Abstract
We have shown that the mammalian target of rapamycin (mTOR) down-regulates thrombin-induced ICAM-1 expression in endothelial cells by suppressing the activation of NF-κB. However, the mechanisms by which mTOR is activated to modulate these responses remain to be addressed. Here, we show that thrombin engages protein kinase C (PKC)-δ and phosphattidylinositol 3-kinase (PI3K)/Akt pathways to activate mTOR and thereby dampens NF-κB activation and intercellular adhesion molecule 1 (ICAM-1) expression. Stimulation of human vascular endothelial cells with thrombin induced the phosphorylation of mTOR and its downstream target p70 S6 kinase in a PKC-δ- and PI3K/Akt-dependent manner. Consistent with this, thrombin-induced phosphorylation of p70 S6 kinase was defective in embryonic fibroblasts from mice with targeted disruption of PKC-δ (Pkc-δ–/–), p85α and p85β subunits of the PI3K (p85α–/–β–/–), or Akt1 and Akt2 (Akt1–/–2–/–). Furthermore, we observed that expression of the constitutively active form of PKC-δ or Akt was sufficient to induce NF-κB activation and ICAM-1 expression, and that co-expression of mTOR suppressed these responses. In reciprocal experiments, inhibition/depletion of mTOR augmented NF-κB activation and ICAM-1 expression induced by PKC-δ or Akt. In control experiments, increasing or impairing mTOR signaling by the above approaches produced similar effects on NF-κB activation and ICAM-1 expression induced by thrombin. Thus, these data reveal an important role of PKC-δ and PI3K/Akt pathways in activating mTOR as an endogenous modulator to ensure a tight regulation of NF-κB signaling of ICAM-1 expression in endothelial cells.
Stable adhesion of polymorphonuclear leukocytes (PMN)3 to the endothelium constitutes a crucial step in the mechanism of PMN recruitment from blood to the site of inflammation, and involves the expression of intercellular adhesion molecule-1 (ICAM-1; CD54) on the endothelial cell surface and activation of its counter-receptor β2 integrins (CD11/CD18) on the PMN surface (1). The interaction of ICAM-1 with β2 integrins enables PMN to adhere firmly to the vascular endothelium and migrate across the endothelial barrier (2, 3). Although ICAM-1 is constitutively expressed in low levels in endothelial cells, its expression can be induced by proinflammatory mediators such as the procoagulant thrombin (4, 5), released during intravascular coagulation initiated by tissue injury or sepsis (6–9). We have shown that the transcription factor NF-κB p65 (RelA/p65) is an essential regulator of ICAM-1 gene transcription in endothelial cells (4). Signals mediating NF-κB activation are initiated by the ligation of the protease-activated receptor-1 and are relayed through heterotrimeric G-protein Gαq and Gβγ (10). Activation of Gαq and dissociation of Gβγ complex results in stimulation of PKCδ, and PI3K and the downstream kinase Akt-dependent pathways (4, 10). These pathways converge to activate IκBβ kinase (IKKβ), which in turn catalyzes the phosphorylation and consequently, degradation of IκBα (11–14), the prototype of a family of inhibitory proteins that sequester NF-κB as an inactive complex in the cytoplasm (15). The released NF-κB undergoes rapid nuclear translocation and subsequent binding to the promoter activates transcription of the ICAM-1 gene (4, 5).
Mammalian target of rapamycin (mTOR) is a highly conserved serine/threonine kinase belonging to the family of phosphatidylinositol kinase-like kinases (16). mTOR is the master regulator of cell growth, predominantly by virtue of controlling the phosphorylation of at least two regulators of protein synthesis: p70 S6 kinase and an inhibitor of translation initiation, eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) (17, 18). mTOR was originally discovered during studies into the mechanism of action of the immunosuppressant rapamycin (19–21). Rapamycin complexes with high affinity with its cellular receptor FK506-binding protein 12 (FKBP12) and the resulting rapamycin-FKBP12 complex specifically binds to mTOR and prevents mTOR-dependent downstream signaling (17, 19, 22). Using rapamycin, we recently showed that inhibition of mTOR potentiates thrombin-induced ICAM-1 expression by augmenting IKK/NF-κB activation in endothelial cells (23), suggesting an inhibitory role of mTOR in these responses.
In view of the above findings showing an stimulatory role for PKC-δ and PI3K/Akt (5, 10), and an inhibitory role for mTOR (23) in the mechanism of ICAM-1 expression, we sought to determine whether there is cross-talk between these stimulatory and inhibitory pathways that leads to a tight regulation of NF-κB activation and ICAM-1 expression in endothelial cells. Our results show that thrombin engages PKC-δ and PI3K/Akt to activate mTOR, in addition to mediating NF-κB activation and ICAM-1 expression (5, 10). We further show that activation of mTOR by these kinases serves to dampen NF-κB signaling of ICAM-1 expression in endothelial cells.
EXPERIMENTAL PROCEDURES
Reagents—Human α-thrombin was purchased from Enzyme Research Laboratories (South Bend, IN). Rapamycin chelerythrine, Go6976, and LY 294002 were all purchased from Calbiochem-Novabiochem Corp. (La Jolla, CA). Polyclonal antibodies to RelA/p65 and β-actin, and monoclonal antibodies to ICAM-1 and p70 S6 kinase were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). A polyclonal antibody that detects p70 S6 kinase when phosphorylated at Thr421 and Ser424 and mTOR when phosphorylated at Ser2448 were obtained from Cell Signaling Technology (Beverly, MA). In addition, polyvinylidene difluoride membrane was from Millipore Corp. (Bradford, MA); plasmid maxi kit from Qiagen Inc. (Valencia, CA); DEAE-dextran from Sigma; and the protein assay kit and nitrocellulose membrane were from Bio-Rad. Lipofectamine 2000 transfection reagent was purchased from Invitrogen. All other materials were from VWR Scientific Products Corporation (Bridgeport, NJ).
cDNA and siRNA Constructs—The construct pNF-κB-LUC containing 5 copies of the consensus NF-κB sequences linked to a minimal E1B promoter-luciferase gene was obtained from Stratagene (La Jolla, CA). The constructs pcDNA3.1-mTOR-shRNA encoding mTOR short hairpin RNA (mTOR-shRNA) and pRK5-myc-mTOR encoding the myc-tagged wild type mTOR (mTOR-WT) (24, 25) were gifts from David M. Sabatini (MIT, Boston, MA). Expression vector encoding the hemagglutinin (HA)-tagged constitutively active form of Akt (Akt-CAT) is described elsewhere (26, 27). The constructs pcDNA3HA-PKC-δ-CAT and pcDNA3HA-PKC-δ-KD encoding HA-tagged constitutively active and kinase-defective forms of mouse PKC-δ, respectively, were gifts from Jae-Won Soh and I. B. Weinstein (Columbia University, NY) (28). The constitutively active form of PKC-δ (PKC-δ-CAT) contains only the catalytic domain and was generated by deletion of the regulatory domain (28). The kinase-deficient mutant of PKC-δ (PKC-δ-KD) was created by replacing Lys376 with Arg (K376R) (28).
