype, which includes resistance to diet-induced obesity and altered energy metabolism but also includes prominent phenotypic findings in the skin (16–19). Retinoids play key roles in skin and hair biology (20), and excess retinoids induce epidermal hyperplasia, inhibit sebocyte proliferation and differentiation, and alter hair growth (21). Notably, the skin manifestations of Dgat1-/- mice, which include alopecia and sebaceous gland atrophy (18), resemble those of retinoid toxicity (22, 23). Thus, we hypothesized that DGAT1 functions as an ARAT in murine skin and that the absence of DGAT1 alters retinoid homeostasis. In this study, we tested this hypothesis by examining retinoid metabolism in the skin of DGAT1-deficient mice.
EXPERIMENTAL PROCEDURES
Mice and Diets—Male Dgat1-/- and wild-type mice (C57BL/6J genetic background) were genotyped as described (16). Mice were housed in a pathogen-free barrier facility (12-h light/12-h dark cycle) and fed a retinoid-abundant chow diet (5053 Pico-Lab Diet; Purina, St. Louis, MO), unless otherwise specified. For studies in which dietary retinol content was controlled, Dgat1+/- dams were fed a retinoid-deficient diet containing 10 kcal% fat and <0.04 IU retinol/g (D03102201; Research Diets, New Brunswick, NJ) throughout gestation and suckling. The first litters of Dgat1-/- and wild-type offspring were weaned and maintained on the retinoid-deficient diet until 3 weeks before sacrifice, when they were switched to a retinoid-sufficient diet containing 10 kcal% fat and 4 IU retinyl palmitate/g (D12450B; Research Diets). To generate retinoid-deficient mice, the second litters of Dgat1+/- dams fed a retinoid-deficient diet were weaned and maintained on the retinoid-deficient diet until sacrifice.
Dgat1flox/flox mice (mixed C57BL/6J and 129/SvJae), generated as described in the supplemental material, were crossed with keratin 14-Cre mice (K14-Cre; mixed Swiss Webster; C57BL/6J; CBA/J; Jackson Laboratory, Bar Harbor, ME) (24). K14-Cre+DGAT1flox/+ mice were crossed with Dgat1flox/flox mice to generate male K14-Cre+DGAT1flox/+, K14-Cre+DGAT1flox/flox, and Dgat1flox/flox littermates (mixed Swiss Webster; 129/SvJae; C57BL/6J; CBA/J) for studies. All experiments were approved by the Committee on Animal Research of the University of California, San Francisco.
Genotyping of Cre and Floxed Dgat1 Alleles—Genomic DNA was extracted from tail epidermis (mechanically separated from the dermis after incubation of skin at 37 °C for 45 min) and dermis, WAT, liver, and testes. Cre was detected with sense primer 5′-GCGGTCTGGCAGTAAAAACTATC-3′ and antisense primer 5′-GTGAAACAGCATTGCTGTCACTT-3′ (100-bp product); interleukin-2 was detected by sense primer 5′-CTAGGCCACAGAATTGAAAGATCT-3′ and antisense primer 5′-GTAGGTGGAAATTCTAGCATCATCC-3′ (324-bp product) and served as an internal PCR control. To identify various alleles of Dgat1, genomic PCR was performed with forward primer 5′-CAGACATGGCAGCAGCAAATG-3′ (in exon 15) and reverse primer 5′-TGCAAGTTGCTGCTGCCACCTG-3′ (in the 3′-untranscribed region). The wild-type Dgat1 allele yields an 895 bp band. The floxed Dgat1 allele yields a 1002 bp band whose absence indicates Cre-mediated recombination of the floxed Dgat1 allele and deletion of exons 14–17.
Retinol Esterification Assays—Mouse skin was homogenized with a Tissue Tearor (model 398, probe 9853G-04; Biospec Products, Bartlesville, OK) in Buffer A (50 mm Tris-HCl, pH 7.4, and 250 mm sucrose) containing proteinase inhibitors (Roche Applied Science). ARAT assays were performed with total protein homogenates (100 μg) in an assay mix containing Buffer A, 5 mm MgCl2, 1.25 mg/ml bovine serum albumin, 200 μm all-trans-retinol (Sigma) in acetone, and 25 μm [14C]oleoyl-CoA (55.0 mCi/mmol). After 10 min at 37 °C, lipids were extracted with chloroform/methanol (2:1, v/v) and separated by silica gel G-60 TLC plates with hexane/ethyl ether/acetic acid (80:20:1). Retinyl ester, triacylglycerol, and cholesterol ester bands were scraped, and radioactivity was measured by scintillation counting.
Retinoid Analyses—Retinol, retinyl ester, and all-trans-RA were quantified as described (25) with modifications. Mouse tissue samples were harvested under yellow light and immediately frozen in liquid N2. Tissues were homogenized on ice with ice-cold 0.9% saline to make a ∼25% homogenate. Tissues were homogenized in ground glass vessels (Kontes; size 21) either manually or with a Heidolph motorized homogenizer (at 280 rpm). For skin samples (200–400 mg), a portion (50–100 mg) would not homogenize and was subtracted to obtain tissue mass. Serum was obtained by centrifuging blood at 7000 × g for ∼7 min at 4 °C. Tissue homogenate or serum was added to a disposable glass culture tube (16 × 150 mm), and an internal standard (50–100 nm 4,4-dimethyl-RA in 10 ml of acetonitrile) was added, followed by the addition of 25 mm KOH in ethanol (1 ml for serum, 3 ml for tissue homogenates) and extraction with 10 ml of hexane. The organic phase containing nonpolar retinoids (retinol and retinyl ester) was removed. HCl (4 m, 60–180 ml) was added to the aqueous phase, and polar substances (RA) were removed by extraction with 10 ml of hexane. Organic phases were dried under nitrogen with gentle heating at ∼25–30 °C in a water bath (N-EVAP 112; Organomation Associates, Berlin, MA). Extracts were resuspended in acetonitrile according to analyte (RA in 60 ml, retinol/retinyl ester from liver in 1000 ml; retinol/retinyl ester from all other tissues in 150 ml). Only glass containers, pipettes, and calibrated syringes were used to handle retinoid samples.
Real Time PCR—Whole skin was homogenized, and total RNA was extracted with the RNeasy fibrous tissue minikit (Qiagen, Valencia, CA). RNA (5 μg) was reverse-transcribed with the Superscript III First-Strand Synthesis Supermix kit (Invitrogen). Real time PCR was performed and analyzed with the ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA). Each 20-μl PCR contained 2 μl of cDNA, 10 μl of 2× SYBR Green PCR Master Mix (Applied Biosystems), and 10 pmol each of forward and reverse primer. Relative expression levels were calculated by the comparative CT (cycle of threshold detection) method, as outlined in the manufacturer's technical bulletin. Cyclophilin expression served as control. The primers used for reverse transcription-PCR are listed in supplemental Table 1.
Topical Retinol Treatments—All-trans-retinol (Sigma) was dissolved in ethanol (0.5 nmol/μl, 1 nmol/μl, and 2 nmol/μl), and 50, 100, or 200 nmol was applied topically to the dorsal cephalad skin of shaved mice (n = 3/group). As control, ethanol alone was applied to dorsal caudal skin of the same mice. Four days after treatment, three skin regions from each retinol- and vehicle-treated area were harvested from each mouse. One skin section per region was stained with hematoxylin-eosin and analyzed. Epidermal thickness was measured with SPOT advanced imaging software in three areas per skin section (nine measurements/mouse). To assess susceptibility to retinol toxicity, all-trans-retinol was dissolved in ethanol (1 nmol/ml), and 100 nmol was applied topically to dorsal cephalad skin of shaved mice (n = 5/group) daily for 3 consecutive days. As a control, ethanol was applied to dorsal caudal skin of the same mice.
Histological Analyses—Whole middorsal skin was removed from wild-type and Dgat1-/- mice, fixed in 4% paraformaldehyde/phosphate-buffered saline at 4 °C overnight, and washed for 15 min in phosphate-buffered saline and embedded in paraffin. Tissue sections (6 μm) were stained with hematoxylin-eosin.
Adhesive Tape Test—A 1 × 1.5-inch piece of adhesive tape (VWR, Batavia, IL) was pressed gently on the nape of the neck of mice and pulled off in the direction of hair growth (toward the tail). The tape was photographed and weighed (before and after stripping).
Statistical Analyses—Values are reported as mean ± S.E. Means were compared by t test or analysis of variance followed by the Bonferroni or Tukey test.
RESULTS
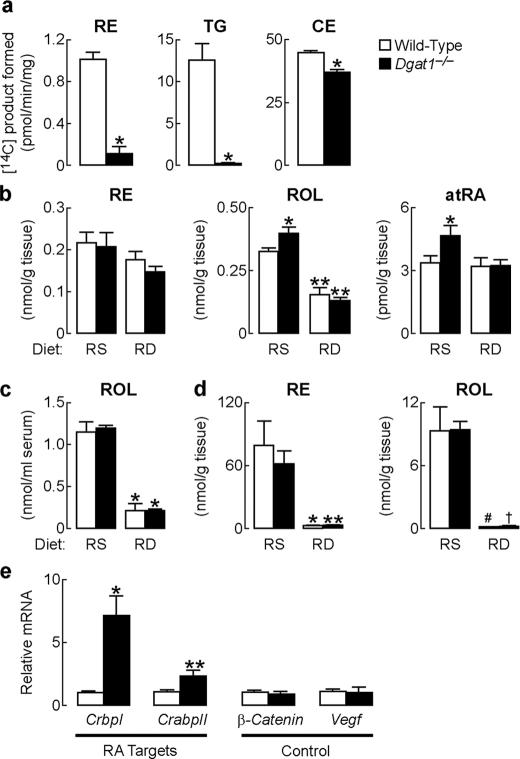
Altered Retinoid Metabolism in Dgat1-/- Skin—To determine if DGAT1 contributes to retinol esterification in skin, we measured ARAT activity in whole skin homogenates of 7-week-old wild-type and Dgat1-/- mice fed a chow diet. ARAT activity was reduced by ∼90% in Dgat1-/- skin (Fig. 1a). DGAT activity was reduced by more than 95%. The activity of a control enzyme, acyl-CoA:cholesterol acyltransferase, which catalyzes cholesterol ester synthesis, was slightly lower in Dgat1-/- skin (Fig. 1a), possibly because of atrophy of sebaceous glands, where acyl-CoA:cholesterol acyltransferases are highly expressed (26).
FIGURE 1.
Altered retinoid homeostasis in Dgat1-/- mice. a, reduced in vitro ARAT activity in whole skin of Dgat1-/- mice (age 7 weeks, n = 7/genotype). *, p < 0.001 versus wild type. Retinyl esters (RE), triacylglycerols (TG), and cholesterol esters (CE) are the respective products of the ARAT, DGAT, and acyl-CoA:cholesterol acyltransferase reactions. b, retinol (ROL) and all-trans-retinoic acid (atRA) concentrations are increased in whole skin in Dgat1-/- mice fed the retinoid-sufficient (RS) diet but not in those fed the retinoid-deficient (RD) diet (age 7.5–14 weeks, n = 4–6/genotype). *, p < 0.05 versus wild type; **, p < 0.001; #, p < 0.05 versus retinoid-sufficient diet. c, serum retinol concentrations are similar in wild-type and Dgat1-/- mice (age 7.5–14 weeks, n = 4–6/genotype). *, p < 0.001 versus retinoid-sufficient diet. d, hepatic retinyl ester and retinol concentrations are similar in wild-type and Dgat1-/- mice (age 7.5–14 weeks, n = 4–6/genotype). *, p < 0.05; **, p < 0.01; #, p = 0.01; †, p < 0.001 versus retinoid-sufficient diet. e, RA target gene expression is increased in the whole skin of Dgat1-/- mice fed a retinoid-abundant chow diet. mRNA levels were quantified by real time PCR (age 7 weeks, n = 5–6/genotype). *, p < 0.01; **, p < 0.05 versus wild type.