Endothelial Cell Culture—Human umbilical vein endothelial cell (HUVEC) cultures were established as described previously (29, 30) by using umbilical cords collected within 48 h of delivery. Human lung microvascular endothelial cells (HLMVEC) were obtained from Clonetics (San Diego, CA). Cells were cultured as described (23) in gelatin-coated flasks using endothelial basal medium 2 (EBM2) with bullet kit™ additives (Bio-Whittaker, Walkersville, MD). In all experiments, unless otherwise indicated, cells were washed twice with serum-free MCDB-131 medium and incubated in the same serum-free medium for 0.5–1 h prior to thrombin challenge. Cells used in the experiments were between 3 and 6 passages.
Mouse Embryonic Fibroblast Cell Culture—Immortalized Akt1–/–2–/– (31) and p85α–/–β–/– (32, 33) mouse embryonic fibroblasts (MEFs) have been previously described. The immortalized p85α–/–β–/– MEFs from the double p85α/p85β knock-out mice (34) were previously provided by the laboratory of Dr. Lewis Cantley (Boston, MA). Mouse embryonic fibroblasts from Pkc-δ–/– knock-out mice (35) and corresponding parental MEFs were immortalized by retroviral infection using E6/E7 oncoproteins of human papillomavirus. Briefly, 1.5 ml of viral supernatant was filtered through a 0.22-μm filter and mixed with 3.5 ml of Dulbecco's modified Eagle's medium and 4 μl of 10 mg/ml Polybrene. After overnight infection, fresh media was added to the plate to keep the immortalized cells in culture. All different immortalized knock-out MEFs were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, penicillin, and streptomycin at 37 °C in 5% CO2.
Cell Lysis, Immunoprecipitation, and Immunoblotting—Cells were lysed in a phosphorylation lysis buffer (50 mm HEPES, 150 mm NaCl, 200 μm sodium orthovanadate, 10 mm sodium pyrophosphate, 1 mm EDTA, 1.5 mm magnesium chloride, 100 mm sodium fluoride, 10% glycerol, 0.5 to 1% Triton X-100, 1 mm phenylmethylsulfonyl fluoride, and protease inhibitor mixture (Sigma)) or in radioimmune precipitation (RIPA) buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 5 mm sodiium fluoride, 0.25 mm EDTA, pH 8.0, 1% deoxycholic acid, 1% Triton X, 1 mm sodium orthovanadate) supplemented with protease inhibitor mixture (Sigma). Cell lysates were resolved on SDS-PAGE and transferred onto nitrocellulose or polyvinylidene difluoride membranes. The residual binding sites on the membranes were blocked by incubation with 5% (w/v) non-fat dry milk in TBST (10 mm Tris, pH 8.0, 150 mm NaCl, and 0.05% Tween 20) for 1 h at room temperature. The membranes were then incubated with appropriate antibodies and developed using an ECL method as previously described (36). For immunoprecipitation, cell lysates were prepared in 300 μl of phosphorylation lysis buffer and then subjected to preclearing with 10 μl of protein A/G-agarose beads (Santa Cruz Biotechnology) for 4 h at 4°C. The precleared lysates were subjected to immunoprecipitation by incubating with 0.6–1 μg of appropriate antibody and 10 μl of the protein A/G-agarose beads at 4 °C overnight with gentle shaking as described (37). The immunoprecipitates were washed 4 times with the same volume of ice-cold phosphorylation lysis buffer. The proteins in the immunoprecipitates were extracted by boiling with SDS sample buffer for 5 min. The extracted proteins were subsequently analyzed by immunoblotting as described above.
Reporter Gene Constructs, Endothelial Cell Transfection, and Luciferase Assay—Transfections were performed using the DEAE-dextran method (38) with slight modifications (5). Briefly, 5 μg of DNA was mixed with 50 μg/ml DEAE-dextran in serum-free EBM2 and the mixture was added onto 60–80% confluent cells. We used 0.125 μg of pTKRLUC plasmid (Promega, Madison, WI) containing the Renilla luciferase gene driven by the constitutively active thymidine kinase promoter to normalize the transfection efficiencies. After 1 h, cells were incubated for 4 min with 10% dimethyl sulfoxide in serum-free EBM2. The cells were then washed 2 times with EBM2, 10% fetal bovine serum and grown to confluence. We achieved transfection efficiency of 16 ± 3 (mean ± S.D.; n = 3) in these cells (36). Cell extracts were prepared and assayed for firefly and Renilla luciferase activities using the Promega Biotech Dual Luciferase Reporter Assay System. The data are expressed as a ratio of firefly to Renilla luciferase activity. In some experiments where pTKRLUC was not used, data are expressed as firefly luciferase activity.
Overexpression of mTOR, PKC-δ-CAT, and Akt-CAT Constructs in Endothelial Cells—The constructs encoding mTOR-WT, PKC-δ-CAT, Akt-CAT, and mTOR-shRNA were transfected in endothelial cells using Lipofectamine reagent (Invitrogen) as per the manufacturer's protocol. Briefly, cDNA (1.5 μg) was mixed with 1.5 μl of Lipofectamine 2000 in 100 μl of serum-free EBM2. After a 15–20-min incubation at room temperature, 0.9 ml of EBM2, 10% fetal bovine serum was added and the mixture applied to 60–70% confluent cells that had been washed once with serum-free EBM2. Three hours later, the medium was replaced with EBM2, 10% fetal bovine serum and the cells were used after 24–30 h for analyses.
Statistical Analysis—Data are expressed as mean ± S.E. Comparisons between experimental groups were made by Student's t test. Differences in mean values were considered significant at p < 0.05.
RESULTS
Thrombin Induces Biphasic Activation of mTOR in Endothelial Cells—We determined the time course of mTOR activation by monitoring its phosphorylation on Ser2448. Results showed that thrombin induced mTOR phosphorylation in a biphasic manner (5 min and 2 h after stimulation) (Fig. 1A). As expected, phosphorylation (Thr421 and Ser424) of p70 S6 kinase, a well established downstream target of mTOR (17, 18, 22) paralleled the time course of mTOR phosphorylation by thrombin (Fig. 1A). Notably, p70 S6 kinase phosphorylation was evident up to 4 h after thrombin challenge, suggesting that the late phase of mTOR activation was sustained (Fig. 1A). Pretreatment of cells with rapamycin, an inhibitor of mTOR prevented the basal as well as thrombin-induced phosphorylation of p70 S6 kinase (Fig. 1B). Similarly, siRNA-mediated depletion of mTOR also inhibited the early phase (5 min) (Fig. 1C) as well as the late phase (2 h) (data not shown) of p70 S6 kinase phosphorylation, confirming the activation of mTOR by thrombin. Therefore, in subsequent experiments the phosphorylation of p70 S6 kinase was used as an indicator of mTOR activation.
FIGURE 1.
A, thrombin induces biphasic phosphorylation of mTOR and p70 S6 kinase in endothelial cells. Confluent HUVEC monolayers were challenged with thrombin (5 units/ml) for the indicated time periods. Total cell lysates were separated by SDS-PAGE and immunoblotted with an anti-phospho-mTOR (Ser2248) or anti-phospho-p70 S6K (Thr421/Ser424) antibody. The blots were subsequently stripped and reprobed with an antibody to p70 S6K to monitor loading. The bar graph represents the level of mTOR and p70 S6K phosphorylation induced by thrombin at different time points. mTOR and p70 S6K phosphorylation normalized to total p70 S6K is expressed relative to the untreated control set at 1. Data are mean ± S.E. (n = 4 for each condition). *, p < 0.05 compared with untreated control. B and C, inhibition or depletion of mTOR prevents p70 S6 kinase phosphorylation in endothelial cells. HUVEC were (B) pretreated with rapamycin (5 ng/ml) for 45 min prior to challenge with thrombin (5 units/ml) for the indicated time periods or (C) transfected with siRNA targeting mTOR (siRNA-mTOR) or control siRNA (siRNA-Con) and then challenged with thrombin (5 units/ml) for 5 min. Total cell lysates were separated by SDS-PAGE, and immunoblotted with an (B and C) anti-phospho-p70 S6K (Thr421/Ser424) antibody or (C) anti-mTOR antibody. Total p70 S6 kinase levels were used to monitor loading. The bar graph represents the effect of rapamycin (A) or RNAi (B) knockdown of mTOR on p70 S6K phosphorylation. p70 S6K phosphorylation normalized to total p70 S6K is expressed relative to the untreated control set at 1. Data are mean ± S.E. (n = 3 for each condition). *, p < 0.05 compared with untreated control; Ψ, p < 0.05 compared with untreated control; #, p < 0.05 compared with thrombin-stimulated control.
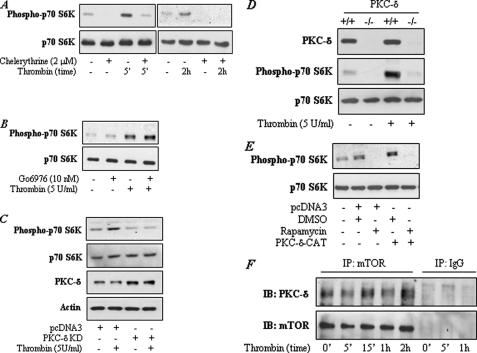
Inhibition or Targeted Deletion of PKC-δ Impairs mTOR Activation—We used pharmacological inhibitors to assess the possible role of PKC-δ in mediating thrombin-induced mTOR activation in endothelial cells. Pretreatment of cells with chelerythrine, a broad-spectrum PKC inhibitor, prevented phosphorylation of p70 S6 kinase indicating the involvement of PKC in the response (Fig. 2A). We next employed Gö6976, a relatively specific inhibitor of PKC-α, to examine the involvement of PKC-α in the response. Inhibition of PKC-α by this approach failed to prevent thrombin-induced (Fig. 2B), but was effective in interfering with PMA-induced p70 S6 kinase phosphorylation (data not shown). Because PKC-α and PKC-δ represent two abundant PKC isoforms in endothelial cells that are activated by thrombin (5, 39), the ability of chelerythrine and the failure of Gö6976 to prevent p70 S6 kinase phosphorylation provided a clue for the involvement of PKC-δ in mTOR activation. We explored this possibility by assessing the effect of expressing the kinase-defective mutant of PKC-δ (PKC-δ-KD) on p70 S6 kinase phosphorylation. Expression of PKC-δ-KD in endothelial cells inhibited p70 S6 kinase phosphorylation by thrombin (Fig. 2C), indicating a role of PKC-δ in the response. To further establish the involvement of PKC-δ in mTOR activation, we used embryonic fibroblasts from mice with targeted disruption of the gene encoding PKC-δ. Stimulation of wild type (Pkc-δ+/+) MEFs with thrombin resulted in increased phosphorylation of p70 S6 kinase and this response was abolished in PKC-δ null (Pkc-δ–/–) MEFs (Fig. 2D). These data confirm an important role of PKC-δ in signaling mTOR activation and also indicate that this pathway is conserved in other cell types and species as well.
FIGURE 2.
A–C, inhibition of PKC-δ prevents thrombin-induced p70 S6 kinase phosphorylation in endothelial cells. HUVEC were pretreated with chelerythrine (2 μm) (A) or Go6976 (10 nm) (B) or transfected with pcDNA3-PKCδ-KD (C) encoding kinase-defective PKC-δ (PKCδ-KD) mutant or empty vector (pcDNA3) using Lipofectamine 2000 as described under “Experimental Procedures.” Cells were then challenged with thrombin (5 units/ml) for (A) the indicated time periods or (B and C) 5 min. Total cell lysates were separated by SDS-PAGE and immunoblotted with an anti-phospho p70 S6K (Thr421/Ser424). Total p70 S6K levels were used to monitor loading. Lysates in C were also immunoblotted with an anti-PKC-δ or anti-β-actin antibody. Results are representative of two to three separate experiments. D, loss of PKC-δ impairs thrombin-induced p70 S6 kinase phosphorylation in mouse embryonic fibroblasts. Pkcδ+/+ and Pkcδ–/– immortalized MEFs were challenged with thrombin for 5 min. Total cell lysates were separated by SDS-PAGE, and immunoblotted with an anti-PKC-δ or anti-phospho-p70 S6K (Thr421/Ser424) antibody. Total p70 S6K levels were used to monitor loading. Results are representative of two separate experiments. E, activation of PKC-δ is sufficient to induce p70 S6 kinase phosphorylation in endothelial cells. HUVEC were transfected with the construct pcDNA3-PKCδ-CAT encoding constitutively active PKC-δ (PKCδ-CAT) mutant or empty vector (pcDNA3) using Lipofectamine 2000 as described under “Experimental Procedures.” Cells were incubated with rapamycin (5 ng/ml) or dimethyl sulfoxide (vehicle) alone for 6–8 h. Total cell lysates were separated by SDS-PAGE, and immunoblotted with an anti-phospho-p70 S6K antibody. Total p70 S6K levels were used to monitor loading. Results are representative of two separate experiments. F, PKC-δ is constitutively associated with mTOR in endothelial cells. HUVEC were challenged with thrombin (5 units/ml) for the indicated times. Total cell lysates were immunoprecipitated with an antibody to mTOR or IgG. The immunoprecipitates were then immunoblotted with an anti-PKC-δ and mTOR antibody. Results are representative of three separate experiments.
We also examined whether activation of PKC-δ is sufficient to induce mTOR activation in endothelial cells. Cells were transfected with a construct encoding an constitutively active PKC-δ mutant (PKC-δ-CAT, which contains the catalytic domain but lacks the regulatory domain) and the phosphorylation status of p70 S6 kinase was determined. Results showed an increased phosphorylation of p70 S6 kinase in cells transfected with PKC-δ-CAT compared with the cells transfected with empty vector. Pretreatment of cells with rapamycin prevented p70 S6 kinase phosphorylation (Fig. 2E). These results show that expression of PKC-δ-CAT is capable of activating mTOR in the absence of thrombin challenge. We next asked whether mTOR associates with PKC-δ for its activation. We determined this possibility by immunoprecipitating mTOR from control and thrombin-challenged cells and then analyzed these precipitates by immunoblotting for the presence of PKC-δ. We found that PKC-δ is constitutively associated with mTOR and that this interaction does not appear to be influenced by thrombin (Fig. 2F). In control IgG immunoprecipitates, we failed to detect the interaction of PKC-δ with mTOR (Fig. 2F), indicating the specificity of the interaction.