To determine if the reduced ARAT activity in Dgat1-/- skin altered retinoid homeostasis, we measured retinoid levels in whole skin of wild-type and Dgat1-/- mice fed a formulated, purified diet containing the amount of retinol recommended by the American Institute of Nutrition (i.e. a retinol-sufficient diet). Unesterified retinol was increased by ∼22%, and all-trans-RA was increased by 40% in Dgat1-/- skin (Fig. 1b). Retinyl ester levels were similar, probably reflecting contributions from LRAT activity. Serum retinol (Fig. 1c) and liver retinoid (Fig. 1d) levels were similar in both genotypes of mice, suggesting that the increased all-trans-RA levels in Dgat1-/- skin resulted from local changes in retinoid metabolism.
All-trans-RA mediates many biological activities of retinol through transcriptional mechanisms (27). We therefore examined mRNA levels of two all-trans-RA target genes, CrbpI and cellular RA-binding protein II (CrabpII) (1, 27), in the synchronized telogen skin of 7-week-old chow-fed Dgat1-/- and wild-type mice. CrbpI mRNA was increased 7-fold and CrabpII mRNA 2-fold in Dgat1-/- skin (Fig. 1e). The mRNA levels of the β-catenin and Vegf (vascular endothelial growth factor) genes, which are hair cycle-regulated genes but not targets of all-trans-RA, were similar in Dgat1-/- and wild-type skin (Fig. 1e).
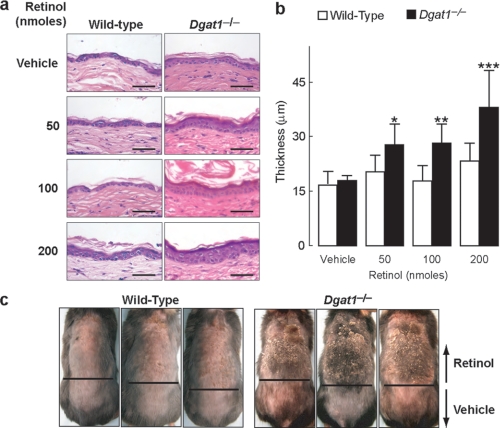
Enhanced Epidermal Hyperplasia in Response to Topical Retinol in Dgat1-/- Mice—To determine whether the biological activity of retinol is enhanced in Dgat1-/- skin, we applied retinol once to dorsal skin of shaved mice and examined the extent of retinoid-induced epidermal hyperplasia 4 days later. Retinol did not exert effects on wild-type skin but induced significant epidermal hyperplasia and increased epidermal thickness in Dgat1-/- skin (Fig. 2, a and b).
FIGURE 2.
Enhanced epidermal response to topical retinol treatment in Dgat1-/- mice. a, enhanced epidermal hyperplasia in Dgat1-/- skin after topical retinol treatment. Retinol (50, 100, or 200 nmol dissolved in 100 μl of ethanol or ethanol alone was applied once topically to dorsal skin. Skin was harvested 4 days after treatment, and sections were stained with hematoxylin-eosin (age 6 months, n = 3/genotype/dose). Scale bars, 50 μm. b, increased epidermal thickness in Dgat1-/- skin treated with retinol. Mice were age 6 months, n = 3/genotype. *, p = 0.0143; **, p = 0.01; ***, p < 0.001 versus vehicle-treated Dgat1-/- skin. c, increased susceptibility of Dgat1-/- skin to retinol-induced irritation. Scaling, cracking, and crusty lesions were prominent in Dgat1-/- skin. Retinol (100 μl of 1 nmol/μl in ethanol) was applied topically once daily for 3 consecutive days to the dorsal cephalad skin. Ethanol was applied to the dorsal caudal skin (age 11 weeks, n = 5/genotype).
To determine if Dgat1-/- skin exhibits increased susceptibility to more chronic retinoid toxicity, we applied retinol topically for 3 consecutive days. Dgat1-/- skin exhibited severe irritation characteristic of retinoid toxicity, including erythema, severe skin scaling and cracking, and crusty lesions (28, 29). In contrast, retinol caused only mild irritation (erythema and some flaking) in wild-type skin (Fig. 2c).
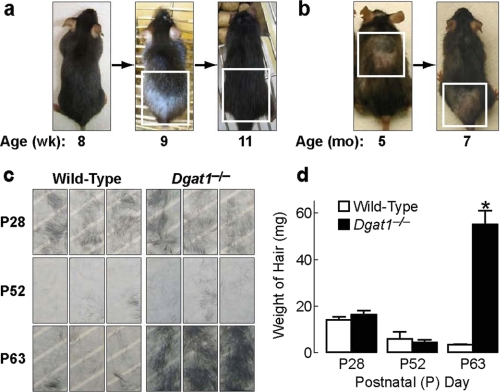
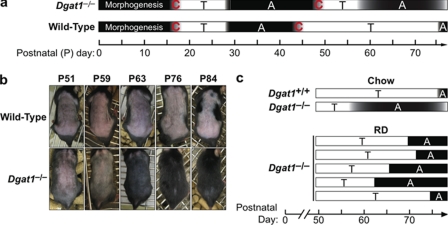
Retinoid Deprivation Prevents Alopecia of Dgat1-/- Mice—Because excess retinoid activity in the skin causes alopecia (29, 30), we hypothesized that the adult onset alopecia in Dgat1-/- mice (18) may be related to increased retinoid activity. To study this, we first examined Dgat1 expression during a depilation-induced, synchronized hair cycle and found that Dgat1 mRNA was detectable in skin and that its levels varied according to cycle stage (supplemental Fig. 1). We next characterized in detail the alopecia phenotype of Dgat1-/- mice. Alopecia appeared first as a prominent bald patch in the dorsal caudal region around 9 weeks of age. The skin within the patch was gray; new hairs emerged a few days later, and the hair regrew by 11 weeks of age (Fig. 3a). However, patchy alopecia in a different location always ensued and persisted in 5–7-month-old mice, which also exhibited generalized hair thinning (Fig. 3b). The cyclic nature of alopecia suggested that excessive hair shedding, not hair cycle arrest, was the primary cause of alopecia in Dgat1-/- mice. To test for excessive hair shedding, an adhesive tape hair removal test (31) was performed. Before 9 weeks of age, similar amounts of hair were removed by tape in wild-type and Dgat1-/- mice; thereafter, more hairs were removed from Dgat1-/- mice than wild type (Fig. 3, c and d). Microscopy revealed that the hair club ends were intact (not shown), consistent with shedding rather than breakage as the cause of hair loss.