In reciprocal experiments, we assessed the effect of depleting mTOR on thrombin-induced activation of PKC-δ, as determined by the phosphorylation of PKC-δ at Thr505. Thrombin induced the phosphorylation of PKC-δ in a time-dependent manner, with maximal phosphorylation occurring at 5 min after thrombin challenge (supplemental data Fig. S1A). RNAi knockdown of mTOR failed to influence PKC-δ activation by thrombin (supplemental data Fig. S1B). Together, these data indicate the requirement of PKC-δ in mTOR activation by thrombin.
Inhibition or Targeted Deletion of PI3K and Akt Impairs mTOR Activation—We determined whether PI3K also participates in thrombin activation of mTOR. Pretreatment with LY294002, a relatively specific PI3K inhibitor, inhibited p70 S6 kinase phosphorylation in both HUVEC and HLMVEC (Fig. 3, A and B), suggesting a role of PI3K in the response. In a related experiment, we used wortmannin, another PI3K inhibitor, to verify the effect of LY294002 on mTOR signaling. Inhibition of PI3K by this approach also impaired p70 S6 kinase phosphorylation (Fig. 3C). Additionally, we found that p70 S6 kinase phosphorylation was defective in embryonic fibroblasts from mice with targeted disruption of the p85α and p85β subunits of the PI3K (p85α–/–β–/–) (Fig. 3D), confirming the involvement of PI3K in mTOR activation by thrombin.
FIGURE 3.
A–C, inhibition of PI3K impairs thrombin-induced p70 S6 kinase phosphorylation. Confluent (A) HUVEC or (B) HLMVEC monolayers were pretreated with LY294002 (50 μm) prior to challenge with thrombin (5 units/ml) for the indicated time periods. C, confluent HUVEC monolayers were pretreated with wortmannin (50 nm) prior to challenge with thrombin (5 units/ml) for the indicated time periods. Total cell lysates were immunoblotted with an anti-phospho-p70 S6K (Thr421/Ser424) antibody. Total p70 S6K levels were used to monitor loading. The bar graph represents the effect of wortmannin on thrombin-induced p70 S6K phosphorylation. p70 S6K phosphorylation normalized to total p70 S6K is expressed relative to the untreated control set at 1. Data are mean ± S.E. (n = 3 for each condition). *, p < 0.05 compared with untreated control; #, p < 0.05 compared with thrombin-stimulated control. D, loss of PI3K prevents thrombin-induced p70 S6 kinase phosphorylation. p85α+/+/β+/+ and p85α–/–/β–/– MEFs were challenged with thrombin (5 units/ml) for 5 min. Total cell lysates were immunoblotted with an anti-phospho-p70 S6K (Thr421/Ser424) antibody. Total p70 S6K levels were used to monitor loading. Results are representative of two to three separate experiments.
We next examined whether PI3K activates mTOR via Akt. To this end, we first determined the involvement of PI3K in mediating the activation of Akt by thrombin. Results showed that thrombin induced the phosphorylation of Akt in a time-dependent manner and that this response was inhibited in cells pretreated with LY294002 (Fig. 4, A and B). Similarly, thrombin-induced Akt phosphorylation was impaired in p85α–/– β–/– MEFs (Fig. 4C). To address the role of Akt in mTOR activation by thrombin, we used MEFs from mice with targeted disruption of the Akt1 and Akt2 subunits (Akt1–/–2–/–). As shown in Fig. 4D, thrombin failed to induce p70 S6 kinase phosphorylation in Akt1–/–2–/– MEFs. These results demonstrate that thrombin engages PI3K/Akt, in addition to PKC-δ, to activate mTOR signaling.
FIGURE 4.
A–C: A and B, inhibition of PI3K prevents thrombin-induced Akt phosphorylation. HUVEC were (A) challenged with thrombin (5 units/ml) for the indicated time periods or (B) pretreated with LY294002 (50 μm) prior to challenge with thrombin (5 units/ml) for 5 min. Total cell lysates were separated with SDS-PAGE and immunoblotted with an anti-phospho-Akt (Ser473) antibody. Total Akt levels were used to monitor loading. The bar graphs represent (A) the time course of Akt phosphorylation induced by thrombin and (B) the effect of LY294002 on thrombin-induced Akt phosphorylation. Akt phosphorylation normalized to total Akt is expressed relative to the untreated control set at 1. Data are mean ± S.E. (n = 3 for each condition). *, p < 0.05 compared with untreated control; #, p < 0.05 compared with thrombin-stimulated control. C, loss of PI3K prevents thrombin-induced Akt phosphorylation. p85α+/+β+/+ and p85α–/–β–/– MEFs were challenged with thrombin (5 units/ml) for 5 min. Total cell lysates were separated with SDS-PAGE and immunoblotted with an anti-phospho-Akt (Ser473) antibody. Total Akt or actin levels were used to monitor loading. Results are representative of two to three separate experiments. D, loss of Akt1 and Akt2 subunits prevents thrombin-induced p70 S6 kinase phosphorylation. Akt1+/+2+/+ and Akt1–/–2–/– MEFs were challenged with thrombin (5 units/ml) for 5 min. Total cell lysates were immunoblotted with an anti-Akt or anti-phospho-p70 S6K antibody. Total p70 S6K levels were used to monitor loading. Results are representative of three separate experiments.
Overexpression of mTOR Dampens NF-κB Activation and ICAM-1 Expression Induced by PKC-δ and Akt—The above findings led us to test the hypothesis that activation of mTOR by PKC-δ and PI3K/Akt serves to tightly regulate NF-κB activation and ICAM-1 expression following thrombin challenge of endothelial cells. To test this possibility, we determined the effects of augmenting mTOR signaling on NF-κB activity and ICAM-1 expression induced by PKC-δ or Akt. We observed that expression of PKC-δ-CAT or Akt-CAT, each was capable of inducing NF-κB activity in the absence of thrombin challenge and that overexpression of mTOR inhibited this response (Fig. 5, A and B).
FIGURE 5.
Overexpression of mTOR suppresses PKC-δ- and Akt-induced NF-κB activity in endothelial cells. A, HUVEC were transfected with NF-κBLUC in combination with pcDNA3-PKCδ-CAT encoding constitutively active PKC-δ (PKC-δ-CAT) and pRK5-mTOR-WT encoding wild type mTOR (mTOR-WT) using DEAE-dextran as described under “Experimental Procedures.” B, HUVEC were transfected with NF-κB LUC in combination with pcDNA3-Akt-CAT encoding constitutively active Akt (Akt-CAT) and pRK5-mTOR-WT using Lipofectamine 2000 as described under “Experimental Procedures.” After 18 h, cell extracts were prepared and assayed for firefly and Renilla luciferase activities. Data are mean ± S.E. (n = 6 to 9 for each condition). *, p < 0.05 compared with vector-transfected control; #, p < 0.01 compared with PKCδ-CAT- or Akt-CAT-transfected control.