FIGURE 3.
Cyclical alopecia and excessive hair shedding in Dgat1-/- mice. a, a Dgat1-/- mouse photographed at 8, 9, and 11 weeks of age. Note the first appearance of alopecia (white box) in the dorsal caudal skin region at 9 weeks of age and complete regrowth of hair in the same region (white box) by 11 weeks of age. b, a Dgat1-/- mouse photographed at 5 and 7 months of age. Note the different locations of alopecia (boxed areas) at two ages. c, adhesive tape test of dorsal hair loss in P28, P52, and P63 wild-type and Dgat1-/- mice (n = 3/genotype/time point; each tape represents hair from a different mouse). d, quantification of hair removed by adhesive tape (n = 3/genotype/time point). *, p < 0.001 versus wild-type.
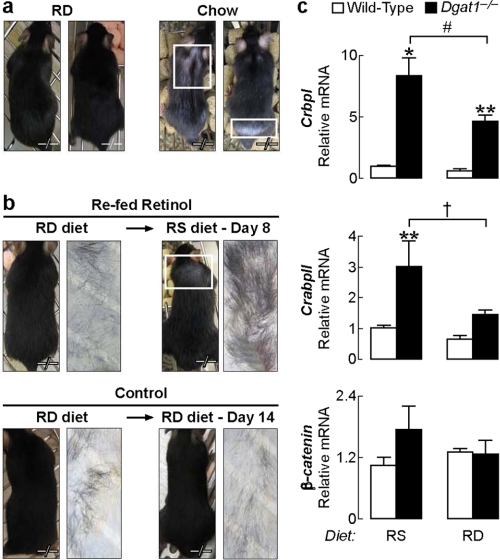
We hypothesized that reducing retinoid levels in the skin would prevent excessive hair shedding and alopecia in Dgat1-/- mice. To test this hypothesis, we depleted skin retinoids by inducing postnatal retinoid deficiency, which was achieved by feeding dams a retinoid-deficient diet during gestation to limit prenatal retinol stores in their offspring, followed by postnatal depletion of retinol stores by maintaining the offspring on a retinoid-deficient diet. The retinoid-deficient diet markedly decreased levels of serum retinol and hepatic retinoids in adult wild-type and Dgat1-/- mice (Fig. 1, c and d). In both genotypes, skin retinyl esters were not significantly reduced, but skin retinol content was reduced >50%. With retinol deprivation, skin levels of all-trans-RA were no longer elevated in Dgat1-/- mice (Fig. 1b).
Dgat1-/- mice fed the retinoid-deficient diet until 15 weeks of age did not develop alopecia, whereas chow-fed Dgat1-/- controls did after 9 weeks of age (Fig. 4a). When retinoid-depleted Dgat1-/- mice resumed the retinoid-sufficient diet, alopecia developed within a week, and adhesive tape tests revealed excessive hair shedding (Fig. 4b). This was not seen in Dgat1-/- mice maintained on the retinoid-deficient diet. The lack of alopecia in retinoid-depleted mice was accompanied by reduced levels of CrbpI and CrabpII mRNAs (44 and 52%, respectively) in Dgat1-/- skin, indicating reduced retinoid activity (Fig. 4c).
FIGURE 4.
Retinoid deprivation prevents alopecia in Dgat1-/- mice. a, retinoid-depleted (retinoid-deficient diet) Dgat1-/- mice (n = 6) did not develop alopecia by 13 weeks of age, whereas chow-fed Dgat1-/- mice (n = 5) had prominent alopecia by 13 weeks of age. b, in retinoid-depleted Dgat1-/- mice, resumption of the retinoid-sufficient diet at age 13 weeks induced alopecia within 8 days (n = 3/group). c, mRNA levels of CrbpI were lower, and CrabpII trended lower in whole skin in Dgat1-/- mice fed the retinoid-deficient diet than in those fed the retinoid-sufficient diet (age 7.5–14 weeks, n = 2–6 mice/group). *, p < 0.001; **, p < 0.05 versus wild type; #, p < 0.05; †, p = 0.079 versus retinoid-sufficient diet. RD, retinoid-deficient; RS, retinoid-sufficient.
Retinoid Deprivation Partially Corrects Altered Hair Cycling in Dgat1-/- Mice—During the first two postnatal (P) murine hair cycles that occur over several months, dorsal skin follicles cycle synchronously (32) (Fig. 5a). Based on the cyclic nature of alopecia in Dgat1-/- mice, we hypothesized that DGAT1 deficiency altered hair cycling. Histological analyses revealed that hair cycling progressed similarly in wild-type and Dgat1-/- follicles from morphogenesis to the first telogen-to-anagen transition (not shown). The appearance of follicles at postnatal days 39 and 41 (P39 and P41) (late first anagen) was also similar (supplemental Fig. 2a). However, at P44, Dgat1-/- follicles were in anagen rather than telogen, suggesting that the first anagen was prolonged (supplemental Fig. 2a). This was confirmed by increased bromodeoxyuridine staining in the bulbs of P44 Dgat1-/- follicles (supplemental Fig. 2b), indicating active proliferation of hair matrix keratinocytes (32). By P50, Dgat1-/- follicles, like those of wild-type mice, had entered telogen (supplemental Fig. 2a). However, Dgat1-/- follicles precociously entered the second anagen. Wild-type mice remained in telogen from P51 to P76, as evidenced by lack of hair growth after shaving, but Dgat1-/- mice exhibited hair growth by P63 (Fig. 5b). Retinoid depletion induced by the retinoid-deficient diet delayed the early onset of the second anagen in Dgat1-/- mice by 5–12 days (Fig. 5c).