We evaluated whether the suppressive effect of mTOR overexpression on NF-κB activity causes decreased expression of ICAM-1 induced by PKC-δ-CAT or Akt-CAT. For this purpose, we first verified whether transfection of endothelial cells with constructs encoding PKC-δ-CAT Akt-CAT or mTOR-WT yields adequate expression of these proteins. As shown in Fig. 6A, expression of PKC-δ-CAT or Akt-CAT mutants and overexpression of mTOR was noted in cells transduced with the corresponding construct of each molecule. Using this strategy, we found that expression of PKCδ-CAT or Akt-CAT was sufficient to induce ICAM-1 expression and that this response was inhibited in cells overexpressing mTOR (Fig. 6, B and C). In control experiments, we also examined the effect of augmented mTOR signaling on ICAM-1 expression by thrombin. As expected, mTOR overexpression was effective in inhibiting thrombin-induced ICAM-1 expression (supplemental data Fig. S2).
FIGURE 6.
Overexpression of mTOR suppresses PKC-δ- and Akt-induced ICAM-1 expression in endothelial cells. A, HUVEC were transfected with pcDNA3-PKCδ-CAT, pcDNA3-Akt-CAT, or pRK5-mTOR-WT using Lipofectamine 2000 as described under “Experimental Procedures.” After 24 h, total cell lysates were prepared and analyzed by immunoblotting using an anti-PKC-δ, anti-HA, or anti-mTOR antibody to verify the expression of PKCδ-CAT, Akt-CAT, or mTOR, respectively. PKC-δ-CAT corresponds to ∼47 kDa as it contains only the catalytic domain and therefore can be distinguished from the endogenous PKC-δ (76 kDa). Anti-HA antibody allowed the detection of Akt-CAT but not the endogenous Akt. Increased expression of mTOR in mTOR-WT-transduced cells was due to overexpressed mTOR. Actin levels were used to monitor loading. B, HUVEC were transfected with pCDNA3-PKCδ-CAT in combination with pRK5-mTOR-WT using Lipofectamine 2000 as described under “Experimental Procedures.” Total cell lysates were resolved by SDS-PAGE and immunoblotted with an antibody to ICAM-1. Actin levels were used to monitor loading. Results are representative of two separate experiments. C, HUVEC were transfected with pCDNA3-Akt-CAT in combination with pRK5-mTOR-WT using Lipofectamine 2000 as described under “Experimental Procedures.” Total cell lysates were resolved by SDS-PAGE and immunoblotted with an antibody to ICAM-1. Actin levels were used to monitor loading. Results are representative of two separate experiments.
Inhibition or Depletion of mTOR Augments NF-κB Activation and ICAM-1 Expression Induced by PKC-δ and Akt—We next asked if suppressing mTOR signaling augments PKC-δ-CAT- and Akt-CAT-induced NF-κB activity and ICAM-1 expression in endothelial cells. To this end, we determined the effect of inhibiting or depleting mTOR on NF-κB activity and ICAM-1 expression induced by PKC-δ-CAT or Akt-CAT. Pretreatment of cells with rapamycin augmented NF-κB activity induced by PKC-δ-CAT or Akt-CAT (Fig. 7, A and B). In related experiments, co-transfection of short hairpin RNA targeting mTOR (mTOR shRNA) also resulted in potentiation of NF-κB activity induced by PKC-δ-CAT or Akt-CAT (Fig. 7, C and D). Similarly, in control experiments, co-transfection of mTOR shRNA augmented NF-κB activity in HUVEC challenged with thrombin (supplemental data Fig. S3). Consistent with these data, impairing mTOR signaling in HUVEC or HLMVEC augmented ICAM-1 expression induced by PKC-δ-CAT or Akt-CAT (Fig. 8, A–C). As expected, siRNA-mediated depletion of mTOR potentiated thrombin-induced ICAM-1 expression (supplemental data Fig. S4). We also determined whether mTOR influences ICAM-1 expression in response to other proinflammatory mediators such as tumor necrosis factor-α (TNF-α). Results showed that interfering with mTOR signaling also potentiated ICAM-1 expression following TNF-α challenge of endothelial cells.4
FIGURE 7.
A and B, inhibition of mTOR augments PKC-δ- and Akt-induced NF-κB activity in endothelial cells. HUVEC were co-transfected with NF-κBLUC and pcDNA3-PKCδ-CAT (A) or NF-κBLUC and pcDNA3-Akt-CAT (B) using Lipofectamine 2000 as described under “Experimental Procedures.” Cells were treated with rapamycin (5 ng/ml) for 12–18 h, and the cell extracts were assayed for firefly and Renilla luciferase activities. Data are mean ± S.E. (n = 6 to 9 for each condition). *, p < 0.05 compared with vector (pcDNA3)-transfected control; #, p < 0.05 compared with PKC-δ-CAT- or Akt-CAT-transfected control. C and D, depletion of mTOR augments PKC-δ- and Akt-induced NF-κB activity in endothelial cells. C, HUVEC were transfected with NF-κBLUC in combination with pCDNA3-PKCδ-CAT and pCDNA3.1-mTOR shRNA encoding short hairpin RNA targeting mTOR (mTOR shRNA) using DEAE-dextran. D, HUVEC were transfected with NF-κBLUC in combination with pCDNA3-Akt-CAT and pCDNA3.1-mTOR shRNA using Lipofectamine 2000. After 18 h, cells extracts were prepared and assayed for firefly and Renilla luciferase activities. Data are mean ± S.E. (n = 6 to 8 for each condition). *, p < 0.05 compared with vector-transfected control; #, p < 0.05 compared with PKC-δ-CAT- or Akt-CAT-transfected control.
FIGURE 8.
Inhibition or depletion of mTOR augments PKC-δ- and Akt-induced ICAM-1 expression in endothelial cells. HUVEC (A) or HLMVEC (B) were transfected with pCDNA3-PKCδ-CAT or empty vector (pCDNA3) using Lipofectamine 2000 as described under “Experimental Procedures.” Cells were incubated with rapamycin (5 ng/ml) or dimethyl sulfoxide (vehicle) alone for 6–8 h. Total cell lysates were resolved by SDS-PAGE and immunoblotted with an anti-ICAM-1 antibody. Actin levels were used to monitor loading. Results are representative of two separate experiments. C, HUVEC were co-transfected with pCDNA3-Akt-CAT and pCDNA3.1-mTOR shRNA using Lipofectamine 2000. Total cell lysates were resolved by SDS-PAGE and immunoblotted with an anti-ICAM-1 antibody. Actin levels were used to monitor loading (upper panel). The same lysates were re-electrophoresed and immunoblotted with an anti-mTOR antibody to assess the depletion of mTOR (lower panel). Results are representative of two separate experiments.