FIGURE 5.
Altered hair cycling in Dgat1-/- mice. a, schematic of the first two postnatal hair cycles in Dgat1-/- and wild-type mice (P44, n = 6/genotype; P63, n = 5/genotype; n = 3/genotype at all other time points). A, anagen (black); C, catagen (black gradient); T, telogen (white). b, precocious onset of second anagen in Dgat1-/- mice. Dgat1-/- and wild-type mice were shaved on P51 and photographed at various time points thereafter. Precocious anagen onset is evident in Dgat1-/- mice by the appearance of new hair at P63 and complete regrowth of hair by P76 (n = 5/genotype). c, retinoid deprivation modulates the onset of the second anagen phase in Dgat1-/- mice. Schematic time line depicting the onset of the second postnatal anagen phase in five retinoid-depleted Dgat1-/- mice compared with the average age of onset of second anagen in chow-fed Dgat1-/- and wild-type mice (32). T, telogen (white bar); A, anagen (black bar). RD, retinoid-deficient; RS, retinoid-sufficient.
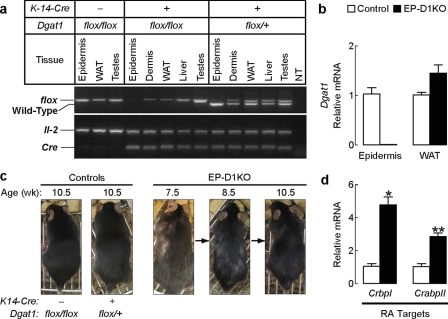
DGAT1 Deficiency in Epidermal Compartment Causes Alopecia and Alters Retinoid Activity in Skin—The effects of DGAT1 deficiency on retinoid homeostasis in the skin may be due to systemic changes in metabolism or to the loss of the enzyme in the epidermal compartment. To distinguish between these possibilities, we generated mice lacking Dgat1 specifically in the epidermis and epidermal appendages, including hair follicles (EP-D1KO mice), by crossing Dgat1flox/flox mice with transgenic mice expressing Cre recombinase under the human keratin 14 promoter (33). PCR of DNA from wild-type and EP-D1KO mice demonstrated specific deletion of the floxed Dgat1 allele in the epidermis (Fig. 6a). Dgat1 mRNA levels were undetectable in epidermis of EP-D1KO mice but were not reduced in a control tissue (WAT) (Fig. 6b). EP-D1KO mice developed prominent alopecia as early as 7 weeks (Fig. 6c), whereas control mice did not develop alopecia (Fig. 6c). The earlier development of alopecia in EP-D1KO compared with Dgat1-/- mice may be due to differences in their genetic background. CrbpI and CrabpII mRNA levels in whole skin were ∼4.5- and ∼3-fold higher, respectively, in EP-D1KO mice (Fig. 6d). Thus, deletion of the enzyme in skin was sufficient to cause altered retinoid metabolism and the alopecia phenotype.
FIGURE 6.
Effects of Dgat1 deficiency on retinoid homeostasis are epidermis-autonomous. a, specific deletion of Dgat1 in epidermis. Shown is PCR detection of the wild-type and floxed allele of Dgat1 and Cre transgene in genomic DNA. Interleukin-2 (IL-2) served as an internal PCR control. The absence of a PCR band indicates Cre-mediated recombination. b, absence of Dgat1 mRNA in the epidermis of K14-Cre+Dgat1flox/flox (EP-D1KO) mice. mRNA levels of Dgat1 in the tail epidermis and WAT were quantified by real time PCR. (age 13 weeks, n = 3–6 mice/group). c, cyclical alopecia in EP-D1KO mice. Alopecia was detectable by 7.5 weeks of age. Note partial hair regrowth by 10.5 weeks of age. Alopecia was not observed in control mice. (n = 6/genotype). d, increased mRNA expression of RA target genes in whole skin of EP-D1KO mice. mRNA levels were quantified by real time PCR (age 13 weeks, n = 5/genotype). *, p < 0.0001; **, p = 0.0002 versus control.
DISCUSSION
We show here that DGAT1 functions as an ARAT in murine skin. In mice lacking DGAT1 activity that were fed a retinol-sufficient diet, RA levels were elevated in the skin, as were RAR target genes, consistent with retinoid toxicity. These mice exhibit alopecia that is probably from increased shedding, which in turn may relate to sebaceous gland dysfunction (18). Lowering retinol levels with a retinoid-deficient diet lowered RA levels, resulted in the appearance of small and possibly immature sebaceous glands,3 and prevented alopecia and abnormal hair cycling in Dgat1-/- mice. Further, Dgat1-/- skin was more susceptible to toxicity from topically applied retinol. These results demonstrate that DGAT1 functions as an ARAT in vivo in murine skin, where it plays an important role in maintaining normal retinoid homeostasis.
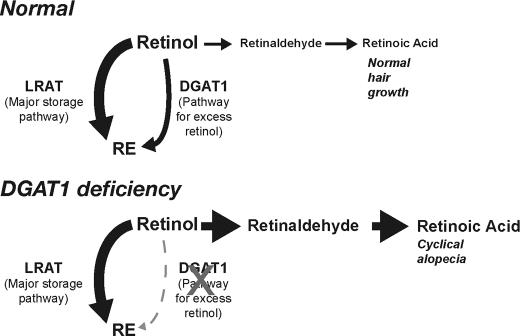
Studies of Lrat-/- mice revealed that LRAT is the predominant retinol acyltransferase for maintaining adequate supplies of retinol (8–10, 15) particularly in the liver, where most retinol is stored. Consistent with these findings, our study shows that Dgat1 inactivation did not predispose mice to retinol deficiency. Retinyl ester levels in liver and other tissues (WAT, brown adipose tissue, skeletal muscle, and brain; not shown) and serum retinol levels in Dgat1-/- mice fed a retinoid-sufficient or retinoid-deficient diet were similar to levels of wild-type mice. In contrast to the storage function of LRAT, our study suggests that the ARAT activity of DGAT1 functions in the epidermis primarily to limit local RA concentrations and protect against retinoid toxicity (see model in Fig. 7). Dgat1-/- skin exhibited enhanced epidermal hyperplasia and irritation in response to a range of retinol concentrations that did not affect wild-type skin. Additionally, the cyclical alopecia and excessive hair shedding in Dgat1-/- mice were probably due to excessive retinoid action, since lowering retinol levels in the skin through retinol deprivation largely prevented these phenotypic manifestations. Supporting the idea that ARAT activity prevents retinoid toxicity, the Km for retinol as a substrate for the ARAT reaction is higher than that for the LRAT reaction (34), and ARAT utilizes predominantly unbound retinol as a substrate (12).
FIGURE 7.
Model of how DGAT1 deficiency modulates RA signaling in skin. LRAT is the major pathway for maintaining adequate stores of retinol, as retinyl esters, in the skin. However, the ARAT activity of DGAT1 is important for generating retinyl esters when retinol levels are excessive and preventing retinol toxicity. In DGAT1 deficiency in murine skin, excess retinol is converted to retinaldehyde and subsequently to RAs, which modulate gene expression and hair cycling. If retinol is depleted from the diet, the effects of DGAT1 deficiency are minimized.
Our study adds to accumulating evidence that the ARAT function of DGAT1 is biologically relevant in vivo. Recently, Wongsiriroj et al. (15) reported that Dgat1 functions as an ARAT in murine intestine. Retinol absorption in the small intestines of Dgat1-/- mice was impaired when the mice were challenged with pharmacological doses of retinol, supporting the idea that Dgat1 is important for handling free retinol that exceeds the capacity of LRAT. Thus, studies with Dgat1-/- mice have now demonstrated the importance of ARAT activity by DGAT1 in two tissues, the small intestine and skin.
Studies of retinoid metabolism have previously suggested a role for retinol esterification in maintaining retinoid homeostasis. In human skin and cultured keratinocytes, higher retinol concentrations increase retinol esterification without increasing RA levels (35, 36). Interestingly, treating cultured human keratinocytes with RA increases the conversion of retinol to retinyl esters and reduces endogenous RA production (37). In our study, DGAT1 deficiency increased RA levels in the skin of Dgat1-/- mice and increased the expression of RA target genes, Crbp and CrabpII. These changes may have exacerbated the retinoid toxicity phenotype. ApoCRBP is a potent inhibitor of LRAT (38), and increased expression may therefore further limit retinol esterification. CRABPII delivers RA to RAR in the nucleus (39) and therefore may further sensitize cells to RA.
Our study identifies DGAT1, through its modulation of retinoid homeostasis, as a potential regulator of hair cycling in mice. In the absence of DGAT1, mice exhibited altered hair cycling, characterized by an increased propensity of follicles to be in anagen. These findings are consistent with a study in mice, in which topical retinoids applied to telogen hair follicles rapidly stimulated and prolonged anagen (40). Our results are also consistent with observations that retinoids exert diverse effects on hair, including promoting hair loss and, paradoxically, hair growth (40, 41). Precisely how retinoids regulate hair cycling is unclear. However, several important molecules of retinoid metabolism (e.g. CRABPII, retinol dehydrogenase, and aldehyde dehydrogenases) and retinoid signaling (RARβ and RARα) exhibit spatiotemporal expression patterns in the hair follicle that are cycle-dependent, suggesting that local retinoid production and signaling are required in different structures of the follicle at each stage of the cycle (42, 43). Of note, Dgat1 mRNA levels were highest during telogen (supplemental Fig. 1), suggesting a role for Dgat1 in maintaining hair follicles in this quiescent phase and preventing them from entering anagen. Although our study highlights the role of retinoids in regulating hair cycling, it also highlights the need for more mechanistic studies in this area, including those aimed at delineating the role of DGAT1 in follicle biology.
Although the retinoid-deficient diet normalized all-trans-RA levels in the skin and prevented alopecia in Dgat1-/- mice, it did not completely normalize the increased expression of RA target genes or prevent the precocious onset of second anagen. A possible explanation is that increased all-trans-RA levels in specific subcompartments of the skin were not detectable in whole skin. Alternatively, other biologically active retinoids in skin, such as 3,4-didehydro-retinoic acid (44), which was not measured, may have been increased in Dgat1-/- skin.
Our study shows that at least one aspect of the pleiotropic phenotype of murine Dgat1 inactivation, alopecia, results in part from changes in retinoid homeostasis. It does not exclude the possibility that other functions of DGAT1, such as triacylglycerol synthesis, may contribute to this phenotype. Nevertheless, it will be of interest to determine if other aspects of the Dgat1 knock-out phenotype are similarly related to retinoid metabolism. For example, Dgat1-/- mice exhibit increased insulin sensitivity and resistance to diet-induced obesity (17). Circulating retinol-binding protein 4 levels are linked to insulin resistance (45), and other studies show connections between retinol metabolism and adiposity (46, 47). Thus, aspects of the metabolic phenotype in Dgat1-/- mice may be retinoid-dependent.
Finally, it will be interesting to determine if our findings concerning DGAT1, retinoid homeostasis, and hair biology are relevant to humans. DGAT1 has not yet been studied in human skin; our studies clearly highlight the need for further investigation. DGAT1 inhibitors have been developed as potential agents to treat metabolic diseases, such as obesity and hyperlipidemia (48, 49). Thus, our findings are relevant to potential side effects of such compounds. They also suggest that these compounds may have utility for the treatment of skin or hair disorders.