DISCUSSION
The major findings of this study are that thrombin activates mTOR via PKC-δ- and PI3K/Akt-dependent pathways, and that this event is crucial in modulating activation of NF-κB and expression of ICAM-1 expression in endothelial cells. Our results establish that mTOR is activated in a biphasic manner with an early and transient phase occurring at 5 min followed by a late and sustained phase that begins at 2 h after thrombin stimulation of endothelial cells. We also show that inhibition/deletion of PKC-δ or PI3K/Akt impairs thrombin-induced mTOR activation and that expression of PKC-δ-CAT or Akt-CAT bypasses the requirement of thrombin challenge for mTOR activation, NF-κB activation, and ICAM-1 expression. Augmenting mTOR signaling dampens whereas inhibiting mTOR signaling potentiates PKC-δ-CAT- as well as Akt-CAT-induced NF-κB activation and ICAM-1 expression. These data reveal an important role of PKC-δ and PI3K/Akt pathways in activating mTOR to modulate thrombin-induced NF-κB signaling of ICAM-1 expression in endothelial cells. Importantly, inhibition of mTOR also augmented ICAM-1 expression induced by TNF-α, suggesting that mTOR is a general modifier of these responses.
We used multiple approaches to address the involvement of PKC-δ and PI3K/Akt pathways in the mechanism of thrombin-induced mTOR activation. Inhibition of PKC-δ or PI3K impaired the phosphorylation of p70 S6 kinase. Similarly, p70 S6 kinase phosphorylation was defective in MEFs lacking PKC-δ, p85α and p85β subunits of PI3K, or Akt1 and Akt2 subunits. Notably, thrombin-induced phosphorylation of Akt was inhibited in endothelial cells pretreated with LY294002 as well as in MEFs lacking p85α and p85β subunits of PI3K. Considered together, these data indicate that activation of PKC-δ or PI3K/Akt by thrombin is required to activate mTOR signaling in endothelial cells. Additionally, these data show that PKC-δ or PI3K/Akt signaling of mTOR activation is not restricted only to endothelial cells but is functional in other cells types as well. Indeed, studies have shown that mTOR and other members of phosphatidylinositol kinase-like kinase family are regulated by PKC-δ in various cell types. For example, PKC-δ constitutively interacts with mTOR to regulate phosphorylation of 4E-BP1 and cap-dependent initiation of protein translation in 293T cells (40). PKC-δ and mTOR interaction is also implicated in regulation of stress and insulin-like growth factor 1-induced insulin receptor substrate-1 phosphorylation (Ser312) in MCF-7 cells (41). Besides mTOR, PKC-δ associates with the catalytic subunit of DNA-dependent protein kinase, a member of phosphatidylinositol kinase-like kinases family, and this interaction inhibits the function of the catalytic subunit of DNA-dependent protein kinase to form complexes with DNA and to phosphorylate its downstream target, tumor suppressor p53, and thus contributes to DNA damage-induced apoptosis in U-937 monoblastic leukemia cells (42). Similarly, the regulation of mTOR by PI3K/Akt is well established in a variety of cellular contexts (43–45).
Our results show that PKC-δ and PI3K/Akt each regulates both the early and the late phase of mTOR activation by thrombin. The early phase of mTOR activation parallels the peak activation of PKC-δ or Akt that occurs at 5 min after thrombin challenge. This observation is also consistent with the constitutive association of PKC-δ with mTOR; it is likely that such an interaction provides an efficient mechanism of mTOR phosphorylation upon stimulation with thrombin. Studies have shown a similar association of PKC-δ with c-Src and activation of PKC-δ in this complex leads to rapid phosphorylation and increased activity of c-Src (46). Intriguingly, the late component of mTOR activation is not consistent with markedly reduced activation of PKC-δ at 2 h after thrombin challenge. To account for the dependence of the late phase of mTOR activation on PKC-δ as well as PI3K/Akt, we propose a model wherein activated PKC-δ or Akt, in addition to catalyzing the early phosphorylation of mTOR, participates in the activation of an intermediate kinase(s) that may in turn control the late onset of mTOR/p70 S6 kinase phosphorylation. However, additional studies are required to verify this model.
The activation of mTOR by PKC-δ or PI3K/Akt prompted us to investigate the functional importance of this relationship in regulating NF-κB activation and ICAM-1 expression following thrombin challenge of endothelial cells. Because activation of PKC-δ, PI3K, and Akt mediate IKK/NF-κB signaling of ICAM-1 expression (5, 10), whereas the activation of mTOR is implicated in dampening IKK/NF-κB signaling of ICAM-1 expression (23), we reasoned that engagement of PKC-δ/mTOR and PI3K/Akt/mTOR pathways by thrombin may lead to a tight regulation of NF-κB activation and ICAM-1 in endothelial cells. In pursuit of this possibility, we carried out a series of experiments in which mTOR signaling was altered either by overexpressing or inhibiting/depleting mTOR, and the effects of altering mTOR signaling by these approaches were assessed on NF-κB activation and ICAM-1 expression induced by PKC-δ-CAT or Akt-CAT. Results showed that promoting mTOR signaling in endothelial cells markedly reduced PKC-δ-CAT- or Akt-CAT-induced NF-κB activation and ICAM-1 expression. Conversely, suppressing mTOR signaling in these cells resulted in augmentation of NF-κB activation and ICAM-1 expression in response to PKC-δ-CAT or Akt-CAT expression. Importantly, these results are consistent with the effects of altering mTOR signaling on NF-κB activation and ICAM-1 expression induced by thrombin in control experiments and the previous study (23). Together, these data indicate that thrombin-induced NF-κB activation and ICAM-1 expression are tightly regulated through the functional coupling of PKC-δ and PI3K/Akt to mTOR.
Analysis of time course of mTOR and NF-κB activation provides more insight into the modifier role of mTOR in these responses. Although thrombin activates mTOR in a biphasic manner, activation of NF-κB begins at 0.5 h, peaks between 1 and 2 h, and declines by 4 h after thrombin challenge (23). We also showed that inhibition of mTOR by rapamycin alters the kinetics of thrombin response such that NF-κB activation occurs within 10 min, peaks between 1 and 2 h, and remains significantly elevated at 4 h after thrombin challenge (23). We interpreted these results to indicate that mTOR dampens thrombin-induced ICAM-1 expression in endothelial cells by controlling a delayed and transient activation of NF-κB (23). Notably, these findings are consistent with the early phase (5 min) of mTOR activation that precedes NF-κB activation and the late phase (2 h) of mTOR activation, after which NF-κB activation begins to decline. Thus, activation of mTOR appears to serve as a “bump” in the pathway to limit the strength and duration of NF-κB signaling of ICAM-1 expression. However, the precise mechanism by which mTOR contributes to suppression of IKK/NF-κB activation and thereby ICAM-1 expression is unclear. One possible mechanism for mTOR modulation of IKK/NF-κB signaling may involve activation of serine/threonine protein phosphatases. It should be noted that multiple serine/threonine protein phosphatases including PP2A, PP2B, and PPM1B (formerly PP2Cβ) are implicated in negative regulation of the IKK/NF-κB pathway (47, 48). For example, PP2A is associated with IKKγ and this interaction is responsible for rapid deactivation of IKK, and thereby phosphorylation and degradation of IκB, and activation of NF-κB (47). Similarly, PPM1B is also associated with the IKK complex and this association decreases at early times and is restored at later times after TNF-α treatment to control activation/deactivation of IKK (48). Furthermore, PP2B has been shown to decrease NF-κB activity by promoting dephosphorylation of IκB in response to insulin-like growth factor 1 (49). In view of these findings and the ability of mTOR to regulate serine/threonine phosphatase (50, 51), it is possible that mTOR activates serine/threonine phosphatase(s) to facilitate the inactivation of IKK by catalyzing its dephosphorylation. Our finding that RNAi knockdown of PPM1B augments thrombin-induced NF-κB activity4 is consistent with this possibility.
In contrast to our findings, Dan et al. (52) have recently shown a role of mTOR in mediating NF-κB activation. The exact reason for the differential regulation of NF-κB activation by mTOR is not clear; one notable difference between these studies, however, is the use of different cell types. Given that the signaling mechanism of mTOR activation depends, at least in part, upon the stimulus and the cell-type used (53), it is likely that the observed differences between our study and that of Dan et al. (52) derive from the different cell types (primary endothelial cells versus HeLa and the prostrate cancer cell lines) used in these studies.
In summary, the present study demonstrates that stimulation of PKC-δ and PI3K/Akt activity by thrombin results in activation of two signaling pathways; one stimulatory pathway that involves IKK/NF-κB-dependent ICAM-1 expression (5, 10), and the other inhibitory pathway involving biphasic activation of mTOR, which is engaged to restrict the duration and intensity of NF-κB activation and thereby ICAM-1 expression in endothelial cells. These findings are consistent with the notion that activation of stimulatory and inhibitory pathways by the same upstream signal, but with different kinetics, may be a mechanism of ensuring the tight regulation of cellular responses. Such a notion finds further support from a recent report (54) showing that a dynamic activation of the mitogen-activated protein kinases (p38/JNK) and mitogen-activated protein kinase phosphatase (MKP-1) by the same upstream signals controls the immune balance by temporally regulating both pro-(TNF-α) and anti-inflammatory (interleukin-10) mediators of Toll-like receptor signaling. Thus, augmenting mTOR signaling may be a useful strategy for dampening ICAM-1 expression to limit excessive PMN infiltration associated with inflammatory disease states such as acute respiratory distress syndrome.
Supplementary Material
Acknowledgments
We are grateful to Drs. I. B. Weinstein, Jae-Won Soh, and David M. Sabatini for kindly providing the constructs used in this study.
This work was supported, in whole or in part, by National Institutes of Health Grants CA77816, CA121192, and CA100579 (to L. C. P.), NHLBI Grant HL67424 (to A. R.), and NIEHSC Grant ES-01247. This work was also supported by a Biomedical Research grant from the American Lung Association (to F. F.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
Footnotes
The abbreviations used are: PMN, polymorphonuclear leukocytes; ICAM-1, intercellular adhesion molecule-1; HUVEC, human umbilical vein endothelial cells; HLMVEC, human lung microvascular endothelial cells; NF-κB, nuclear factor-κB; IKK, IκB kinase; mTOR, mammalian target of rapamycin; PKC, protein kinase C; 4E-BP1, eukaryotic initiation factor 4E binding protein; FKBP12, FK506-binding protein 12; PI3K, phosphatidylinositol 3-kinase; LUC, luciferase; siRNA, small interfering RNA; HA, heamgglutinin; MEF, mouse embryonic fibroblast; CAT, chloramphenicol; TNF-α, tumor necrosis factor α; EBM2, endothelial basal medium 2.
M. Minhajuddin, unpublished observations.
References
- 1.Springer, T. A. (1994) Cell 76 301–314 [DOI] [PubMed] [Google Scholar]
- 2.Smith, C. W., Marlin, S. D., Rothlein, R., Toman, C., and Anderson, D. C. (1989) J. Clin. Investig. 83 2008–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doerschuk, C. M., Tasaka, S., and Wang, Q. (2000) Am. J. Respir. Cell Mol. Biol. 23 133–136 [DOI] [PubMed] [Google Scholar]
- 4.Rahman, A., Anwar, K. N., True, A. L., and Malik, A. B. (1999) J. Immunol. 162 5466–5476 [PubMed] [Google Scholar]
- 5.Rahman, A., Anwar, K. N., Uddin, S., Xu, N., Ye, R. D., Platanias, L. C., and Malik, A. B. (2001) Mol. Cell. Biol. 21 5554–5565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schouten, M., Wiersinga, W. J., Levi, M., and van der Poll, T. (2008) J. Leukocyte Biol. 83 536–545 [DOI] [PubMed] [Google Scholar]
- 7.Fenton, J. W., 2nd (1981) Ann. N. Y. Acad. Sci. 370 468–495 [DOI] [PubMed] [Google Scholar]
- 8.Dickneite, G., and Paques, E. P. (1993) Thromb. Haemostasis 69 98–102 [PubMed] [Google Scholar]
- 9.Aird, W. C. (2005) Crit. Care Clin. 21 417–431 [DOI] [PubMed] [Google Scholar]
- 10.Rahman, A., True, A. L., Anwar, K. N., Ye, R. D., Voyno-Yasenetskaya, T. A., and Malik, A. B. (2002) Circ. Res. 91 398–405 [DOI] [PubMed] [Google Scholar]
- 11.Hayden, M. S., and Ghosh, S. (2004) Genes Dev. 18 2195–2224 [DOI] [PubMed] [Google Scholar]
- 12.Karin, M., and Ben-Neriah, Y. (2000) Annu. Rev. Immunol. 18 621–663 [DOI] [PubMed] [Google Scholar]
- 13.Bonizzi, G., and Karin, M. (2004) Trends Immunol. 25 280–288 [DOI] [PubMed] [Google Scholar]
- 14.Aggarwal, B. B. (2004) Cancer Cell 6 203–208 [DOI] [PubMed] [Google Scholar]
- 15.Baldwin, A. S., Jr. (1996) Annu. Rev. Immunol. 14 649–683 [DOI] [PubMed] [Google Scholar]
- 16.Keith, C. T., and Schreiber, S. L. (1995) Science 270 50–51 [DOI] [PubMed] [Google Scholar]
- 17.Gingras, A. C., Raught, B., and Sonenberg, N. (2001) Genes Dev. 15 807–826 [DOI] [PubMed] [Google Scholar]
- 18.Fingar, D. C., Salama, S., Tsou, C., Harlow, E., and Blenis, J. (2002) Genes Dev. 16 1472–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabatini, D. M., Erdjument-Bromage, H., Lui, M., Tempst, P., and Snyder, S. H. (1994) Cell 78 35–43 [DOI] [PubMed] [Google Scholar]
- 20.Brown, E. J., Albers, M. W., Shin, T. B., Ichikawa, K., Keith, C. T., Lane, W. S., and Schreiber, S. L. (1994) Nature 369 756–758 [DOI] [PubMed] [Google Scholar]