Supplementary Material
Acknowledgments
We thank H. Chen for early contributions to this study, J. D. Fish for histological assistance, A. E. Folias for assistance in retinoid measurements, R. Bituin for mouse husbandry, S. Espineda for blastocyst microinjections, J. Carroll for graphics, G. Howard and S. Ordway for editorial assistance, D. Jones for manuscript preparation, members of the Farese laboratory for discussions and comments, and K. Feingold, J. Herz, D. Pearce, B. Yu, and D. Srivastava for helpful comments on the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants DK-056084 (to R. F.) and DK36870 (to J. L. N.). This work was also supported by National Center for Research Resources extramural research facilities Grant C06 RR018928 and by the J. David Gladstone Institutes. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1 and Figs. 1–3.
Footnotes
The abbreviations used are: RA, retinoic acid; RAR, RA receptor; WAT, white adipose tissue; LRAT, lecithin:retinol acyltransferase; CRBP, cellular retinol-binding protein; ARAT, acyl-CoA:retinol acyltransferase; DGAT, acyl-CoA: diacylglycerol acyltransferase; Pn, postnatal day n.
M. Shih, unpublished observations.
References
- 1.Balmer, J. E., and Blomhoff, R. (2002) J. Lipid Res. 43 1773-1808 [DOI] [PubMed] [Google Scholar]
- 2.Mark, M., Ghyselinck, N. B., and Chambon, P. (2006) Annu. Rev. Pharmacol. Toxicol. 46 451-480 [DOI] [PubMed] [Google Scholar]
- 3.Azais-Braesco, V., and Pascal, G. (2000) Am. J. Clin. Nutr. 71 1325S-1333S [DOI] [PubMed] [Google Scholar]
- 4.Hansen, L. A., Sigman, C. C., Andreola, F., Ross, S. A., Kelloff, G. J., and De Luca, L. M. (2000) Carcinogenesis 21 1271-1279 [PubMed] [Google Scholar]
- 5.Napoli, J. L. (1999) Biochim. Biophys. Acta 1440 139-162 [DOI] [PubMed] [Google Scholar]
- 6.Ong, D. E., Kakkad, B., and MacDonald, P. N. (1987) J. Biol. Chem. 262 2729-2736 [PubMed] [Google Scholar]
- 7.MacDonald, P. N., and Ong, D. E. (1988) J. Biol. Chem. 263 12478-12482 [PubMed] [Google Scholar]
- 8.Batten, M. L., Imanishi, Y., Maeda, T., Tu, D. C., Moise, A. R., Bronson, D., Possin, D., Van Gelder, R. N., Baehr, W., and Palczewski, K. (2004) J. Biol. Chem. 279 10422-10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu, L., and Gudas, L. J. (2005) J. Biol. Chem. 280 40226-40234 [DOI] [PubMed] [Google Scholar]
- 10.O'Byrne, S. M., Wongsiriroj, N., Libien, J., Vogel, S., Goldberg, I. J., Baehr, W., Palczewski, K., and Blaner, W. S. (2005) J. Biol. Chem. 280 35647-35657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross, A. C. (1982) J. Lipid Res. 23 133-144 [PubMed] [Google Scholar]
- 12.Torma, H., and Vahlquist, A. (1987) J. Invest. Dermatol. 88 398-402 [DOI] [PubMed] [Google Scholar]
- 13.Orland, M. D., Anwar, K., Cromley, D., Chu, C. H., Chen, L., Billheimer, J. T., Hussain, M. M., and Cheng, D. (2005) Biochim. Biophys. Acta 1737 76-82 [DOI] [PubMed] [Google Scholar]
- 14.Yen, C. L., Monetti, M., Burri, B. J., and Farese, R. V., Jr. (2005) J. Lipid Res. 46 1502-1511 [DOI] [PubMed] [Google Scholar]
- 15.Wongsiriroj, N., Piantedosi, R., Palczewski, K., Goldberg, I. J., Johnston, T. P., Li, E., and Blaner, W. S. (2008) J. Biol. Chem. 283 13510-13519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith, S. J., Cases, S., Jensen, D. R., Chen, H. C., Sande, E., Tow, B., Sanan, D. A., Raber, J., Eckel, R. H., and Farese, R. V., Jr. (2000) Nat. Genet. 25 87-90 [DOI] [PubMed] [Google Scholar]
- 17.Chen, H. C., Smith, S. J., Ladha, Z., Jensen, D. R., Ferreira, L. D., Pulawa, L. K., McGuire, J. G., Pitas, R. E., Eckel, R. H., and Farese, R. V., Jr. (2002) J. Clin. Invest. 109 1049-1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen, H. C., Smith, S. J., Tow, B., Elias, P. M., and Farese, R. V., Jr. (2002) J. Clin. Invest. 109 175-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cases, S., Zhou, P., Shillingford, J. M., Wiseman, B. S., Fish, J. D., Angle, C. S., Hennighausen, L., Werb, Z., and Farese, R. V., Jr. (2004) Development (Camb.) 131 3047-3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reichrath, J., Lehmann, B., Carlberg, C., Varani, J., and Zouboulis, C. C. (2007) Horm. Metab. Res. 39 71-84 [DOI] [PubMed] [Google Scholar]
- 21.Zouboulis, C. C. (2001) Skin Pharmacol. Appl. Skin Physiol. 14 303-315 [DOI] [PubMed] [Google Scholar]
- 22.Berth-Jones, J., and Hutchinson, P. E. (1995) Br. J. Dermatol. 132 367-375 [DOI] [PubMed] [Google Scholar]
- 23.Clarke, S. B., Nelson, A. M., George, R. E., and Thiboutot, D. M. (2007) Dermatol. Clin. 25 137-146 [DOI] [PubMed] [Google Scholar]
- 24.Dassule, H. R., Lewis, P., Bei, M., Maas, R., and McMahon, A. P. (2000) Development (Cambr.) 127 4775-4785 [DOI] [PubMed] [Google Scholar]
- 25.Kane, M. A., Chen, N., Sparks, S., and Napoli, J. L. (2005) Biochem. J. 388 363-369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meiner, V., Tam, C., Gunn, M. D., Dong, L. M., Weisgraber, K. H., Novak, S., Myers, H. M., Erickson, S. K., and Farese, R. V., Jr. (1997) J. Lipid Res. 38 1928-1933 [PubMed] [Google Scholar]
- 27.Fisher, G. J., and Voorhees, J. J. (1996) FASEB J. 10 1002-1013 [DOI] [PubMed] [Google Scholar]
- 28.Elias, P. M. (1986) J. Am. Acad. Dermatol. 15 797-809 [DOI] [PubMed] [Google Scholar]
- 29.Look, J., Landwehr, J., Bauer, F., Hoffmann, A. S., Bluethmann, H., and LeMotte, P. (1995) Am. J. Physiol. 269 E91-E98 [DOI] [PubMed] [Google Scholar]
- 30.Foitzik, K., Spexard, T., Nakamura, M., Halsner, U., and Paus, R. (2005) J. Invest. Dermatol. 124 1119-1126 [DOI] [PubMed] [Google Scholar]
- 31.Hanakawa, Y., Matsuyoshi, N., and Stanley, J. R. (2002) J. Invest. Dermatol. 119 27-31 [DOI] [PubMed] [Google Scholar]
- 32.Muller-Rover, S., Handjiski, B., van der Veen, C., Eichmuller, S., Foitzik, K., McKay, I. A., Stenn, K. S., and Paus, R. (2001) J. Invest. Dermatol. 117 3-15 [DOI] [PubMed] [Google Scholar]
- 33.Gritli-Linde, A., Hallberg, K., Harfe, B. D., Reyahi, A., Kannius-Janson, M., Nilsson, J., Cobourne, M. T., Sharpe, P. T., McMahon, A. P., and Linde, A. (2007) Dev. Cell 12 99-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Randolph, R. K., Winkler, K. E., and Ross, A. C. (1991) Arch. Biochem. Biophys. 288 500-508 [DOI] [PubMed] [Google Scholar]
- 35.Randolph, R. K., and Simon, M. (1993) J. Biol. Chem. 268 9198-9205 [PubMed] [Google Scholar]
- 36.Kang, S., Duell, E. A., Fisher, G. J., Datta, S. C., Wang, Z.-Q., Reddy, A. P., Tavakkol, A., Yi, J. Y., Griffiths, C. E. M., Elder, J. T., and Voorhees, J. J. (1995) J. Invest. Dermatol. 105 549-556 [DOI] [PubMed] [Google Scholar]
- 37.Kurlandsky, S. B., Duell, E. A., Kang, S., Voorhees, J. J., and Fisher, G. J. (1996) J. Biol. Chem. 271 15346-15352 [DOI] [PubMed] [Google Scholar]
- 38.Herr, F. M., and Ong, D. E. (1992) Biochemistry 31 6748-6755 [DOI] [PubMed] [Google Scholar]
- 39.Budhu, A. S., and Noy, N. (2002) Mol. Cell Biol. 22 2632-2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bazzano, G., Terezakis, N., Attia, H., Bazzano, A., Dover, R., Fenton, D., Mandir, N., Celleno, L., Tamburro, M., and Jaconi, S. (1993) J. Invest. Dermatol. 101 138S-142S [DOI] [PubMed] [Google Scholar]
- 41.Bazzano, G. S., Terezakis, N., and Galen, W. (1986) J. Am. Acad. Dermatol. 15 880-883, 890–893 [DOI] [PubMed] [Google Scholar]
- 42.Everts, H. B., King, L. E., Jr., Sundberg, J. P., and Ong, D. E. (2004) J. Investig. Dermatol. 123 258-263 [DOI] [PubMed] [Google Scholar]
- 43.Everts, H. B., Sundberg, J. P., King, L. E., Jr., and Ong, D. E. (2007) J. Investig. Dermatol. 127 1593-1604 [DOI] [PubMed] [Google Scholar]
- 44.Randolph, R. K., and Simon, M. (1997) J. Lipid Res. 38 1374-1383 [PubMed] [Google Scholar]
- 45.Yang, Q., Graham, T. E., Mody, N., Preitner, F., Peroni, O. D., Zabolotny, J. M., Kotani, K., Quadro, L., and Kahn, B. B. (2005) Nature 436 356-362 [DOI] [PubMed] [Google Scholar]
- 46.Zhang, M., Hu, P., Krois, C. R., Kane, M. A., and Napoli, J. L. (2007) FASEB J. 21 2886-2896 [DOI] [PubMed] [Google Scholar]
- 47.Ziouzenkova, O., Orasanu, G., Sharlach, M., Akiyama, T. E., Berger, J. P., Viereck, J., Hamilton, J. A., Tang, G., Dolnikowski, G. G., Vogel, S., Duester, G., and Plutzky, J. (2007) Nat. Med. 13 695-702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao, G., Souers, A. J., Voorbach, M., Falls, H. D., Droz, B., Brodjian, S., Lau, Y. Y., Iyengar, R. R., Gao, J., Judd, A. S., Wagaw, S. H., Ravn, M. M., Engstrom, K. M., Lynch, J. K., Mulhern, M. M., Freeman, J., Dayton, B. D., Wang, X., Grihalde, N., Fry, D., Beno, D. W., Marsh, K. C., Su, Z., Diaz, G. J., Collins, C. A., Sham, H., Reilly, R. M., Brune, M. E., and Kym, P. R. (2008) J. Med. Chem. 51 380-383 [DOI] [PubMed] [Google Scholar]
- 49.Matsuda, D., and Tomoda, H. (2007) Curr. Opin. Investig. Drugs 8 836-841 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.