- 21.Sabers, C. J., Martin, M. M., Brunn, G. J., Williams, J. M., Dumont, F. J., Wiederrecht, G., and Abraham, R. T. (1995) J. Biol. Chem. 270 815–822 [DOI] [PubMed] [Google Scholar]
- 22.Jacinto, E., and Hall, M. N. (2003) Nat. Rev. Mol. Cell. Biol. 4 117–126 [DOI] [PubMed] [Google Scholar]
- 23.Minhajuddin, M., Fazal, F., Bijli, K. M., Amin, M. R., and Rahman, A. (2005) J. Immunol. 174 5823–5829 [DOI] [PubMed] [Google Scholar]
- 24.Sarbassov, D. D., Ali, S. M., Kim, D. H., Guertin, D. A., Latek, R. R., Erdjument-Bromage, H., Tempst, P., and Sabatini, D. M. (2004) Curr. Biol. 14 1296–1302 [DOI] [PubMed] [Google Scholar]
- 25.Sarbassov, D. D., Guertin, D. A., Ali, S. M., and Sabatini, D. M. (2005) Science 307 1098–1101 [DOI] [PubMed] [Google Scholar]
- 26.Gingras, A. C., Kennedy, S. G., O'Leary, M. A., Sonenberg, N., and Hay, N. (1998) Genes Dev. 12 502–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed, N. N., Grimes, H. L., Bellacosa, A., Chan, T. O., and Tsichlis, P. N. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 3627–3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soh, J. W., Lee, E. H., Prywes, R., and Weinstein, I. B. (1999) Mol. Cell. Biol. 19 1313–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gimbrone, M. A., Jr., Cotran, R. S., and Folkman, J. (1974) J. Cell Biol. 60 673–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner, D. D., Olmsted, J. B., and Marder, V. J. (1982) J. Cell Biol. 95 355–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng, X. D., Xu, P. Z., Chen, M. L., Hahn-Windgassen, A., Skeen, J., Jacobs, J., Sundararajan, D., Chen, W. S., Crawford, S. E., Coleman, K. G., and Hay, N. (2003) Genes Dev. 17 1352–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fruman, D. A., Mauvais-Jarvis, F., Pollard, D. A., Yballe, C. M., Brazil, D., Bronson, R. T., Kahn, C. R., and Cantley, L. C. (2000) Nat. Genet. 26 379–382 [DOI] [PubMed] [Google Scholar]
- 33.Lekmine, F., Uddin, S., Sassano, A., Parmar, S., Brachmann, S. M., Majchrzak, B., Sonenberg, N., Hay, N., Fish, E. N., and Platanias, L. C. (2003) J. Biol. Chem. 278 27772–27780 [DOI] [PubMed] [Google Scholar]
- 34.Brachmann, S. M., Yballe, C. M., Innocenti, M., Deane, J. A., Fruman, D. A., Thomas, S. M., and Cantley, L. C. (2005) Mol. Cell. Biol. 25 2593–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyamoto, A., Nakayama, K., Imaki, H., Hirose, S., Jiang, Y., Abe, M., Tsukiyama, T., Nagahama, H., Ohno, S., Hatakeyama, S., and Nakayama, K. I. (2002) Nature 416 865–869 [DOI] [PubMed] [Google Scholar]
- 36.Anwar, K. N., Fazal, F., Malik, A. B., and Rahman, A. (2004) J. Immunol. 173 6965–6972 [DOI] [PubMed] [Google Scholar]
- 37.Bijli, K. M., Minhajuddin, M., Fazal, F., O'Reilly, M. A., Platanias, L. C., and Rahman, A. (2007) Am. J. Physiol. 292 L396–L404 [DOI] [PubMed] [Google Scholar]
- 38.Lopata, M. A., Cleveland, D. W., and Sollner-Webb, B. (1984) Nucleic Acids Res. 12 5707–5717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta, D., Rahman, A., and Malik, A. B. (2001) J. Biol. Chem. 276 22614–22620 [DOI] [PubMed] [Google Scholar]
- 40.Kumar, V., Pandey, P., Sabatini, D., Kumar, M., Majumder, P. K., Bharti, A., Carmichael, G., Kufe, D., and Kharbanda, S. (2000) EMBO J. 19 1087–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mingo-Sion, A. M., Ferguson, H. A., Koller, E., Reyland, M. E., and Van Den Berg, C. L. (2005) Breast Cancer Res. Treat. 91 259–269 [DOI] [PubMed] [Google Scholar]
- 42.Bharti, A., Kraeft, S. K., Gounder, M., Pandey, P., Jin, S., Yuan, Z. M., Lees-Miller, S. P., Weichselbaum, R., Weaver, D., Chen, L. B., Kufe, D., and Kharbanda, S. (1998) Mol. Cell. Biol. 18 6719–6728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhaskar, P. T., and Hay, N. (2007) Dev. Cell 12 487–502 [DOI] [PubMed] [Google Scholar]
- 44.Marinov, M., Fischer, B., and Arcaro, A. (2007) Crit. Rev. Oncol. Hematol. 63 172–182 [DOI] [PubMed] [Google Scholar]
- 45.Phung, T. L., Ziv, K., Dabydeen, D., Eyiah-Mensah, G., Riveros, M., Perruzzi, C., Sun, J., Monahan-Earley, R. A., Shiojima, I., Nagy, J. A., Lin, M. I., Walsh, K., Dvorak, A. M., Briscoe, D. M., Neeman, M., Sessa, W. C., Dvorak, H. F., and Benjamin, L. E. (2006) Cancer Cell 10 159–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song, J. S., Swann, P. G., Szallasi, Z., Blank, U., Blumberg, P. M., and Rivera, J. (1998) Oncogene 16 3357–3368 [DOI] [PubMed] [Google Scholar]
- 47.Fu, D. X., Kuo, Y. L., Liu, B. Y., Jeang, K. T., and Giam, C. Z. (2003) J. Biol. Chem. 278 1487–1493 [DOI] [PubMed] [Google Scholar]
- 48.Prajapati, S., Verma, U., Yamamoto, Y., Kwak, Y. T., and Gaynor, R. B. (2004) J. Biol. Chem. 279 1739–1746 [DOI] [PubMed] [Google Scholar]
- 49.Pons, S., and Torres-Aleman, I. (2000) J. Biol. Chem. 275 38620–38625 [DOI] [PubMed] [Google Scholar]
- 50.Nien, W. L., Dauphinee, S. M., Moffat, L. D., and Too, C. K. (2007) Mol. Cell. Endocrinol. 263 10–17 [DOI] [PubMed] [Google Scholar]
- 51.Huang, S., Shu, L., Easton, J., Harwood, F. C., Germain, G. S., Ichijo, H., and Houghton, P. J. (2004) J. Biol. Chem. 279 36490–36496 [DOI] [PubMed] [Google Scholar]
- 52.Dan, H. C., Cooper, M. J., Cogswell, P. C., Duncan, J. A., Ting, J. P., and Baldwin, A. S. (2008) Genes Dev. 22 1490–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dan, H. C., and Baldwin, A. S. (2008) J. Immunol. 180 7582–7589 [DOI] [PubMed] [Google Scholar]
- 54.Chi, H., Barry, S. P., Roth, R. J., Wu, J. J., Jones, E. A., Bennett, A. M., and Flavell, R. A. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 2274–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.